 Found 1111 hits with Last Name = 'goldstein' and Initial = 'd'
Found 1111 hits with Last Name = 'goldstein' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR2 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated with enzyme followed by peptide substrate addition by ca... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR3 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314073
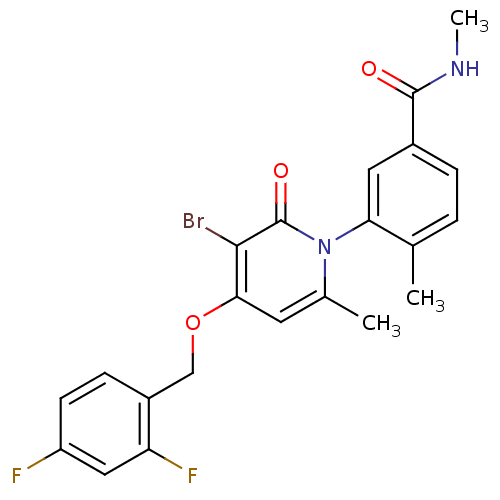
(3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...)Show SMILES CNC(=O)c1ccc(C)c(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(29.32,-4.45,;29.32,-2.91,;27.99,-2.14,;26.65,-2.91,;27.99,-.6,;29.32,.17,;29.31,1.72,;27.98,2.48,;27.97,4.02,;26.66,1.71,;26.65,.17,;25.32,2.48,;25.32,4.03,;26.65,4.8,;23.98,4.79,;22.65,4.02,;21.32,4.79,;19.99,4.02,;18.65,4.79,;18.65,6.33,;17.32,7.1,;16,6.33,;14.66,7.1,;15.99,4.78,;17.32,4.02,;17.33,2.48,;22.65,2.48,;21.32,1.71,;23.99,1.71,;23.99,.17,)| Show InChI InChI=1S/C22H19BrF2N2O3/c1-12-4-5-14(21(28)26-3)9-18(12)27-13(2)8-19(20(23)22(27)29)30-11-15-6-7-16(24)10-17(15)25/h4-10H,11H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha |
J Med Chem 53: 2345-53 (2010)
Article DOI: 10.1021/jm9012906
BindingDB Entry DOI: 10.7270/Q27H1JQC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50418610
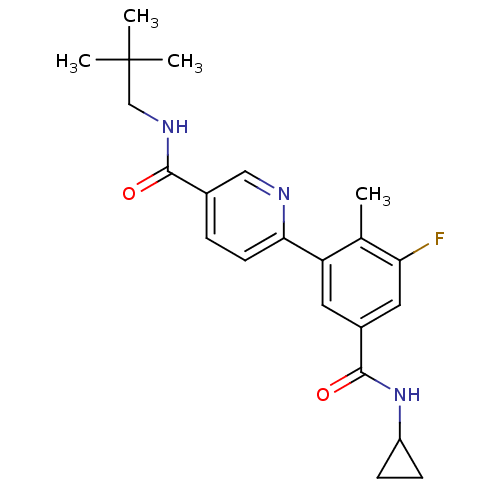
(GW-856553 | GW856553X | LOSMAPIMOD | US10550073, C...)Show SMILES Cc1c(F)cc(cc1-c1ccc(cn1)C(=O)NCC(C)(C)C)C(=O)NC1CC1 Show InChI InChI=1S/C22H26FN3O2/c1-13-17(9-15(10-18(13)23)21(28)26-16-6-7-16)19-8-5-14(11-24-19)20(27)25-12-22(2,3)4/h5,8-11,16H,6-7,12H2,1-4H3,(H,25,27)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha |
J Med Chem 53: 2345-53 (2010)
Article DOI: 10.1021/jm9012906
BindingDB Entry DOI: 10.7270/Q27H1JQC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50418610

(GW-856553 | GW856553X | LOSMAPIMOD | US10550073, C...)Show SMILES Cc1c(F)cc(cc1-c1ccc(cn1)C(=O)NCC(C)(C)C)C(=O)NC1CC1 Show InChI InChI=1S/C22H26FN3O2/c1-13-17(9-15(10-18(13)23)21(28)26-16-6-7-16)19-8-5-14(11-24-19)20(27)25-12-22(2,3)4/h5,8-11,16H,6-7,12H2,1-4H3,(H,25,27)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity to p38beta |
J Med Chem 53: 2345-53 (2010)
Article DOI: 10.1021/jm9012906
BindingDB Entry DOI: 10.7270/Q27H1JQC |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human FGFR4 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394918
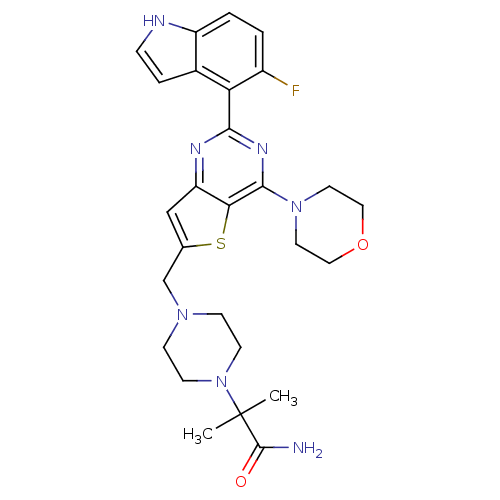
