 Found 332 hits with Last Name = 'gonsiorek' and Initial = 'w'
Found 332 hits with Last Name = 'gonsiorek' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329148
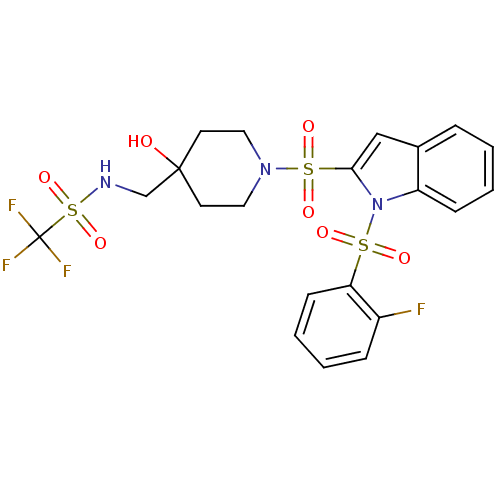
(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES OC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H21F4N3O7S3/c22-16-6-2-4-8-18(16)36(30,31)28-17-7-3-1-5-15(17)13-19(28)37(32,33)27-11-9-20(29,10-12-27)14-26-38(34,35)21(23,24)25/h1-8,13,26,29H,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50305990
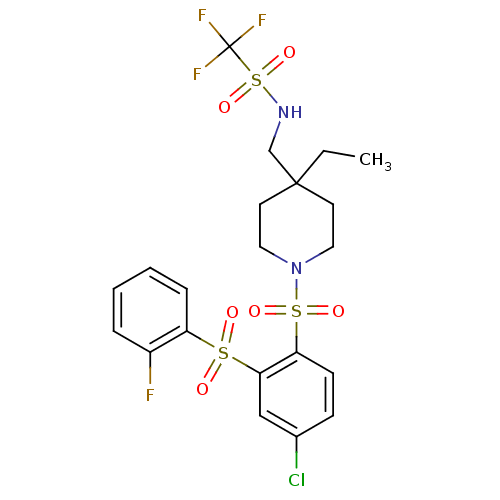
(CHEMBL596388 | N-((1-(4-chloro-2-(2-fluorophenylsu...)Show SMILES CCC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H23ClF4N2O6S3/c1-2-20(14-27-37(33,34)21(24,25)26)9-11-28(12-10-20)36(31,32)18-8-7-15(22)13-19(18)35(29,30)17-6-4-3-5-16(17)23/h3-8,13,27H,2,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329147
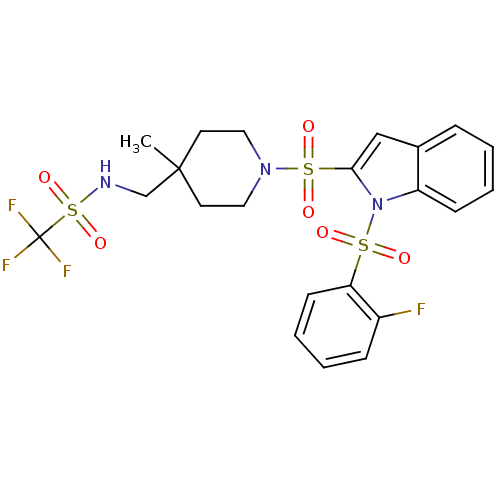
(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES CC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C22H23F4N3O6S3/c1-21(15-27-38(34,35)22(24,25)26)10-12-28(13-11-21)37(32,33)20-14-16-6-2-4-8-18(16)29(20)36(30,31)19-9-5-3-7-17(19)23/h2-9,14,27H,10-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM85739
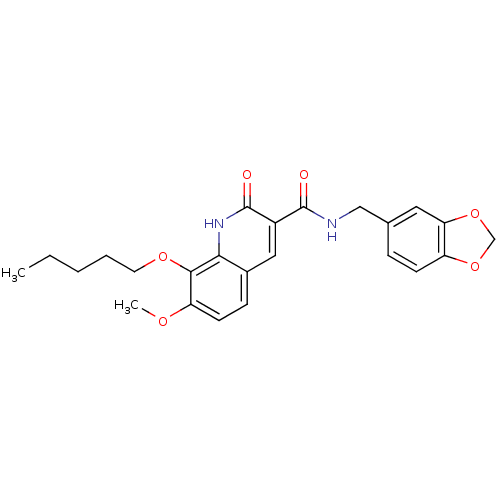
(CHEMBL178372 | JTE-907 | N-(1,3-Benzodioxole-5-ylm...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCc3ccc4OCOc4c3)c(=O)[nH]c12 Show InChI InChI=1S/C24H26N2O6/c1-3-4-5-10-30-22-19(29-2)9-7-16-12-17(24(28)26-21(16)22)23(27)25-13-15-6-8-18-20(11-15)32-14-31-18/h6-9,11-12H,3-5,10,13-14H2,1-2H3,(H,25,27)(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 783-6 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.007
BindingDB Entry DOI: 10.7270/Q2MW2GN7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329151

(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES Fc1ccccc1S(=O)(=O)n1c(cc2ccccc12)S(=O)(=O)N1CCC(CNS(=O)(=O)C(F)(F)F)CC1 Show InChI InChI=1S/C21H21F4N3O6S3/c22-17-6-2-4-8-19(17)35(29,30)28-18-7-3-1-5-16(18)13-20(28)36(31,32)27-11-9-15(10-12-27)14-26-37(33,34)21(23,24)25/h1-8,13,15,26H,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50305989
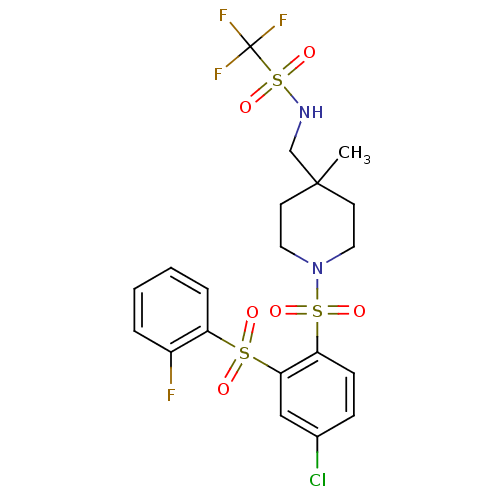
(CHEMBL596387 | N-((1-(4-chloro-2-(2-fluorophenylsu...)Show SMILES CC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C20H21ClF4N2O6S3/c1-19(13-26-36(32,33)20(23,24)25)8-10-27(11-9-19)35(30,31)17-7-6-14(21)12-18(17)34(28,29)16-5-3-2-4-15(16)22/h2-7,12,26H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160446
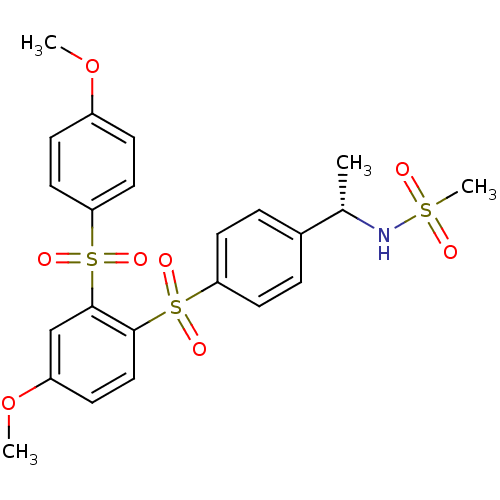
