 Found 37 hits with Last Name = 'gopi mohan' and Initial = 'c'
Found 37 hits with Last Name = 'gopi mohan' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM25524
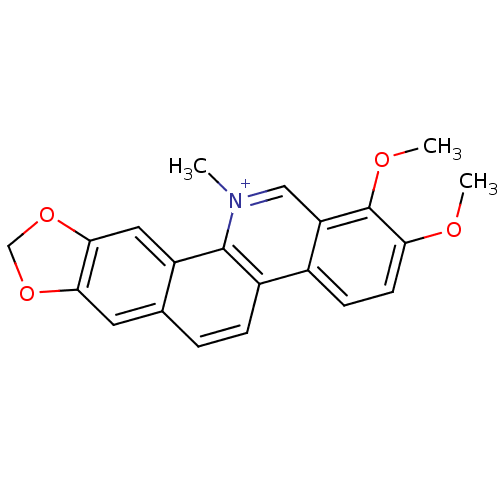
(17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyc...)Show SMILES COc1ccc2c(c[n+](C)c3c4cc5OCOc5cc4ccc23)c1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human AChE using acetyl thiocholine iodide as substrate by Lineweaver-Burk double-reciprocal analysis |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM25524

(17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyc...)Show SMILES COc1ccc2c(c[n+](C)c3c4cc5OCOc5cc4ccc23)c1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Competitive inhibition of electric eel AChE using acetyl thiocholine iodide as substrate by Lineweaver-Burk double-reciprocal analysis |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM25524

(17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyc...)Show SMILES COc1ccc2c(c[n+](C)c3c4cc5OCOc5cc4ccc23)c1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of electric eel AChE using acetyl thiocholine iodide as substrate by Lineweaver-Burk double-reciprocal analysis |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM25524

(17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyc...)Show SMILES COc1ccc2c(c[n+](C)c3c4cc5OCOc5cc4ccc23)c1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human AChE using acetyl thiocholine iodide as substrate by Lineweaver-Burk double-reciprocal analysis |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50396008

(CHEMBL1512936)Show SMILES C[C@@H]1CC[C@H]2[C@H](C)[C@H]3CC[C@@]4(O)[C@@H]5CC(=O)[C@H]6CC(=O)CC[C@]6(C)[C@H]5C[C@H]4[C@@H]3CN2C1 Show InChI InChI=1S/C27H41NO3/c1-15-4-5-24-16(2)18-7-9-27(31)20(19(18)14-28(24)13-15)11-21-22(27)12-25(30)23-10-17(29)6-8-26(21,23)3/h15-16,18-24,31H,4-14H2,1-3H3/t15-,16-,18-,19-,20+,21+,22-,23-,24+,26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50203126
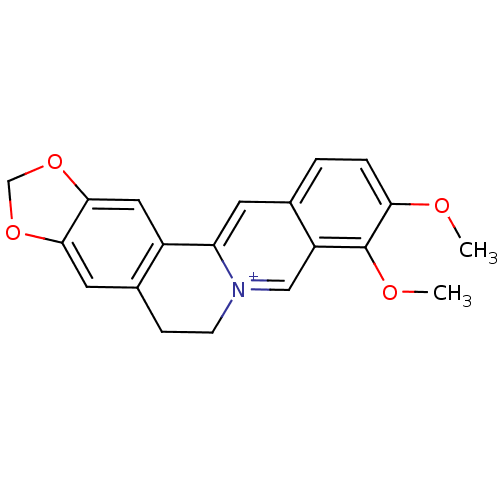
(3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...)Show InChI InChI=1S/C20H18NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM25524