(CHEMBL2165504)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-9-7-33(8-10-35)16-17-15-21-23(38-17)25(34-11-13-37-14-12-34)32-24(31-21)22-18-5-6-30-20(18)4-3-19(22)28/h3-6,15,30H,7-14,16H2,1-2H3,(H2,29,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394917
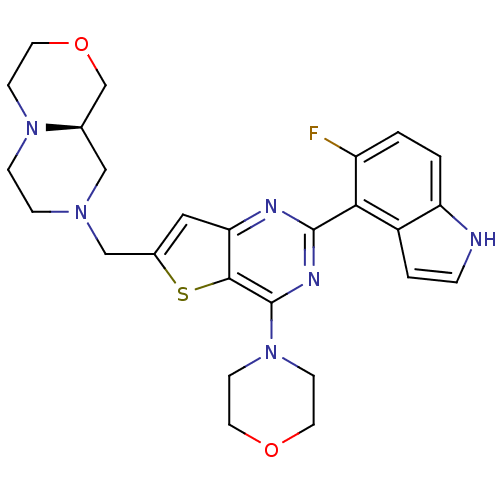
(CHEMBL2165505)Show SMILES Fc1ccc2[nH]ccc2c1-c1nc(N2CCOCC2)c2sc(CN3CCN4CCOC[C@H]4C3)cc2n1 |r| Show InChI InChI=1S/C26H29FN6O2S/c27-20-1-2-21-19(3-4-28-21)23(20)25-29-22-13-18(15-31-5-6-32-7-12-35-16-17(32)14-31)36-24(22)26(30-25)33-8-10-34-11-9-33/h1-4,13,17,28H,5-12,14-16H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589205
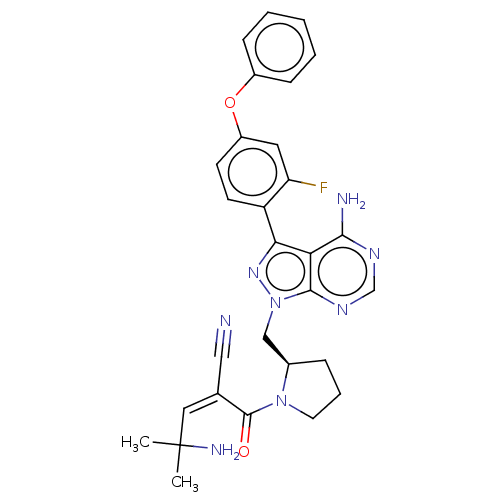
(CHEMBL5174021)Show SMILES CC(C)(N)\C=C(\C#N)C(=O)N1CCC[C@@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192809

(US9187487, 71)Show SMILES CN(C)C(=O)C(=Cc1ccc(Nc2cnc3[nH]cc(C(=O)NC(C)(C)C)c3n2)cc1)C#N |w:6.6| Show InChI InChI=1S/C23H25N7O2/c1-23(2,3)29-21(31)17-12-25-20-19(17)28-18(13-26-20)27-16-8-6-14(7-9-16)10-15(11-24)22(32)30(4)5/h6-10,12-13H,1-5H3,(H,25,26)(H,27,28)(H,29,31)/b15-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192804

(US9187487, 64)Show SMILES CN1CCN(CC1)C(=O)C(=Cc1ccc(Nc2cnc3[nH]cc(C(=O)C(C)(C)C)c3n2)cc1)C#N |w:10.11| Show InChI InChI=1S/C26H29N7O2/c1-26(2,3)23(34)20-15-28-24-22(20)31-21(16-29-24)30-19-7-5-17(6-8-19)13-18(14-27)25(35)33-11-9-32(4)10-12-33/h5-8,13,15-16H,9-12H2,1-4H3,(H,28,29)(H,30,31)/b18-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192818

(US9187487, 83)Show SMILES CC(C)NC(=O)c1c[nH]c2ncc(Nc3ccc(C=C(C#N)C(=O)N4CCNCC4)cc3)nc12 |w:18.17| Show InChI InChI=1S/C24H26N8O2/c1-15(2)29-23(33)19-13-27-22-21(19)31-20(14-28-22)30-18-5-3-16(4-6-18)11-17(12-25)24(34)32-9-7-26-8-10-32/h3-6,11,13-15,26H,7-10H2,1-2H3,(H,27,28)(H,29,33)(H,30,31)/b17-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM521835
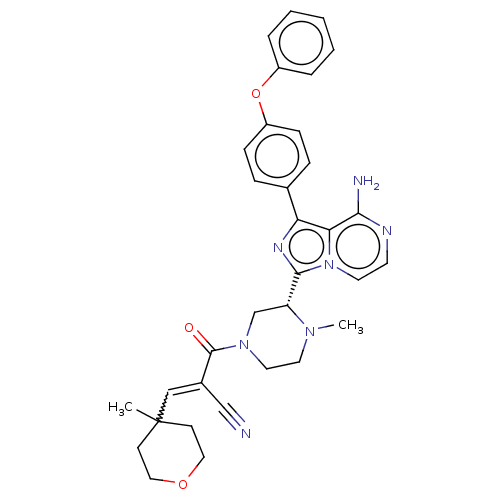
(US11155544, Compound 67)Show SMILES CN1CCN(C[C@@H]1c1nc(-c2ccc(Oc3ccccc3)cc2)c2c(N)nccn12)C(=O)C(=CC1(C)CCOCC1)C#N |w:33.38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of BTK kinase activity of a compound of the pre... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P92J3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192799

(US9187487, 50)Show SMILES CC(C)NC(=O)c1c[nH]c2ncc(Nc3ccc(C=C(C#N)C(=O)N(C)C)cc3)nc12 |w:18.17| Show InChI InChI=1S/C22H23N7O2/c1-13(2)26-21(30)17-11-24-20-19(17)28-18(12-25-20)27-16-7-5-14(6-8-16)9-15(10-23)22(31)29(3)4/h5-9,11-13H,1-4H3,(H,24,25)(H,26,30)(H,27,28)/b15-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50388189