(CHEMBL180465 | N-((S)-1-{4-[4-Methoxy-2-(4-methoxy...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1cc(OC)ccc1S(=O)(=O)c1ccc(cc1)[C@H](C)NS(C)(=O)=O Show InChI InChI=1S/C23H25NO8S3/c1-16(24-33(4,25)26)17-5-10-20(11-6-17)34(27,28)22-14-9-19(32-3)15-23(22)35(29,30)21-12-7-18(31-2)8-13-21/h5-16,24H,1-4H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172146
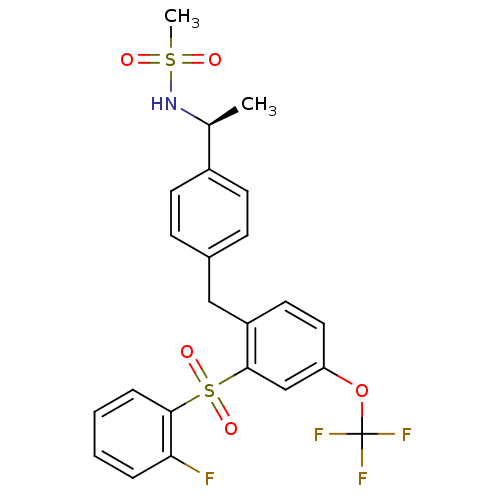
(CHEMBL381669 | N-((S)-1-{4-[2-(2-Fluoro-benzenesul...)Show SMILES C[C@H](NS(C)(=O)=O)c1ccc(Cc2ccc(OC(F)(F)F)cc2S(=O)(=O)c2ccccc2F)cc1 Show InChI InChI=1S/C23H21F4NO5S2/c1-15(28-34(2,29)30)17-9-7-16(8-10-17)13-18-11-12-19(33-23(25,26)27)14-22(18)35(31,32)21-6-4-3-5-20(21)24/h3-12,14-15,28H,13H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329150

(1,1,1-trifluoro-N-(1-(1-(1-(2-fluorophenylsulfonyl...)Show SMILES CC(NS(=O)(=O)C(F)(F)F)C1CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C22H23F4N3O6S3/c1-15(27-38(34,35)22(24,25)26)16-10-12-28(13-11-16)37(32,33)21-14-17-6-2-4-8-19(17)29(21)36(30,31)20-9-5-3-7-18(20)23/h2-9,14-16,27H,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329130
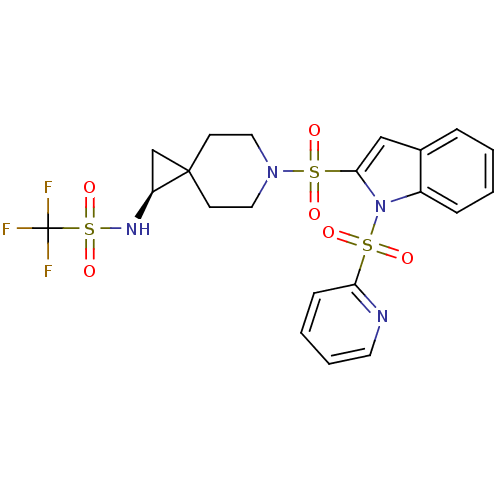
((S)-1,1,1-trifluoro-N-(6-(1-(pyridin-2-ylsulfonyl)...)Show SMILES FC(F)(F)S(=O)(=O)N[C@H]1CC11CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C21H21F3N4O6S3/c22-21(23,24)37(33,34)26-17-14-20(17)8-11-27(12-9-20)36(31,32)19-13-15-5-1-2-6-16(15)28(19)35(29,30)18-7-3-4-10-25-18/h1-7,10,13,17,26H,8-9,11-12,14H2/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329129
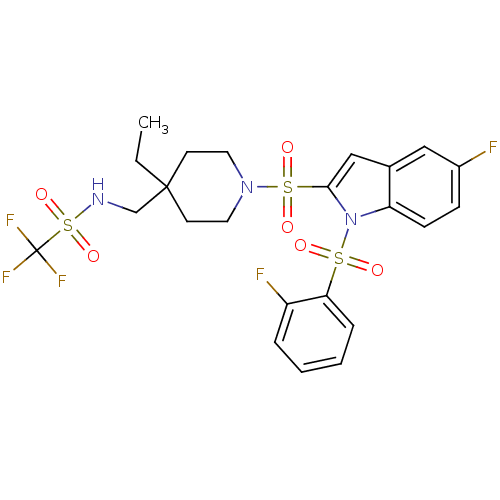
(CHEMBL1271093 | N-((4-ethyl-1-(5-fluoro-1-(2-fluor...)Show SMILES CCC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2cc(F)ccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C23H24F5N3O6S3/c1-2-22(15-29-40(36,37)23(26,27)28)9-11-30(12-10-22)39(34,35)21-14-16-13-17(24)7-8-19(16)31(21)38(32,33)20-6-4-3-5-18(20)25/h3-8,13-14,29H,2,9-12,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172182
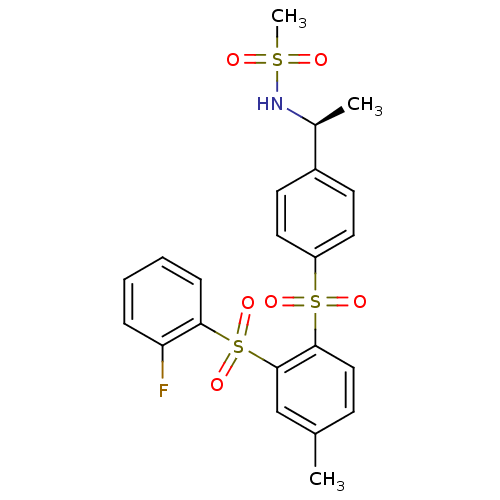
(CHEMBL371575 | N-((S)-1-{4-[2-(2-Fluoro-benzenesul...)Show SMILES C[C@H](NS(C)(=O)=O)c1ccc(cc1)S(=O)(=O)c1ccc(C)cc1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C22H22FNO6S3/c1-15-8-13-21(22(14-15)33(29,30)20-7-5-4-6-19(20)23)32(27,28)18-11-9-17(10-12-18)16(2)24-31(3,25)26/h4-14,16,24H,1-3H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329144
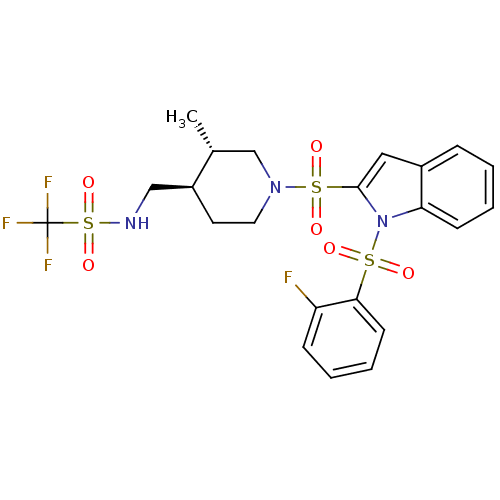
(1,1,1-trifluoro-N-(((3S,4R)-1-(1-(2-fluorophenylsu...)Show SMILES C[C@@H]1CN(CC[C@H]1CNS(=O)(=O)C(F)(F)F)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F |r| Show InChI InChI=1S/C22H23F4N3O6S3/c1-15-14-28(11-10-17(15)13-27-38(34,35)22(24,25)26)37(32,33)21-12-16-6-2-4-8-19(16)29(21)36(30,31)20-9-5-3-7-18(20)23/h2-9,12,15,17,27H,10-11,13-14H2,1H3/t15-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172164