(17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyc...)Show SMILES COc1ccc2c(c[n+](C)c3c4cc5OCOc5cc4ccc23)c1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50292332
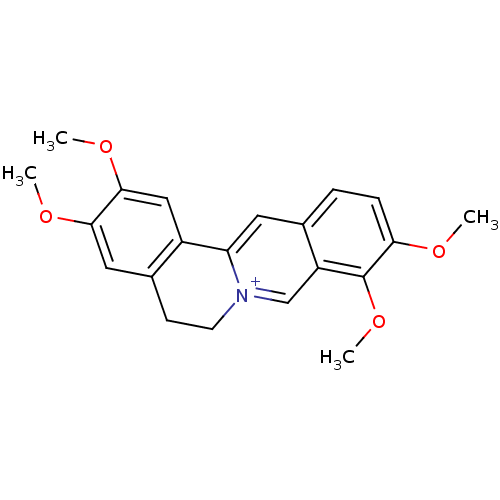
(2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...)Show InChI InChI=1S/C21H22NO4/c1-23-18-6-5-13-9-17-15-11-20(25-3)19(24-2)10-14(15)7-8-22(17)12-16(13)21(18)26-4/h5-6,9-12H,7-8H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50396005
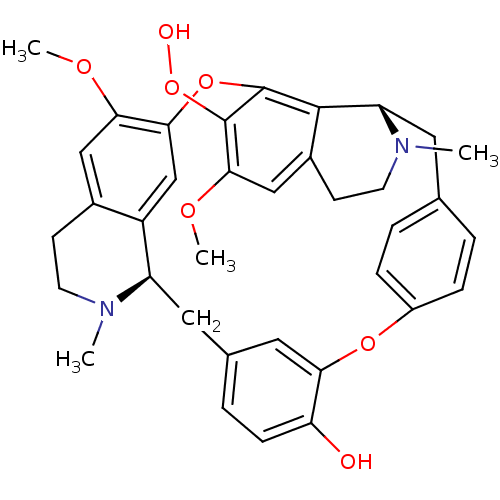
(CHEMBL2163794)Show SMILES COc1cc2CCN(C)[C@@H]3Cc4ccc(O)c(Oc5ccc(C[C@@H]6N(C)CCc7cc(OC)c(OO)c(Oc1cc23)c67)cc5)c4 |r| Show InChI InChI=1S/C36H38N2O7/c1-37-13-11-23-18-31(41-3)32-20-26(23)27(37)16-22-7-10-29(39)30(17-22)43-25-8-5-21(6-9-25)15-28-34-24(12-14-38(28)2)19-33(42-4)35(45-40)36(34)44-32/h5-10,17-20,27-28,39-40H,11-16H2,1-4H3/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50396011
(CHEMBL327134)Show SMILES CC[C@H]1CN2CCc3c([nH]c4ccccc34)[C@H]2C[C@@H]1\C(=C/OC)C(=O)OC Show InChI InChI=1S/C22H28N2O3/c1-4-14-12-24-10-9-16-15-7-5-6-8-19(15)23-21(16)20(24)11-17(14)18(13-26-2)22(25)27-3/h5-8,13-14,17,20,23H,4,9-12H2,1-3H3/b18-13+/t14-,17-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50396006
(CHEMBL405686)Show SMILES COc1cc2CCN(C)[C@H]3Cc4ccc(Oc5cc(C[C@H]6N(C)CCc7cc(OC)c(OC)c(Oc1cc23)c67)ccc5OC(=O)c1ccc(cc1)[N+]([O-])=O)cc4 Show InChI InChI=1S/C44H43N3O9/c1-45-18-16-29-23-37(51-3)39-25-33(29)34(45)20-26-6-13-32(14-7-26)54-38-22-27(8-15-36(38)56-44(48)28-9-11-31(12-10-28)47(49)50)21-35-41-30(17-19-46(35)2)24-40(52-4)42(53-5)43(41)55-39/h6-15,22-25,34-35H,16-21H2,1-5H3/t34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396012
(CHEMBL2163791)Show InChI InChI=1S/C11H12N2O/c14-10-5-6-13-7-8-3-1-2-4-9(8)12-11(10)13/h1-4,10,14H,5-7H2/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50203126

(3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...)Show InChI InChI=1S/C20H18NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50396012

(CHEMBL2163791)Show InChI InChI=1S/C11H12N2O/c14-10-5-6-13-7-8-3-1-2-4-9(8)12-11(10)13/h1-4,10,14H,5-7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50396009
(CHEMBL2163793)Show SMILES CCC1=C[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@@](C2)([C@@H]13)C(=O)OC |r,t:2,THB:2:20:5.4.19:7.8.9.10| Show InChI InChI=1S/C21H24N2O2/c1-3-14-10-13-11-21(20(24)25-2)18-16(8-9-23(12-13)19(14)21)15-6-4-5-7-17(15)22-18/h4-7,10,13,19,22H,3,8-9,11-12H2,1-2H3/t13-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396008

(CHEMBL1512936)Show SMILES C[C@@H]1CC[C@H]2[C@H](C)[C@H]3CC[C@@]4(O)[C@@H]5CC(=O)[C@H]6CC(=O)CC[C@]6(C)[C@H]5C[C@H]4[C@@H]3CN2C1 Show InChI InChI=1S/C27H41NO3/c1-15-4-5-24-16(2)18-7-9-27(31)20(19(18)14-28(24)13-15)11-21-22(27)12-25(30)23-10-17(29)6-8-26(21,23)3/h15-16,18-24,31H,4-14H2,1-3H3/t15-,16-,18-,19-,20+,21+,22-,23-,24+,26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM25524

(17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyc...)Show SMILES COc1ccc2c(c[n+](C)c3c4cc5OCOc5cc4ccc23)c1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50292332