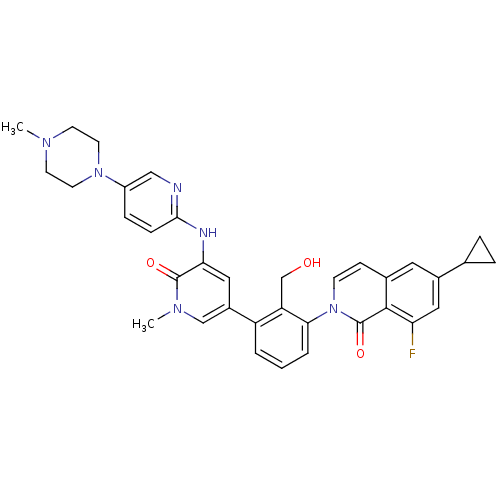
(CHEMBL2057918)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)nc1 Show InChI InChI=1S/C35H35FN6O3/c1-39-12-14-41(15-13-39)26-8-9-32(37-19-26)38-30-18-25(20-40(2)34(30)44)27-4-3-5-31(28(27)21-43)42-11-10-23-16-24(22-6-7-22)17-29(36)33(23)35(42)45/h3-5,8-11,16-20,22,43H,6-7,12-15,21H2,1-2H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of BTK by TR-FRET based competitive assay |
J Med Chem 55: 4539-50 (2012)
Article DOI: 10.1021/jm300035p
BindingDB Entry DOI: 10.7270/Q27H1KMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM521834
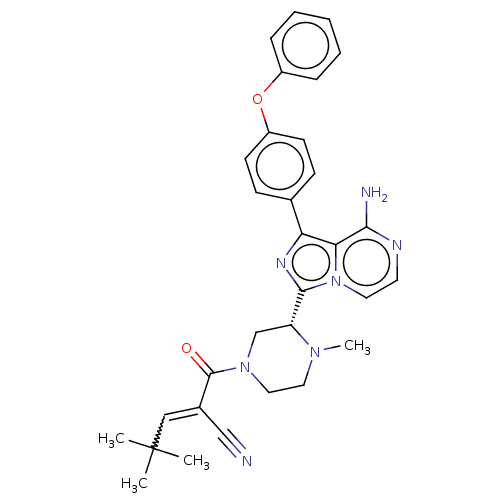
(US11155544, Compound 66)Show SMILES CN1CCN(C[C@@H]1c1nc(-c2ccc(Oc3ccccc3)cc2)c2c(N)nccn12)C(=O)C(=CC(C)(C)C)C#N |w:33.38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of BTK kinase activity of a compound of the pre... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P92J3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589186
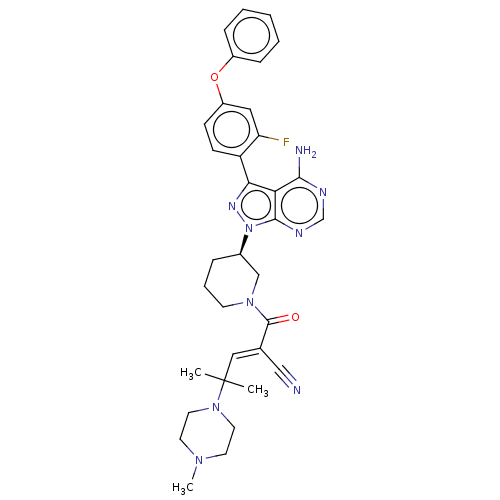
(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM521782
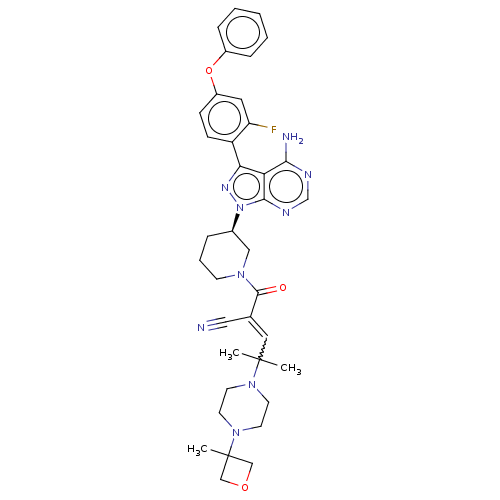
(US11155544, Compound 8)Show SMILES CC(C)(C=C(C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1(C)COC1 |w:3.2| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of BTK kinase activity of a compound of the pre... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P92J3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192802
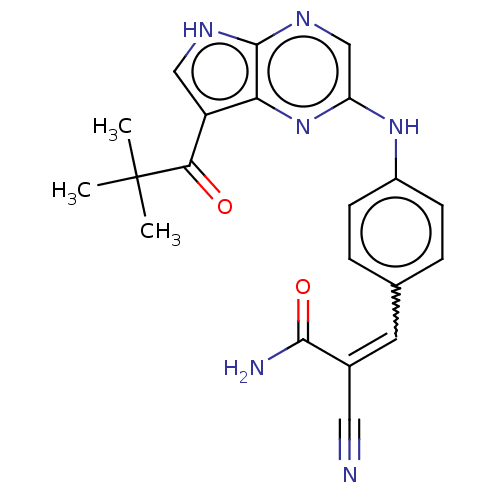
(US9187487, 60)Show SMILES CC(C)(C)C(=O)c1c[nH]c2ncc(Nc3ccc(C=C(C#N)C(N)=O)cc3)nc12 |w:18.17| Show InChI InChI=1S/C21H20N6O2/c1-21(2,3)18(28)15-10-24-20-17(15)27-16(11-25-20)26-14-6-4-12(5-7-14)8-13(9-22)19(23)29/h4-8,10-11H,1-3H3,(H2,23,29)(H,24,25)(H,26,27)/b13-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM197248
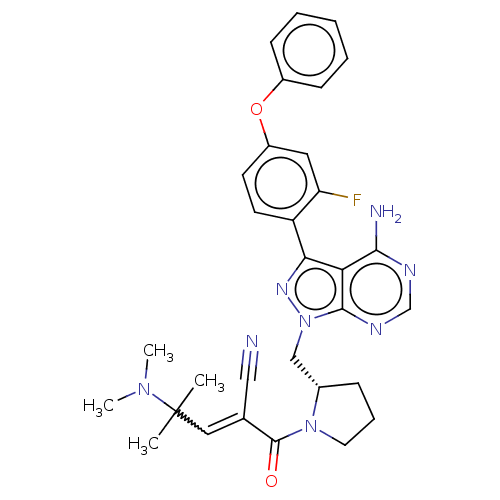
(US9090621, 84B)Show SMILES CN(C)C(C)(C)C=C(C#N)C(=O)N1CCC[C@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r,w:6.5| Show InChI InChI=1S/C31H33FN8O2/c1-31(2,38(3)4)16-20(17-33)30(41)39-14-8-9-21(39)18-40-29-26(28(34)35-19-36-29)27(37-40)24-13-12-23(15-25(24)32)42-22-10-6-5-7-11-22/h5-7,10-13,15-16,19,21H,8-9,14,18H2,1-4H3,(H2,34,35,36)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Principia Biopharma Inc.