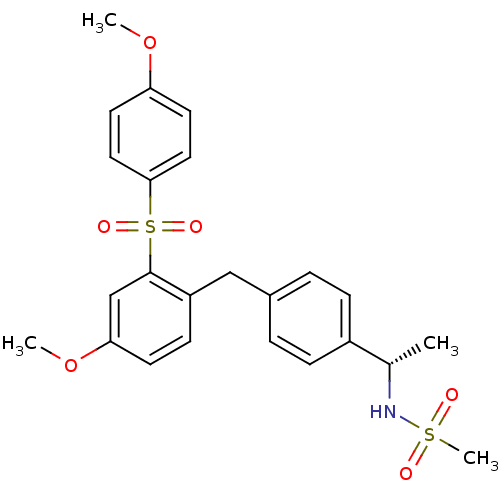
(CHEMBL199048 | N-((S)-1-{4-[4-Methoxy-2-(4-methoxy...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1cc(OC)ccc1Cc1ccc(cc1)[C@H](C)NS(C)(=O)=O Show InChI InChI=1S/C24H27NO6S2/c1-17(25-32(4,26)27)19-7-5-18(6-8-19)15-20-9-10-22(31-3)16-24(20)33(28,29)23-13-11-21(30-2)12-14-23/h5-14,16-17,25H,15H2,1-4H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172163
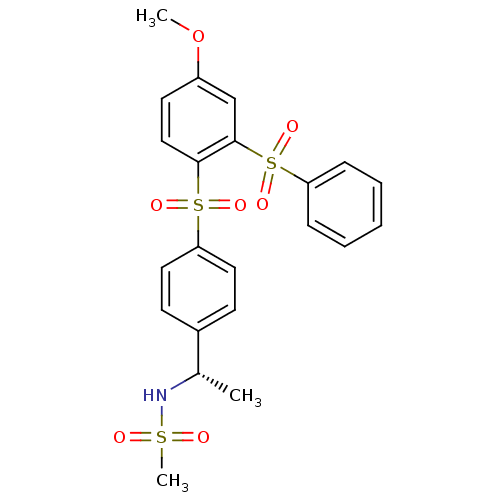
(CHEMBL197955 | N-{(S)-1-[4-(2-Benzenesulfonyl-4-me...)Show SMILES COc1ccc(c(c1)S(=O)(=O)c1ccccc1)S(=O)(=O)c1ccc(cc1)[C@H](C)NS(C)(=O)=O Show InChI InChI=1S/C22H23NO7S3/c1-16(23-31(3,24)25)17-9-12-20(13-10-17)32(26,27)21-14-11-18(30-2)15-22(21)33(28,29)19-7-5-4-6-8-19/h4-16,23H,1-3H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306002
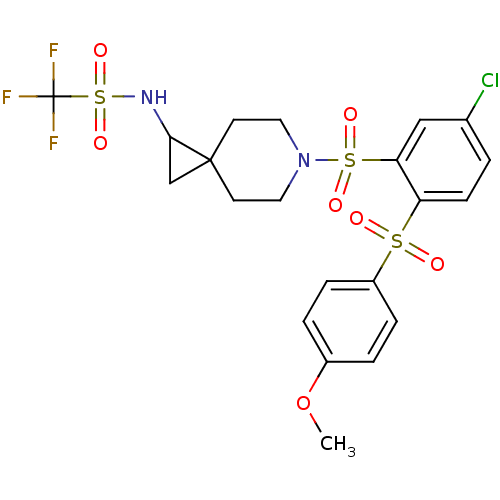
(CHEMBL595705 | N-(6-(5-chloro-2-(4-methoxyphenylsu...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)N1CCC2(CC2NS(=O)(=O)C(F)(F)F)CC1 Show InChI InChI=1S/C21H22ClF3N2O7S3/c1-34-15-3-5-16(6-4-15)35(28,29)17-7-2-14(22)12-18(17)36(30,31)27-10-8-20(9-11-27)13-19(20)26-37(32,33)21(23,24)25/h2-7,12,19,26H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50211605
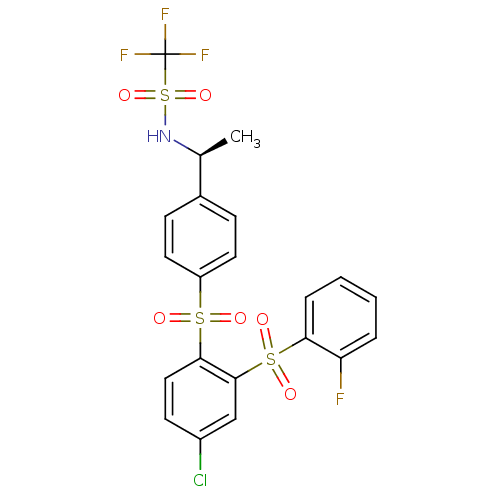
((S)-N-(1-(4-(4-chloro-2-(2-fluorophenylsulfonyl)ph...)Show SMILES C[C@H](NS(=O)(=O)C(F)(F)F)c1ccc(cc1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H16ClF4NO6S3/c1-13(27-36(32,33)21(24,25)26)14-6-9-16(10-7-14)34(28,29)19-11-8-15(22)12-20(19)35(30,31)18-5-3-2-4-17(18)23/h2-13,27H,1H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329159

((R)-N-(1-(4-(4-chloro-2-(2-fluorophenylsulfonyl)ph...)Show SMILES C[C@@H](NS(=O)(=O)C(F)(F)F)c1ccc(cc1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1F |r| Show InChI InChI=1S/C21H16ClF4NO6S3/c1-13(27-36(32,33)21(24,25)26)14-6-9-16(10-7-14)34(28,29)19-11-8-15(22)12-20(19)35(30,31)18-5-3-2-4-17(18)23/h2-13,27H,1H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329137

(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES COc1ccc2n(c(cc2c1)S(=O)(=O)N1CCC(CNS(=O)(=O)C(F)(F)F)CC1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C22H23F4N3O7S3/c1-36-17-6-7-19-16(12-17)13-21(29(19)37(30,31)20-5-3-2-4-18(20)23)38(32,33)28-10-8-15(9-11-28)14-27-39(34,35)22(24,25)26/h2-7,12-13,15,27H,8-11,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172155
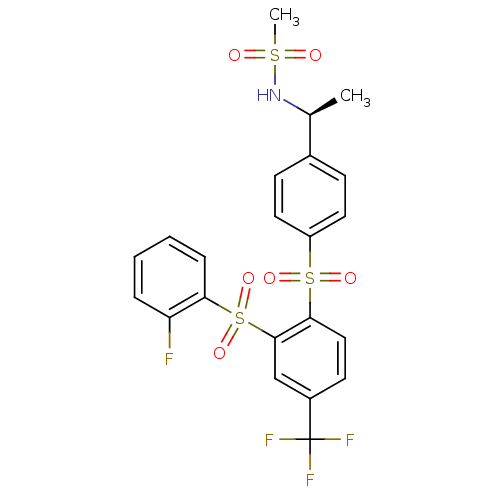
(CHEMBL198246 | N-((S)-1-{4-[2-(2-Fluoro-benzenesul...)Show SMILES C[C@H](NS(C)(=O)=O)c1ccc(cc1)S(=O)(=O)c1ccc(cc1S(=O)(=O)c1ccccc1F)C(F)(F)F Show InChI InChI=1S/C22H19F4NO6S3/c1-14(27-34(2,28)29)15-7-10-17(11-8-15)35(30,31)20-12-9-16(22(24,25)26)13-21(20)36(32,33)19-6-4-3-5-18(19)23/h3-14,27H,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172168