(2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...)Show InChI InChI=1S/C21H22NO4/c1-23-18-6-5-13-9-17-15-11-20(25-3)19(24-2)10-14(15)7-8-22(17)12-16(13)21(18)26-4/h5-6,9-12H,7-8H2,1-4H3/q+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396005

(CHEMBL2163794)Show SMILES COc1cc2CCN(C)[C@@H]3Cc4ccc(O)c(Oc5ccc(C[C@@H]6N(C)CCc7cc(OC)c(OO)c(Oc1cc23)c67)cc5)c4 |r| Show InChI InChI=1S/C36H38N2O7/c1-37-13-11-23-18-31(41-3)32-20-26(23)27(37)16-22-7-10-29(39)30(17-22)43-25-8-5-21(6-9-25)15-28-34-24(12-14-38(28)2)19-33(42-4)35(45-40)36(34)44-32/h5-10,17-20,27-28,39-40H,11-16H2,1-4H3/t27-,28+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396006

(CHEMBL405686)Show SMILES COc1cc2CCN(C)[C@H]3Cc4ccc(Oc5cc(C[C@H]6N(C)CCc7cc(OC)c(OC)c(Oc1cc23)c67)ccc5OC(=O)c1ccc(cc1)[N+]([O-])=O)cc4 Show InChI InChI=1S/C44H43N3O9/c1-45-18-16-29-23-37(51-3)39-25-33(29)34(45)20-26-6-13-32(14-7-26)54-38-22-27(8-15-36(38)56-44(48)28-9-11-31(12-10-28)47(49)50)21-35-41-30(17-19-46(35)2)24-40(52-4)42(53-5)43(41)55-39/h6-15,22-25,34-35H,16-21H2,1-5H3/t34-,35+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396011

(CHEMBL327134)Show SMILES CC[C@H]1CN2CCc3c([nH]c4ccccc34)[C@H]2C[C@@H]1\C(=C/OC)C(=O)OC Show InChI InChI=1S/C22H28N2O3/c1-4-14-12-24-10-9-16-15-7-5-6-8-19(15)23-21(16)20(24)11-17(14)18(13-26-2)22(25)27-3/h5-8,13-14,17,20,23H,4,9-12H2,1-3H3/b18-13+/t14-,17-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396009

(CHEMBL2163793)Show SMILES CCC1=C[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@@](C2)([C@@H]13)C(=O)OC |r,t:2,THB:2:20:5.4.19:7.8.9.10| Show InChI InChI=1S/C21H24N2O2/c1-3-14-10-13-11-21(20(24)25-2)18-16(8-9-23(12-13)19(14)21)15-6-4-5-7-17(15)22-18/h4-7,10,13,19,22H,3,8-9,11-12H2,1-2H3/t13-,19+,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM25524

(17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyc...)Show SMILES COc1ccc2c(c[n+](C)c3c4cc5OCOc5cc4ccc23)c1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50026636

(17alpha-hydroxy-20alpha-yohimban-16beta-carboxylic...)Show SMILES COC(=O)[C@@H]1[C@@H](O)CC[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15+,17+,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50396002
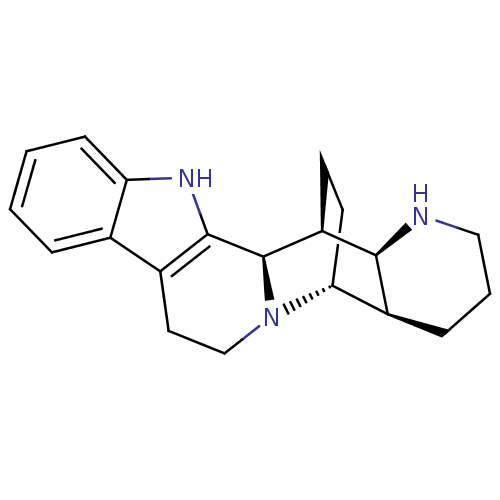
(CHEMBL2165597)Show SMILES C1CN[C@H]2[C@@H](C1)[C@H]1CC[C@@H]2[C@H]2N1CCc1c2[nH]c2ccccc12 |r| Show InChI InChI=1S/C20H25N3/c1-2-6-16-12(4-1)13-9-11-23-17-8-7-15(20(23)19(13)22-16)18-14(17)5-3-10-21-18/h1-2,4,6,14-15,17-18,20-22H,3,5,7-11H2/t14-,15-,17+,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM25524

(17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyc...)Show SMILES COc1ccc2c(c[n+](C)c3c4cc5OCOc5cc4ccc23)c1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396002