US Patent
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of Formula... |
US Patent US9090621 (2015)
BindingDB Entry DOI: 10.7270/Q27M06QW |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM287008
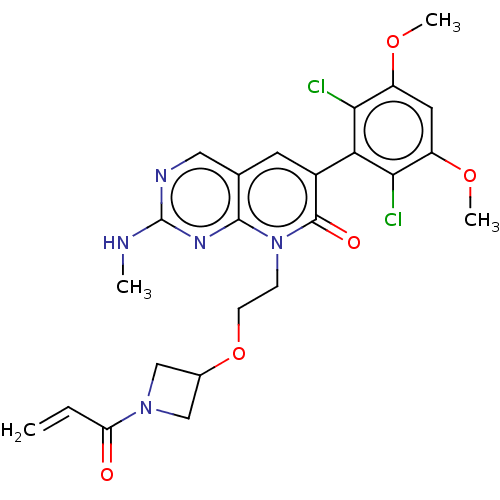
(8-(2-((1-acryloylazetidin-3-yl)oxy)ethyl)-6-(2,6-d...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCOC3CN(C3)C(=O)C=C)c2n1 |(-6.94,-1.38,;-6.94,.16,;-5.61,.93,;-5.61,2.47,;-4.28,3.24,;-2.94,2.47,;-1.61,3.24,;-.28,2.47,;1.06,3.24,;1.06,4.78,;-.28,5.55,;2.39,5.55,;2.39,7.09,;1.06,7.86,;3.72,4.78,;3.72,3.24,;5.06,2.47,;6.39,3.24,;2.39,2.47,;2.39,.93,;-.28,.93,;1.06,.16,;-1.61,.16,;-1.61,-1.38,;-.28,-2.15,;-.28,-3.69,;1.06,-4.46,;1.46,-5.95,;2.94,-5.55,;2.55,-4.06,;4.28,-6.32,;4.28,-7.86,;5.61,-5.55,;6.94,-6.32,;-2.94,.93,;-4.28,.16,)| Show InChI InChI=1S/C24H25Cl2N5O5/c1-5-18(32)30-11-14(12-30)36-7-6-31-22-13(10-28-24(27-2)29-22)8-15(23(31)33)19-20(25)16(34-3)9-17(35-4)21(19)26/h5,8-10,14H,1,6-7,11-12H2,2-4H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM197250
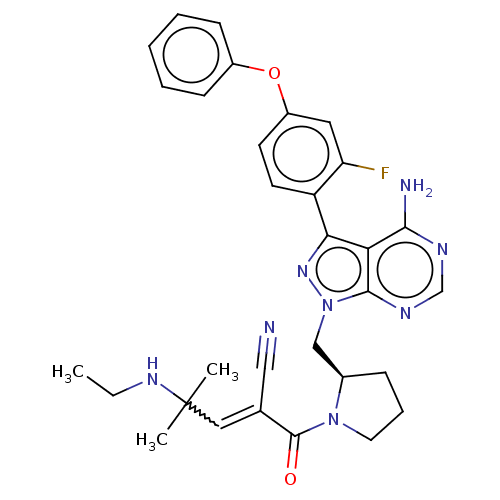
(US9090621, 85A | US9572811, Example 41A)Show SMILES CCNC(C)(C)C=C(C#N)C(=O)N1CCC[C@@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r,w:6.5| Show InChI InChI=1S/C31H33FN8O2/c1-4-37-31(2,3)16-20(17-33)30(41)39-14-8-9-21(39)18-40-29-26(28(34)35-19-36-29)27(38-40)24-13-12-23(15-25(24)32)42-22-10-6-5-7-11-22/h5-7,10-13,15-16,19,21,37H,4,8-9,14,18H2,1-3H3,(H2,34,35,36)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Principia Biopharma Inc.
US Patent
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of Formula... |
US Patent US9090621 (2015)
BindingDB Entry DOI: 10.7270/Q27M06QW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192803

(US9187487, 62)Show SMILES CC(C)(C)C(=O)c1c[nH]c2ncc(Nc3ccc(C=C(C#N)C(=O)N4CCC4)cc3)nc12 |w:18.17| Show InChI InChI=1S/C24H24N6O2/c1-24(2,3)21(31)18-13-26-22-20(18)29-19(14-27-22)28-17-7-5-15(6-8-17)11-16(12-25)23(32)30-9-4-10-30/h5-8,11,13-14H,4,9-10H2,1-3H3,(H,26,27)(H,28,29)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589207
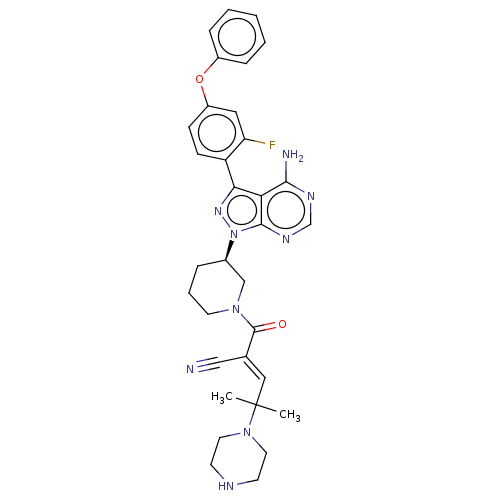
(CHEMBL5169419)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394916
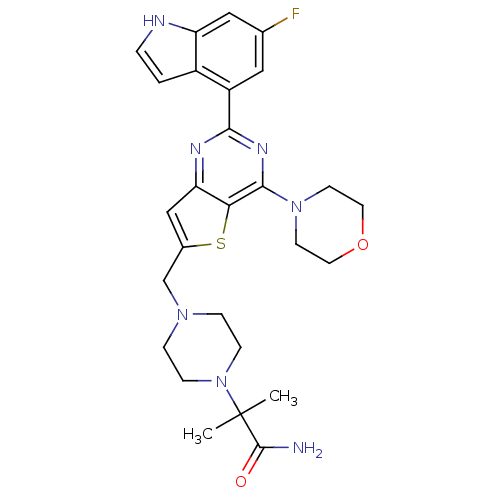
(CHEMBL2165506)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cc(F)cc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-7-5-33(6-8-35)16-18-15-22-23(38-18)25(34-9-11-37-12-10-34)32-24(31-22)20-13-17(28)14-21-19(20)3-4-30-21/h3-4,13-15,30H,5-12,16H2,1-2H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192807
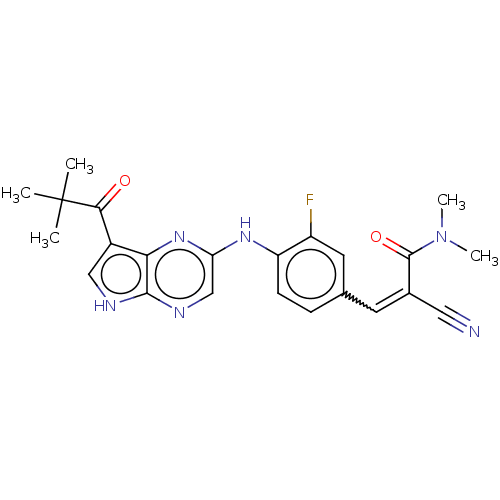
(US9187487, 69)Show SMILES CN(C)C(=O)C(=Cc1ccc(Nc2cnc3[nH]cc(C(=O)C(C)(C)C)c3n2)c(F)c1)C#N |w:6.6| Show InChI InChI=1S/C23H23FN6O2/c1-23(2,3)20(31)15-11-26-21-19(15)29-18(12-27-21)28-17-7-6-13(9-16(17)24)8-14(10-25)22(32)30(4)5/h6-9,11-12H,1-5H3,(H,26,27)(H,28,29)/b14-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BLK |