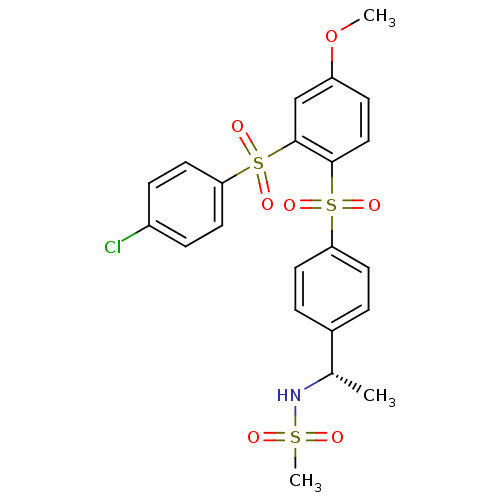
(CHEMBL370824 | N-((S)-1-{4-[2-(4-Chloro-benzenesul...)Show SMILES COc1ccc(c(c1)S(=O)(=O)c1ccc(Cl)cc1)S(=O)(=O)c1ccc(cc1)[C@H](C)NS(C)(=O)=O Show InChI InChI=1S/C22H22ClNO7S3/c1-15(24-32(3,25)26)16-4-9-19(10-5-16)33(27,28)21-13-8-18(31-2)14-22(21)34(29,30)20-11-6-17(23)7-12-20/h4-15,24H,1-3H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306008
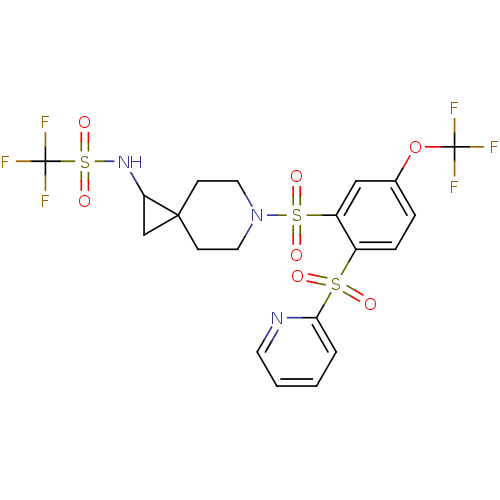
(1,1,1-trifluoro-N-(6-(2-(pyridin-2-ylsulfonyl)-5-(...)Show SMILES FC(F)(F)Oc1ccc(c(c1)S(=O)(=O)N1CCC2(CC2NS(=O)(=O)C(F)(F)F)CC1)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C20H19F6N3O7S3/c21-19(22,23)36-13-4-5-14(37(30,31)17-3-1-2-8-27-17)15(11-13)38(32,33)29-9-6-18(7-10-29)12-16(18)28-39(34,35)20(24,25)26/h1-5,8,11,16,28H,6-7,9-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329133
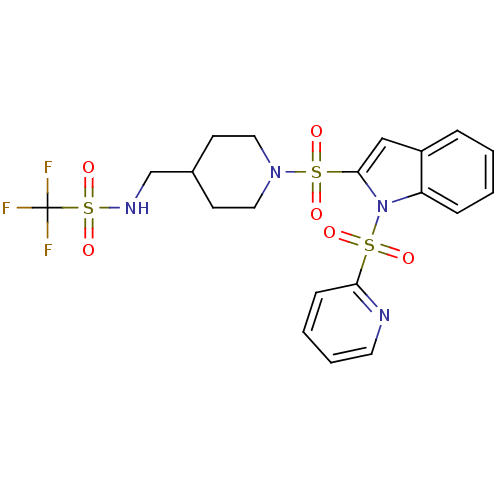
(1,1,1-trifluoro-N-((1-(1-(pyridin-2-ylsulfonyl)-1H...)Show SMILES FC(F)(F)S(=O)(=O)NCC1CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C20H21F3N4O6S3/c21-20(22,23)36(32,33)25-14-15-8-11-26(12-9-15)35(30,31)19-13-16-5-1-2-6-17(16)27(19)34(28,29)18-7-3-4-10-24-18/h1-7,10,13,15,25H,8-9,11-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172158
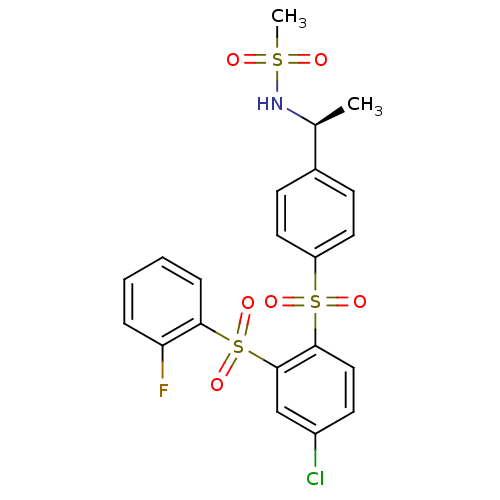
((S)-N-(1-(4-(4-chloro-2-(2-fluorophenylsulfonyl)ph...)Show SMILES C[C@H](NS(C)(=O)=O)c1ccc(cc1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H19ClFNO6S3/c1-14(24-31(2,25)26)15-7-10-17(11-8-15)32(27,28)20-12-9-16(22)13-21(20)33(29,30)19-6-4-3-5-18(19)23/h3-14,24H,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329139
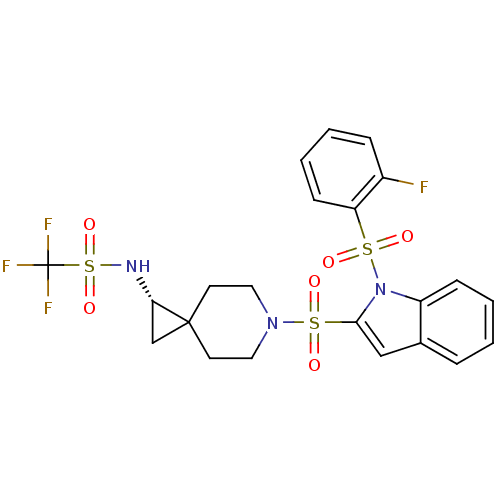
((S)-1,1,1-trifluoro-N-(6-(1-(2-fluorophenylsulfony...)Show SMILES Fc1ccccc1S(=O)(=O)n1c(cc2ccccc12)S(=O)(=O)N1CCC2(C[C@@H]2NS(=O)(=O)C(F)(F)F)CC1 |r| Show InChI InChI=1S/C22H21F4N3O6S3/c23-16-6-2-4-8-18(16)36(30,31)29-17-7-3-1-5-15(17)13-20(29)37(32,33)28-11-9-21(10-12-28)14-19(21)27-38(34,35)22(24,25)26/h1-8,13,19,27H,9-12,14H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172180
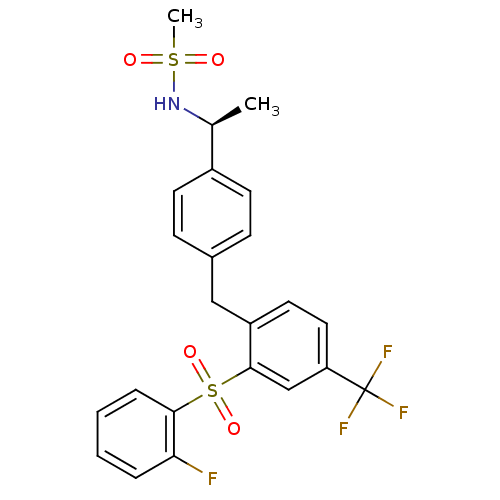
(CHEMBL199102 | N-((S)-1-{4-[2-(2-Fluoro-benzenesul...)Show SMILES C[C@H](NS(C)(=O)=O)c1ccc(Cc2ccc(cc2S(=O)(=O)c2ccccc2F)C(F)(F)F)cc1 Show InChI InChI=1S/C23H21F4NO4S2/c1-15(28-33(2,29)30)17-9-7-16(8-10-17)13-18-11-12-19(23(25,26)27)14-22(18)34(31,32)21-6-4-3-5-20(21)24/h3-12,14-15,28H,13H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172174