(CHEMBL2165597)Show SMILES C1CN[C@H]2[C@@H](C1)[C@H]1CC[C@@H]2[C@H]2N1CCc1c2[nH]c2ccccc12 |r| Show InChI InChI=1S/C20H25N3/c1-2-6-16-12(4-1)13-9-11-23-17-8-7-15(20(23)19(13)22-16)18-14(17)5-3-10-21-18/h1-2,4,6,14-15,17-18,20-22H,3,5,7-11H2/t14-,15-,17+,18-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396007

(VERATRAMINE)Show SMILES C[C@H]([C@@H]1NC[C@@H](C)C[C@H]1O)c1ccc2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3Cc2c1C |r,t:17| Show InChI InChI=1S/C27H39NO2/c1-15-11-25(30)26(28-14-15)17(3)20-7-8-21-22-6-5-18-12-19(29)9-10-27(18,4)24(22)13-23(21)16(20)2/h5,7-8,15,17,19,22,24-26,28-30H,6,9-14H2,1-4H3/t15-,17-,19-,22-,24-,25+,26-,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50396003
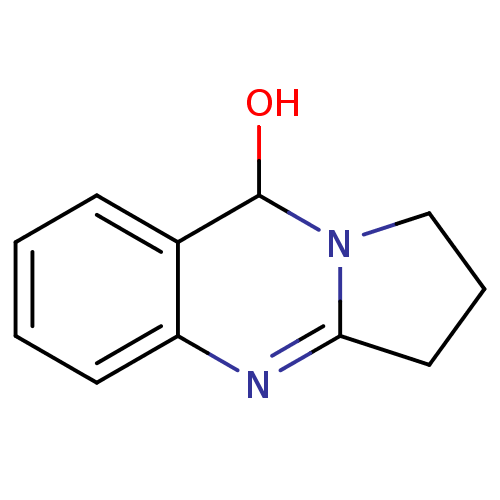
(CHEMBL2165578)Show InChI InChI=1S/C11H12N2O/c14-11-8-4-1-2-5-9(8)12-10-6-3-7-13(10)11/h1-2,4-5,11,14H,3,6-7H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50289102

(1,2,3,9-Tetrahydro-pyrrolo[2,1-b]quinazoline | CHE...)Show InChI InChI=1S/C11H12N2/c1-2-5-10-9(4-1)8-13-7-3-6-11(13)12-10/h1-2,4-5H,3,6-8H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50289102

(1,2,3,9-Tetrahydro-pyrrolo[2,1-b]quinazoline | CHE...)Show InChI InChI=1S/C11H12N2/c1-2-5-10-9(4-1)8-13-7-3-6-11(13)12-10/h1-2,4-5H,3,6-8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of horse BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50026636

(17alpha-hydroxy-20alpha-yohimban-16beta-carboxylic...)Show SMILES COC(=O)[C@@H]1[C@@H](O)CC[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15+,17+,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50396007

(VERATRAMINE)Show SMILES C[C@H]([C@@H]1NC[C@@H](C)C[C@H]1O)c1ccc2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3Cc2c1C |r,t:17| Show InChI InChI=1S/C27H39NO2/c1-15-11-25(30)26(28-14-15)17(3)20-7-8-21-22-6-5-18-12-19(29)9-10-27(18,4)24(22)13-23(21)16(20)2/h5,7-8,15,17,19,22,24-26,28-30H,6,9-14H2,1-4H3/t15-,17-,19-,22-,24-,25+,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50396003

(CHEMBL2165578)Show InChI InChI=1S/C11H12N2O/c14-11-8-4-1-2-5-9(8)12-10-6-3-7-13(10)11/h1-2,4-5,11,14H,3,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human BChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50076089

((3S,3aR,4R,4aS,8aR,9aS)-3-Methyl-4-[2-((R)-1-methy...)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM31904
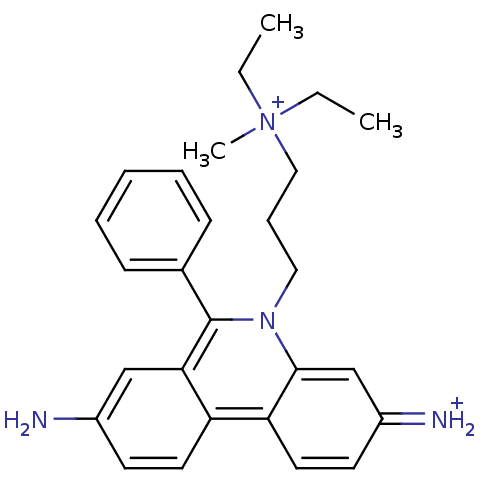
(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's method |
Bioorg Med Chem 20: 6669-79 (2012)
Article DOI: 10.1016/j.bmc.2012.09.040
BindingDB Entry DOI: 10.7270/Q2ZG6TBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data