J Med Chem 55: 4539-50 (2012)
Article DOI: 10.1021/jm300035p
BindingDB Entry DOI: 10.7270/Q27H1KMF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392144
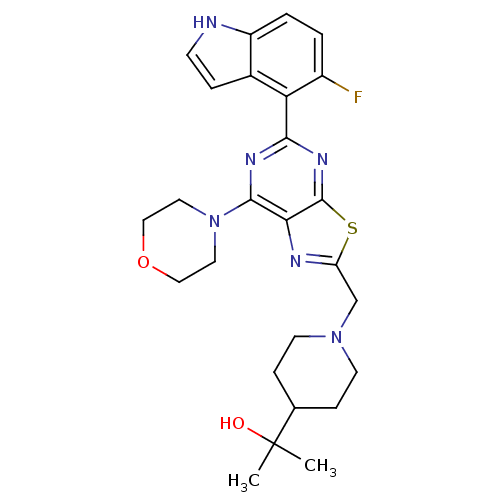
(CHEMBL2152774)Show SMILES CC(C)(O)C1CCN(Cc2nc3c(nc(nc3s2)-c2c(F)ccc3[nH]ccc23)N2CCOCC2)CC1 Show InChI InChI=1S/C26H31FN6O2S/c1-26(2,34)16-6-9-32(10-7-16)15-20-29-22-24(33-11-13-35-14-12-33)30-23(31-25(22)36-20)21-17-5-8-28-19(17)4-3-18(21)27/h3-5,8,16,28,34H,6-7,9-15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 4296-302 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.027
BindingDB Entry DOI: 10.7270/Q2HM59J8 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM521833
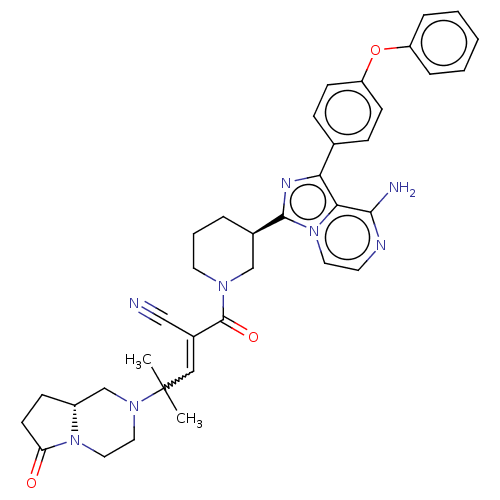
(US11155544, Compound 65)Show SMILES CC(C)(C=C(C#N)C(=O)N1CCC[C@H](C1)c1nc(-c2ccc(Oc3ccccc3)cc2)c2c(N)nccn12)N1CCN2[C@H](CCC2=O)C1 |w:3.2| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of BTK kinase activity of a compound of the pre... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P92J3 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589202
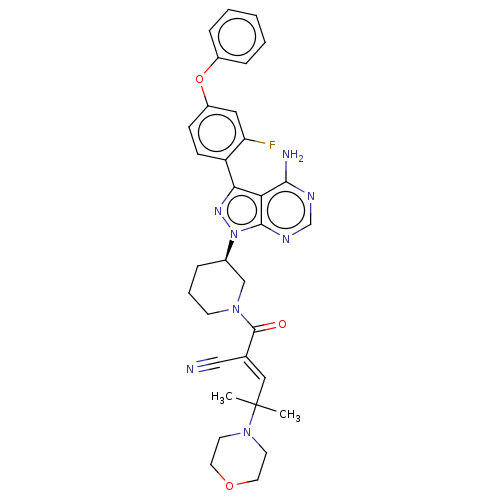
(CHEMBL5191633)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286984

(8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCN3CCN(CC3)C(=O)C=C)c2n1 |(-6.67,-.77,;-6.67,.77,;-5.33,1.54,;-5.33,3.08,;-4,3.85,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;,6.16,;2.67,6.16,;2.67,7.7,;1.33,8.47,;4,5.39,;4,3.85,;5.33,3.08,;6.67,3.85,;2.67,3.08,;2.67,1.54,;,1.54,;1.33,.77,;-1.33,.77,;-1.33,-.77,;,-1.54,;,-3.08,;1.33,-3.85,;1.33,-5.39,;2.67,-6.16,;4,-5.39,;4,-3.85,;2.67,-3.08,;5.33,-6.16,;6.67,-5.39,;5.33,-7.7,;6.67,-8.47,;-2.67,1.54,;-4,.77,)| Show InChI InChI=1S/C26H30Cl2N6O4/c1-5-20(35)33-11-9-32(10-12-33)7-6-8-34-24-16(15-30-26(29-2)31-24)13-17(25(34)36)21-22(27)18(37-3)14-19(38-4)23(21)28/h5,13-15H,1,6-12H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192816
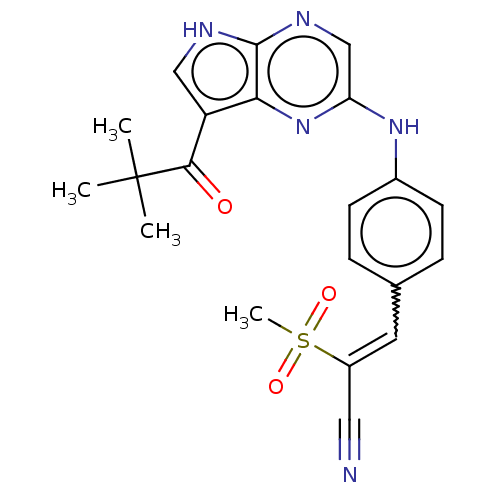
(US9187487, 81)Show SMILES CC(C)(C)C(=O)c1c[nH]c2ncc(Nc3ccc(C=C(C#N)S(C)(=O)=O)cc3)nc12 |w:18.17| Show InChI InChI=1S/C21H21N5O3S/c1-21(2,3)19(27)16-11-23-20-18(16)26-17(12-24-20)25-14-7-5-13(6-8-14)9-15(10-22)30(4,28)29/h5-9,11-12H,1-4H3,(H,23,24)(H,25,26)/b15-9- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
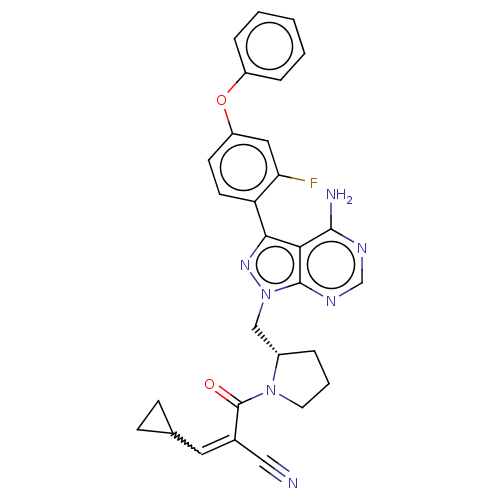
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM197275
(US9090621, 27B | US9572811, Example 34)Show SMILES Nc1ncnc2n(C[C@@H]3CCCN3C(=O)C(=CC3CC3)C#N)nc(-c3ccc(Oc4ccccc4)cc3F)c12 |r,w:16.17| Show InChI InChI=1S/C29H26FN7O2/c30-24-14-22(39-21-6-2-1-3-7-21)10-11-23(24)26-25-27(32)33-17-34-28(25)37(35-26)16-20-5-4-12-36(20)29(38)19(15-31)13-18-8-9-18/h1-3,6-7,10-11,13-14,17-18,20H,4-5,8-9,12,16H2,(H2,32,33,34)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 25 |
Principia Biopharma Inc.