(CHEMBL197777 | N-{4-[4-Methoxy-2-(4-methoxy-benzen...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1cc(OC)ccc1S(=O)(=O)c1ccc(CNS(C)(=O)=O)cc1 Show InChI InChI=1S/C22H23NO8S3/c1-30-17-6-11-20(12-7-17)34(28,29)22-14-18(31-2)8-13-21(22)33(26,27)19-9-4-16(5-10-19)15-23-32(3,24)25/h4-14,23H,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329135

(1,1,1-trifluoro-N-((1-(2-(2-fluorophenylsulfonyl)f...)Show SMILES Fc1ccccc1S(=O)(=O)c1occc1S(=O)(=O)N1CCC(CNS(=O)(=O)C(F)(F)F)CC1 Show InChI InChI=1S/C17H18F4N2O7S3/c18-13-3-1-2-4-14(13)31(24,25)16-15(7-10-30-16)32(26,27)23-8-5-12(6-9-23)11-22-33(28,29)17(19,20)21/h1-4,7,10,12,22H,5-6,8-9,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306017
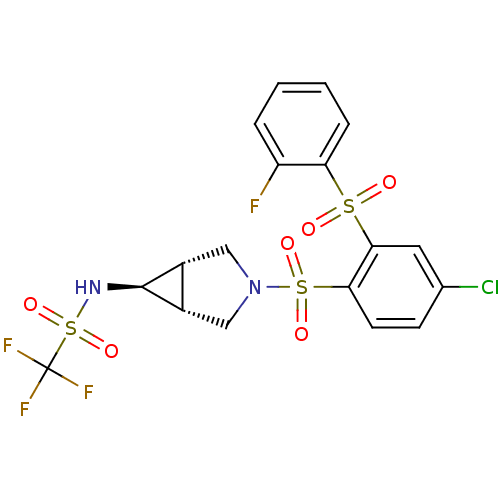
(CHEMBL604704 | N-((1R,5S,6s)-3-(4-chloro-2-(2-fluo...)Show SMILES Fc1ccccc1S(=O)(=O)c1cc(Cl)ccc1S(=O)(=O)N1C[C@H]2[C@@H](C1)[C@@H]2NS(=O)(=O)C(F)(F)F |r| Show InChI InChI=1S/C18H15ClF4N2O6S3/c19-10-5-6-15(16(7-10)32(26,27)14-4-2-1-3-13(14)20)33(28,29)25-8-11-12(9-25)17(11)24-34(30,31)18(21,22)23/h1-7,11-12,17,24H,8-9H2/t11-,12+,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329143
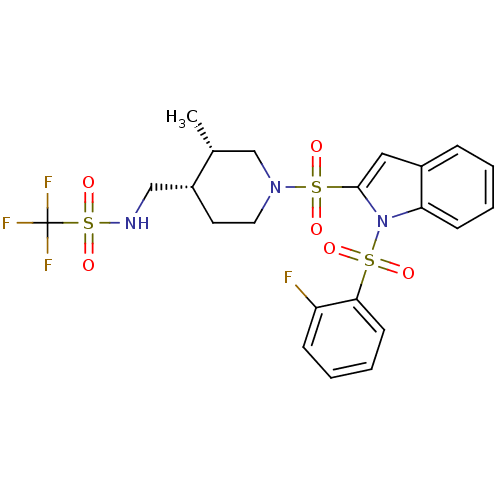
(1,1,1-trifluoro-N-(((3S,4S)-1-(1-(2-fluorophenylsu...)Show SMILES C[C@@H]1CN(CC[C@@H]1CNS(=O)(=O)C(F)(F)F)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F |r| Show InChI InChI=1S/C22H23F4N3O6S3/c1-15-14-28(11-10-17(15)13-27-38(34,35)22(24,25)26)37(32,33)21-12-16-6-2-4-8-19(16)29(21)36(30,31)20-9-5-3-7-18(20)23/h2-9,12,15,17,27H,10-11,13-14H2,1H3/t15-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306007
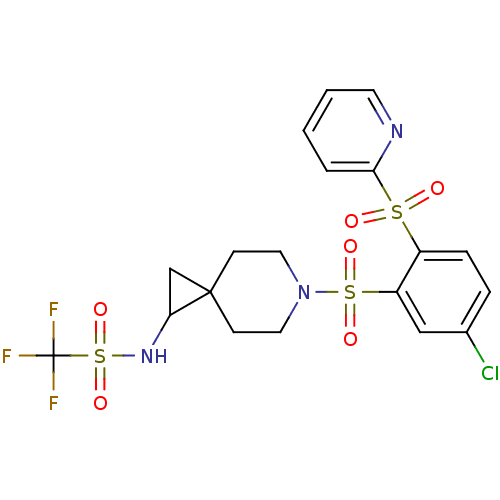
(CHEMBL592896 | N-(6-(5-chloro-2-(pyridin-2-ylsulfo...)Show SMILES FC(F)(F)S(=O)(=O)NC1CC11CCN(CC1)S(=O)(=O)c1cc(Cl)ccc1S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C19H19ClF3N3O6S3/c20-13-4-5-14(33(27,28)17-3-1-2-8-24-17)15(11-13)34(29,30)26-9-6-18(7-10-26)12-16(18)25-35(31,32)19(21,22)23/h1-5,8,11,16,25H,6-7,9-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329128
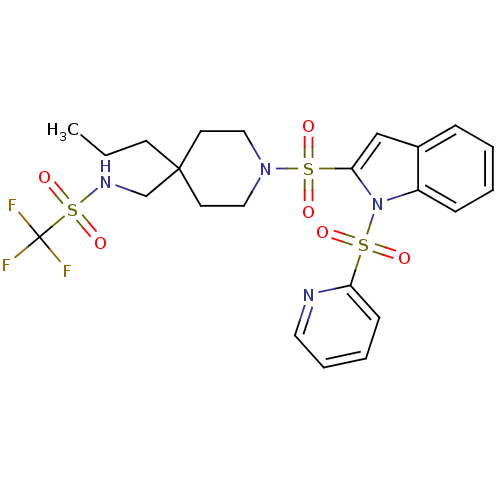
(1,1,1-trifluoro-N-((4-propyl-1-(1-(pyridin-2-ylsul...)Show SMILES CCCC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C23H27F3N4O6S3/c1-2-10-22(17-28-39(35,36)23(24,25)26)11-14-29(15-12-22)38(33,34)21-16-18-7-3-4-8-19(18)30(21)37(31,32)20-9-5-6-13-27-20/h3-9,13,16,28H,2,10-12,14-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306016
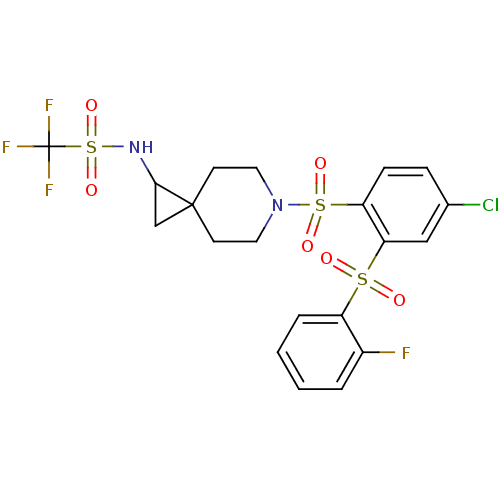
(CHEMBL596579 | N-(6-(4-chloro-2-(2-fluorophenylsul...)Show SMILES Fc1ccccc1S(=O)(=O)c1cc(Cl)ccc1S(=O)(=O)N1CCC2(CC2NS(=O)(=O)C(F)(F)F)CC1 Show InChI InChI=1S/C20H19ClF4N2O6S3/c21-13-5-6-16(17(11-13)34(28,29)15-4-2-1-3-14(15)22)35(30,31)27-9-7-19(8-10-27)12-18(19)26-36(32,33)20(23,24)25/h1-6,11,18,26H,7-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329146

(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES CCCC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C24H27F4N3O6S3/c1-2-11-23(17-29-40(36,37)24(26,27)28)12-14-30(15-13-23)39(34,35)22-16-18-7-3-5-9-20(18)31(22)38(32,33)21-10-6-4-8-19(21)25/h3-10,16,29H,2,11-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172183

(CHEMBL197904 | N-((S)-1-{4-[2-(2,6-Difluoro-benzen...)Show SMILES C[C@H](NS(C)(=O)=O)c1ccc(Cc2ccc(cc2S(=O)(=O)c2c(F)cccc2F)C(F)(F)F)cc1 Show InChI InChI=1S/C23H20F5NO4S2/c1-14(29-34(2,30)31)16-8-6-15(7-9-16)12-17-10-11-18(23(26,27)28)13-21(17)35(32,33)22-19(24)4-3-5-20(22)25/h3-11,13-14,29H,12H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306003
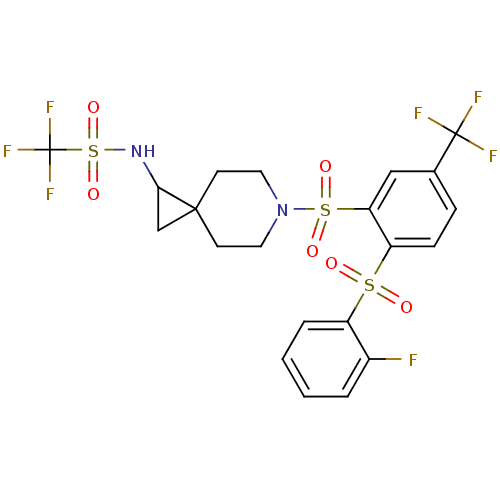
(1,1,1-trifluoro-N-(6-(2-(2-fluorophenylsulfonyl)-5...)Show SMILES Fc1ccccc1S(=O)(=O)c1ccc(cc1S(=O)(=O)N1CCC2(CC2NS(=O)(=O)C(F)(F)F)CC1)C(F)(F)F Show InChI InChI=1S/C21H19F7N2O6S3/c22-14-3-1-2-4-15(14)37(31,32)16-6-5-13(20(23,24)25)11-17(16)38(33,34)30-9-7-19(8-10-30)12-18(19)29-39(35,36)21(26,27)28/h1-6,11,18,29H,7-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172179
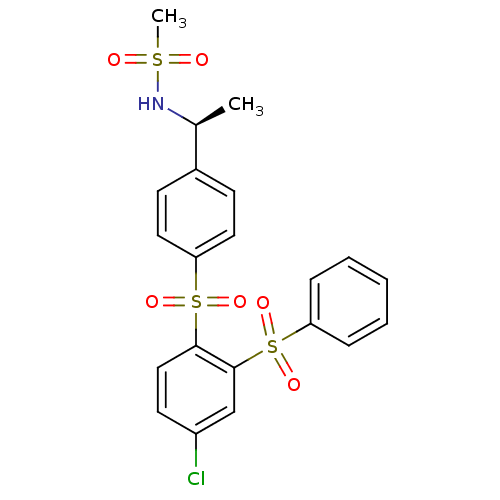
(CHEMBL198164 | N-{(S)-1-[4-(2-Benzenesulfonyl-4-ch...)Show SMILES C[C@H](NS(C)(=O)=O)c1ccc(cc1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H20ClNO6S3/c1-15(23-30(2,24)25)16-8-11-19(12-9-16)31(26,27)20-13-10-17(22)14-21(20)32(28,29)18-6-4-3-5-7-18/h3-15,23H,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329127

(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES COC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2cccnc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H22F4N4O7S3/c1-36-20(14-27-39(34,35)21(23,24)25)8-11-28(12-9-20)38(32,33)18-13-15-5-4-10-26-19(15)29(18)37(30,31)17-7-3-2-6-16(17)22/h2-7,10,13,27H,8-9,11-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50297764

((R)-5-cyano-3-(3,4-dioxo-2-(1-phenylpropylamino)cy...)Show SMILES CC[C@@H](Nc1c(Nc2cc(cc(C(=O)N(C)C)c2O)C#N)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C23H22N4O4/c1-4-16(14-8-6-5-7-9-14)25-18-19(22(30)21(18)29)26-17-11-13(12-24)10-15(20(17)28)23(31)27(2)3/h5-11,16,25-26,28H,4H2,1-3H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of human [125I]IL-8 from human CXCR2 |
Bioorg Med Chem Lett 19: 4446-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.049
BindingDB Entry DOI: 10.7270/Q2H1323X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50305993

(CHEMBL594300 | N-((1-(4-chloro-2-(2-fluorophenylsu...)Show SMILES OCC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C20H21ClF4N2O7S3/c21-14-5-6-17(18(11-14)35(29,30)16-4-2-1-3-15(16)22)36(31,32)27-9-7-19(13-28,8-10-27)12-26-37(33,34)20(23,24)25/h1-6,11,26,28H,7-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50306005