US Patent
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of Formula... |
US Patent US9090621 (2015)
BindingDB Entry DOI: 10.7270/Q27M06QW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394910
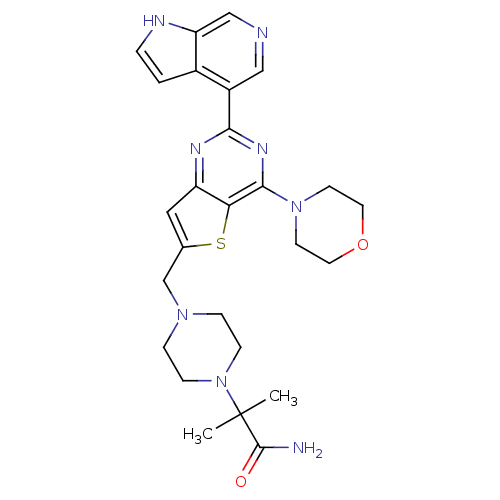
(CHEMBL2165512)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cncc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-7-5-32(6-8-34)16-17-13-20-22(37-17)24(33-9-11-36-12-10-33)31-23(30-20)19-14-28-15-21-18(19)3-4-29-21/h3-4,13-15,29H,5-12,16H2,1-2H3,(H2,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192805
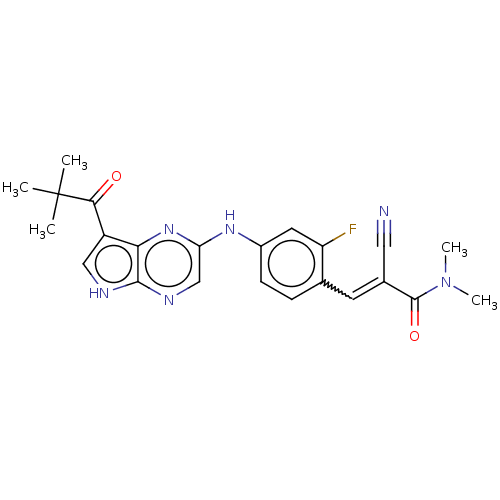
(US9187487, 66)Show SMILES CN(C)C(=O)C(=Cc1ccc(Nc2cnc3[nH]cc(C(=O)C(C)(C)C)c3n2)cc1F)C#N |w:6.6| Show InChI InChI=1S/C23H23FN6O2/c1-23(2,3)20(31)16-11-26-21-19(16)29-18(12-27-21)28-15-7-6-13(17(24)9-15)8-14(10-25)22(32)30(4)5/h6-9,11-12H,1-5H3,(H,26,27)(H,28,29)/b14-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50388185
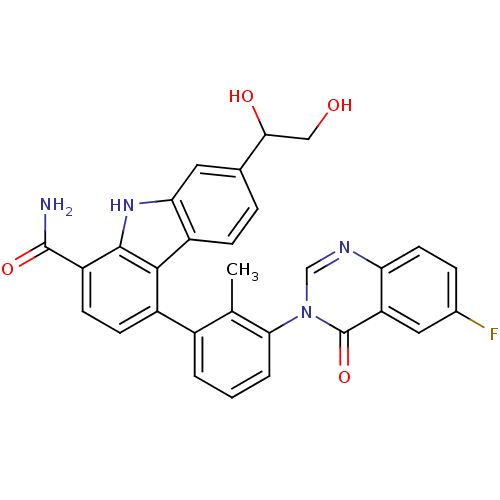
(CHEMBL2057919)Show SMILES Cc1c(cccc1-n1cnc2ccc(F)cc2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(O)CO Show InChI InChI=1S/C30H23FN4O4/c1-15-18(3-2-4-25(15)35-14-33-23-10-6-17(31)12-22(23)30(35)39)19-8-9-21(29(32)38)28-27(19)20-7-5-16(26(37)13-36)11-24(20)34-28/h2-12,14,26,34,36-37H,13H2,1H3,(H2,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BTK by enzymatic assay |
J Med Chem 55: 4539-50 (2012)
Article DOI: 10.1021/jm300035p
BindingDB Entry DOI: 10.7270/Q27H1KMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM197225
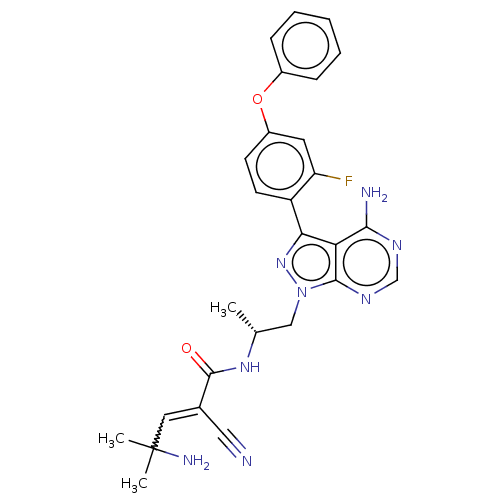
(US9090621, 188 | US9572811, Example 188)Show SMILES C[C@H](Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)NC(=O)C(=CC(C)(C)N)C#N |r,w:31.35| Show InChI InChI=1S/C27H27FN8O2/c1-16(34-26(37)17(13-29)12-27(2,3)31)14-36-25-22(24(30)32-15-33-25)23(35-36)20-10-9-19(11-21(20)28)38-18-7-5-4-6-8-18/h4-12,15-16H,14,31H2,1-3H3,(H,34,37)(H2,30,32,33)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Principia Biopharma Inc.