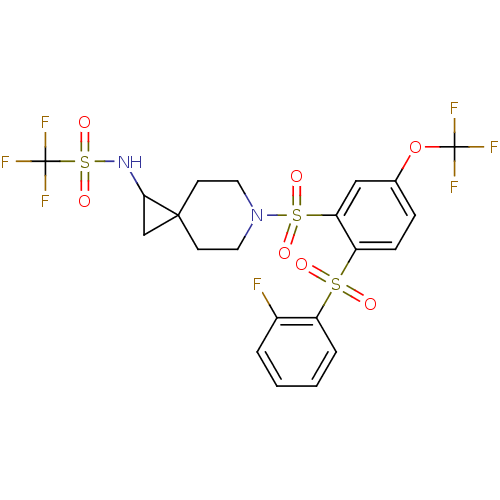
(1,1,1-trifluoro-N-(6-(2-(2-fluorophenylsulfonyl)-5...)Show SMILES Fc1ccccc1S(=O)(=O)c1ccc(OC(F)(F)F)cc1S(=O)(=O)N1CCC2(CC2NS(=O)(=O)C(F)(F)F)CC1 Show InChI InChI=1S/C21H19F7N2O7S3/c22-14-3-1-2-4-15(14)38(31,32)16-6-5-13(37-20(23,24)25)11-17(16)39(33,34)30-9-7-19(8-10-30)12-18(19)29-40(35,36)21(26,27)28/h1-6,11,18,29H,7-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329140
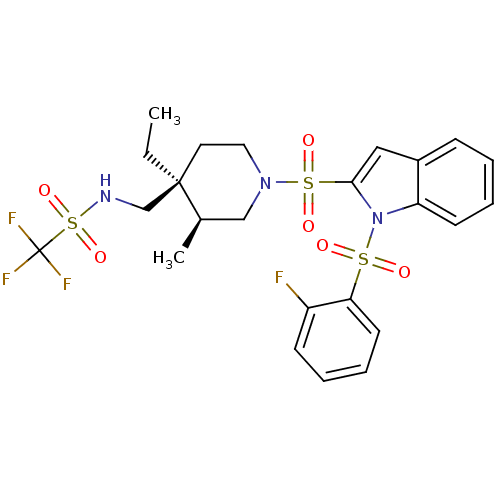
(CHEMBL1270287 | N-(((3S,4S)-4-ethyl-1-(1-(2-fluoro...)Show SMILES CC[C@]1(CNS(=O)(=O)C(F)(F)F)CCN(C[C@H]1C)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F |r| Show InChI InChI=1S/C24H27F4N3O6S3/c1-3-23(16-29-40(36,37)24(26,27)28)12-13-30(15-17(23)2)39(34,35)22-14-18-8-4-6-10-20(18)31(22)38(32,33)21-11-7-5-9-19(21)25/h4-11,14,17,29H,3,12-13,15-16H2,1-2H3/t17-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50172175

(CHEMBL198251 | N-((S)-1-{4-[2-(3-Fluoro-benzenesul...)Show SMILES C[C@H](NS(C)(=O)=O)c1ccc(Cc2ccc(cc2S(=O)(=O)c2cccc(F)c2)C(F)(F)F)cc1 Show InChI InChI=1S/C23H21F4NO4S2/c1-15(28-33(2,29)30)17-8-6-16(7-9-17)12-18-10-11-19(23(25,26)27)13-22(18)34(31,32)21-5-3-4-20(24)14-21/h3-11,13-15,28H,12H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant against Cannabinoid receptor 2 |
Bioorg Med Chem Lett 15: 4417-20 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.023
BindingDB Entry DOI: 10.7270/Q24T6HXQ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50297761
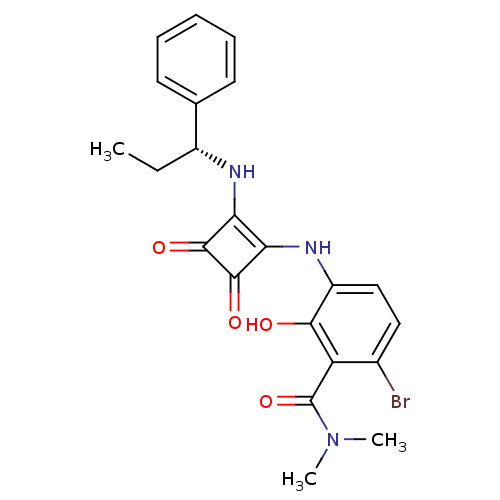
((R)-6-bromo-3-(3,4-dioxo-2-(1-phenylpropylamino)cy...)Show SMILES CC[C@@H](Nc1c(Nc2ccc(Br)c(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C22H22BrN3O4/c1-4-14(12-8-6-5-7-9-12)24-17-18(21(29)20(17)28)25-15-11-10-13(23)16(19(15)27)22(30)26(2)3/h5-11,14,24-25,27H,4H2,1-3H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of human [125I]IL-8 from human CXCR2 |
Bioorg Med Chem Lett 19: 4446-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.049
BindingDB Entry DOI: 10.7270/Q2H1323X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329141
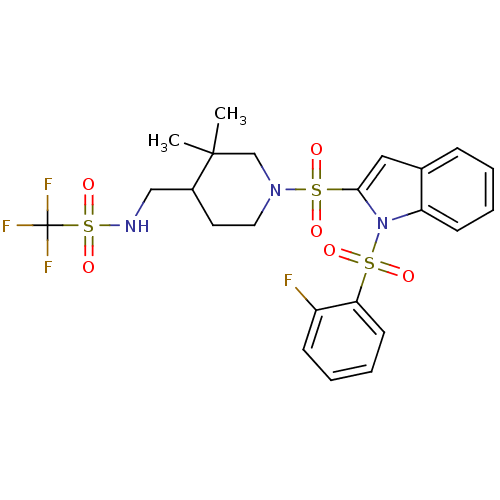
(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES CC1(C)CN(CCC1CNS(=O)(=O)C(F)(F)F)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C23H25F4N3O6S3/c1-22(2)15-29(12-11-17(22)14-28-39(35,36)23(25,26)27)38(33,34)21-13-16-7-3-5-9-19(16)30(21)37(31,32)20-10-6-4-8-18(20)24/h3-10,13,17,28H,11-12,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329156
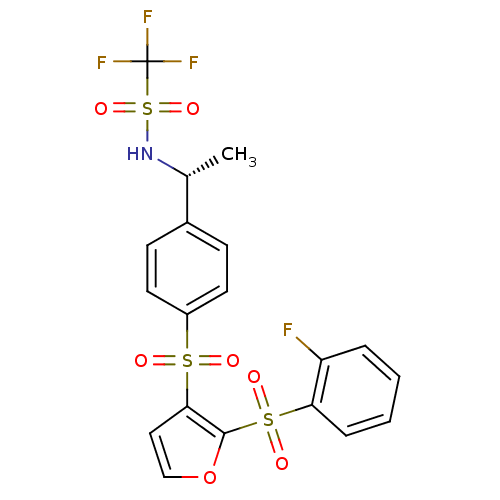
((R)-1,1,1-trifluoro-N-(1-(4-(2-(2-fluorophenylsulf...)Show SMILES C[C@@H](NS(=O)(=O)C(F)(F)F)c1ccc(cc1)S(=O)(=O)c1ccoc1S(=O)(=O)c1ccccc1F |r| Show InChI InChI=1S/C19H15F4NO7S3/c1-12(24-34(29,30)19(21,22)23)13-6-8-14(9-7-13)32(25,26)17-10-11-31-18(17)33(27,28)16-5-3-2-4-15(16)20/h2-12,24H,1H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50236053
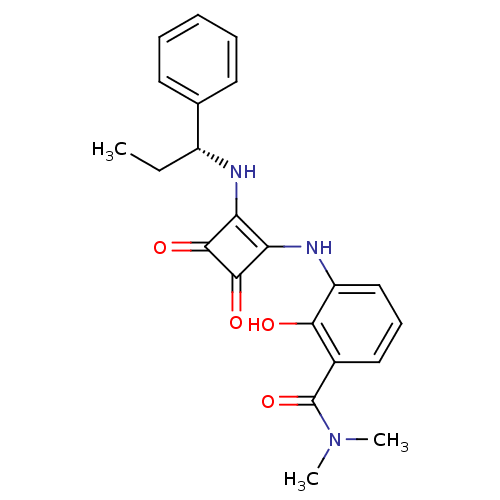
((R)-3-(3,4-dioxo-2-(1-phenylpropylamino)cyclobut-1...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C22H23N3O4/c1-4-15(13-9-6-5-7-10-13)23-17-18(21(28)20(17)27)24-16-12-8-11-14(19(16)26)22(29)25(2)3/h5-12,15,23-24,26H,4H2,1-3H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of human [125I]IL-8 from human CXCR2 |
Bioorg Med Chem Lett 19: 4446-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.049
BindingDB Entry DOI: 10.7270/Q2H1323X |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50297740
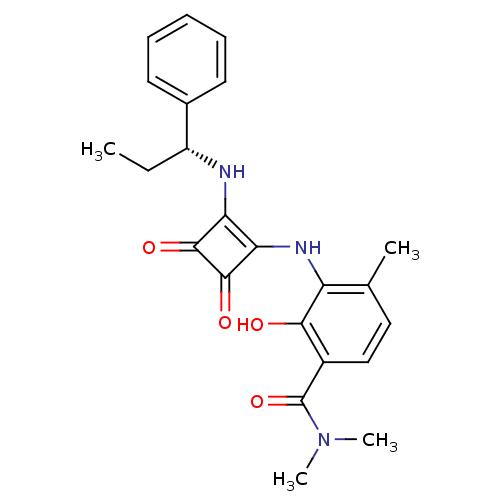
((R)-3-(3,4-dioxo-2-(1-phenylpropylamino)cyclobut-1...)Show SMILES CC[C@@H](Nc1c(Nc2c(C)ccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C23H25N3O4/c1-5-16(14-9-7-6-8-10-14)24-18-19(22(29)21(18)28)25-17-13(2)11-12-15(20(17)27)23(30)26(3)4/h6-12,16,24-25,27H,5H2,1-4H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of human [125I]IL-8 from human CXCR2 |
Bioorg Med Chem Lett 19: 4446-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.049
BindingDB Entry DOI: 10.7270/Q2H1323X |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50297741
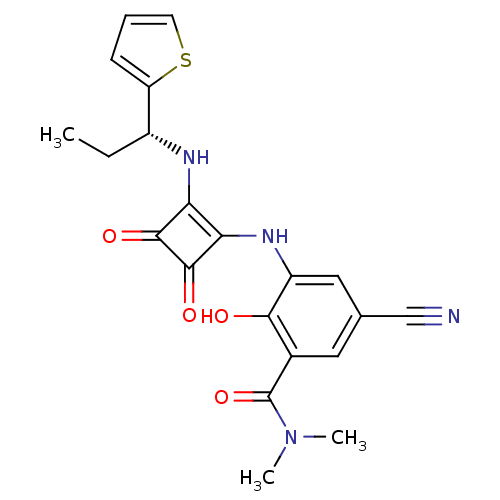
((R)-5-cyano-3-(3,4-dioxo-2-(1-(thiophen-2-yl)propy...)Show SMILES CC[C@@H](Nc1c(Nc2cc(cc(C(=O)N(C)C)c2O)C#N)c(=O)c1=O)c1cccs1 |r| Show InChI InChI=1S/C21H20N4O4S/c1-4-13(15-6-5-7-30-15)23-16-17(20(28)19(16)27)24-14-9-11(10-22)8-12(18(14)26)21(29)25(2)3/h5-9,13,23-24,26H,4H2,1-3H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of human [125I]IL-8 from human CXCR2 |
Bioorg Med Chem Lett 19: 4446-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.049
BindingDB Entry DOI: 10.7270/Q2H1323X |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50200887
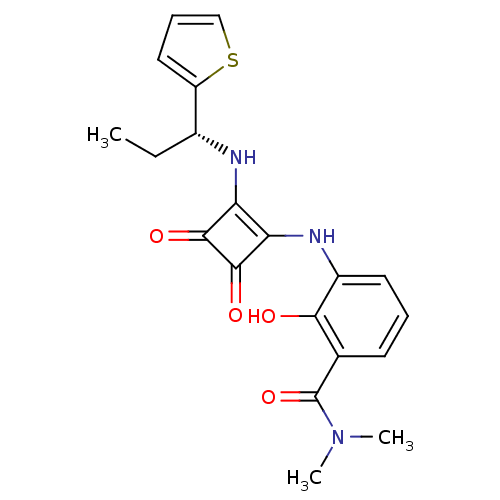
((R)-3-(3,4-dioxo-2-(1-(thiophen-2-yl)propylamino)c...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cccs1 |r| Show InChI InChI=1S/C20H21N3O4S/c1-4-12(14-9-6-10-28-14)21-15-16(19(26)18(15)25)22-13-8-5-7-11(17(13)24)20(27)23(2)3/h5-10,12,21-22,24H,4H2,1-3H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of human [125I]IL-8 from human CXCR2 |
Bioorg Med Chem Lett 19: 4446-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.049
BindingDB Entry DOI: 10.7270/Q2H1323X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data