US Patent
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of Formula... |
US Patent US9090621 (2015)
BindingDB Entry DOI: 10.7270/Q27M06QW |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM286381
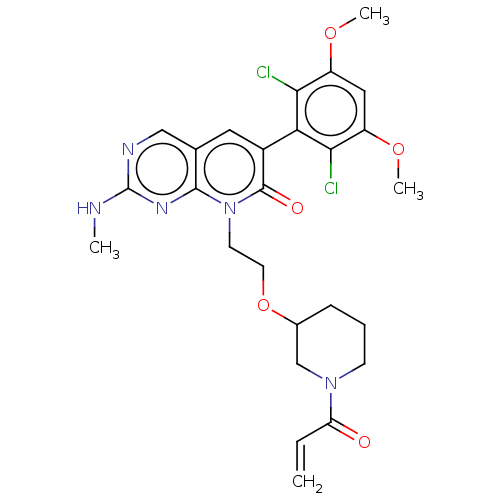
(8-(2-((1-acryloylpiperidin-3-yl)oxy)ethyl)-6-(2,6-...)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCOC3CCCN(C3)C(=O)C=C)c2n1 |(-6.67,,;-6.67,1.54,;-5.33,2.31,;-5.33,3.85,;-4,4.62,;-2.67,3.85,;-1.33,4.62,;,3.85,;1.33,4.62,;1.33,6.16,;,6.93,;2.67,6.93,;2.67,8.47,;4,9.24,;4,6.16,;4,4.62,;5.33,3.85,;6.67,4.62,;2.67,3.85,;2.67,2.31,;,2.31,;1.33,1.54,;-1.33,1.54,;-1.33,,;,-.77,;,-2.31,;1.33,-3.08,;2.67,-2.31,;4,-3.08,;4,-4.62,;2.67,-5.39,;1.33,-4.62,;2.67,-6.93,;1.33,-7.7,;4,-7.7,;4,-9.24,;-2.67,2.31,;-4,1.54,)| Show InChI InChI=1S/C26H29Cl2N5O5/c1-5-20(34)32-8-6-7-16(14-32)38-10-9-33-24-15(13-30-26(29-2)31-24)11-17(25(33)35)21-22(27)18(36-3)12-19(37-4)23(21)28/h5,11-13,16H,1,6-10,14H2,2-4H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 in HUVEC assessed as reduction in FGF2-induced ERK phosphorylation preincubated for 60 mins followed by FGF2 stimulation for 10 m... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM197220
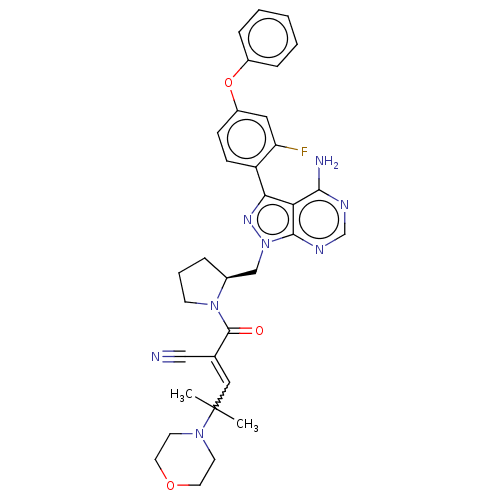
(BDBM197237 | US9090621, 95B | US9572811, Example 9...)Show SMILES CC(C)(C=C(C#N)C(=O)N1CCC[C@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCOCC1 |r,w:3.2| Show InChI InChI=1S/C33H35FN8O3/c1-33(2,40-13-15-44-16-14-40)18-22(19-35)32(43)41-12-6-7-23(41)20-42-31-28(30(36)37-21-38-31)29(39-42)26-11-10-25(17-27(26)34)45-24-8-4-3-5-9-24/h3-5,8-11,17-18,21,23H,6-7,12-16,20H2,1-2H3,(H2,36,37,38)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Principia Biopharma Inc.
US Patent
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of Btk kinase activity of a compound of Formula... |
US Patent US9090621 (2015)
BindingDB Entry DOI: 10.7270/Q27M06QW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3 [781-1124]
(Homo sapiens (Human)) | BDBM192815
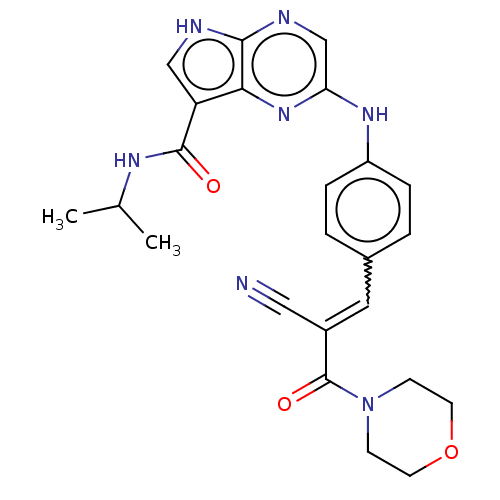
(US9187487, 80)Show SMILES CC(C)NC(=O)c1c[nH]c2ncc(Nc3ccc(C=C(C#N)C(=O)N4CCOCC4)cc3)nc12 |w:18.17| Show InChI InChI=1S/C24H25N7O3/c1-15(2)28-23(32)19-13-26-22-21(19)30-20(14-27-22)29-18-5-3-16(4-6-18)11-17(12-25)24(33)31-7-9-34-10-8-31/h3-6,11,13-15H,7-10H2,1-2H3,(H,26,27)(H,28,32)(H,29,30)/b17-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | -248.15 |
PRINCIPIA BIOPHARMA, INC.
US Patent
| Assay Description
Phosphorylation of the appropriate peptide substrate (fluorescently labeled Srctide) by recombinant JAK3 (catalytic domain, aa 781-1124) was measured... |
US Patent US9187487 (2015)
BindingDB Entry DOI: 10.7270/Q2K936B3 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238813
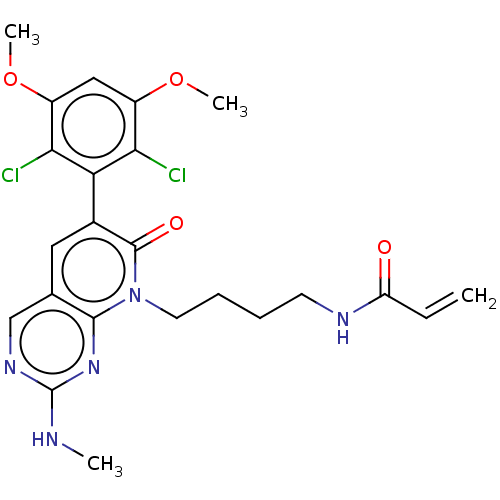
(CHEMBL4083740)Show SMILES CNc1ncc2cc(-c3c(Cl)c(OC)cc(OC)c3Cl)c(=O)n(CCCCNC(=O)C=C)c2n1 |(28.89,-34.94,;28.89,-33.4,;30.23,-32.63,;30.23,-31.09,;31.56,-30.32,;32.89,-31.08,;34.23,-30.3,;35.57,-31.08,;36.9,-30.32,;36.9,-28.79,;35.56,-28.02,;38.23,-28.02,;38.23,-26.48,;39.56,-25.71,;39.57,-28.79,;39.56,-30.33,;40.9,-31.11,;42.23,-30.34,;38.23,-31.1,;38.22,-32.64,;35.56,-32.63,;36.89,-33.4,;34.22,-33.4,;34.22,-34.94,;35.55,-35.71,;35.54,-37.25,;36.88,-38.03,;36.87,-39.57,;38.2,-40.34,;39.54,-39.57,;38.2,-41.88,;39.53,-42.65,;32.89,-32.63,;31.56,-33.4,)| Show InChI InChI=1S/C23H25Cl2N5O4/c1-5-17(31)27-8-6-7-9-30-21-13(12-28-23(26-2)29-21)10-14(22(30)32)18-19(24)15(33-3)11-16(34-4)20(18)25/h5,10-12H,1,6-9H2,2-4H3,(H,27,31)(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM521832
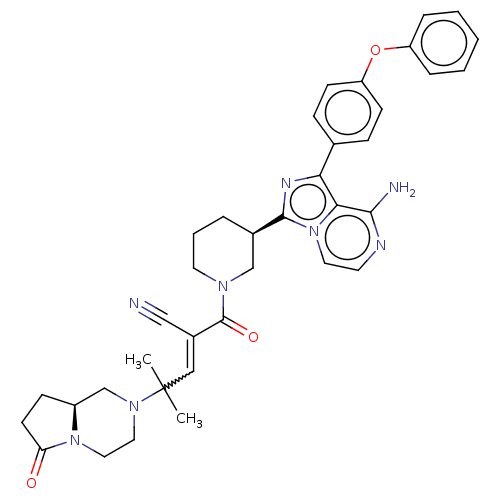
(US11155544, Compound 64)Show SMILES CC(C)(C=C(C#N)C(=O)N1CCC[C@H](C1)c1nc(-c2ccc(Oc3ccccc3)cc2)c2c(N)nccn12)N1CCN2[C@@H](CCC2=O)C1 |w:3.2| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of BTK kinase activity of a compound of the pre... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P92J3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589212
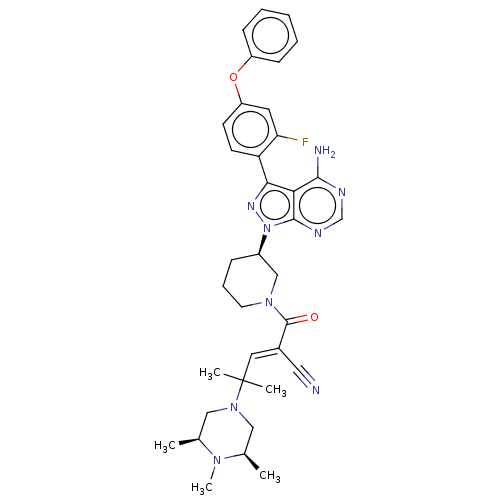
(CHEMBL5195816)Show SMILES C[C@H]1CN(C[C@@H](C)N1C)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50238823
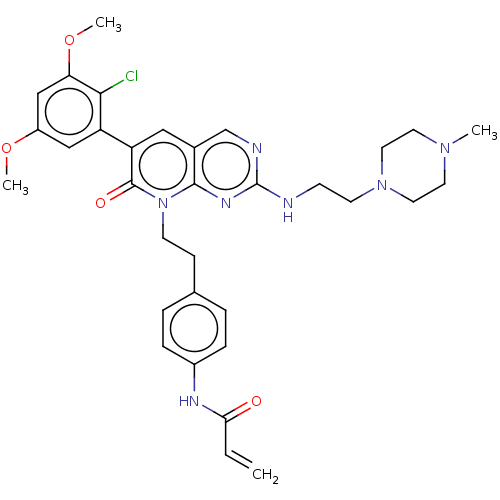
(CHEMBL4086567)Show SMILES COc1cc(OC)c(Cl)c(c1)-c1cc2cnc(NCCN3CCN(C)CC3)nc2n(CCc2ccc(NC(=O)C=C)cc2)c1=O Show InChI InChI=1S/C33H38ClN7O4/c1-5-29(42)37-24-8-6-22(7-9-24)10-12-41-31-23(21-36-33(38-31)35-11-13-40-16-14-39(2)15-17-40)18-27(32(41)43)26-19-25(44-3)20-28(45-4)30(26)34/h5-9,18-21H,1,10-17H2,2-4H3,(H,37,42)(H,35,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated for 15 mins followed by peptide substrate addition measured after 3 h... |
J Med Chem 60: 6516-6527 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00360
BindingDB Entry DOI: 10.7270/Q2C53P3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM521781
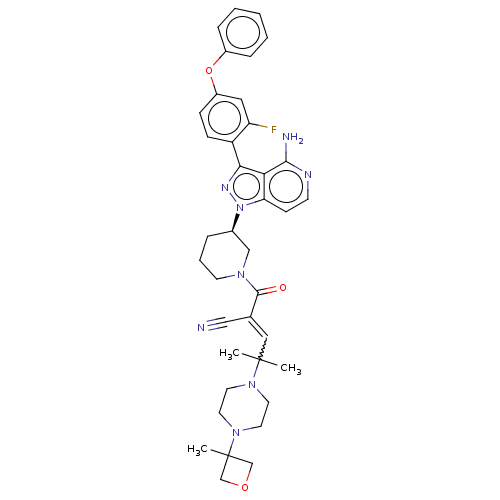
(US11155544, Compound 7)Show SMILES CC(C)(C=C(C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)nccc12)N1CCN(CC1)C1(C)COC1 |w:3.2| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A Caliper-based kinase assay (Caliper Life Sciences, Hopkinton, Mass.) was used to measure inhibition of BTK kinase activity of a compound of the pre... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P92J3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data