 Found 796 hits with Last Name = 'gould' and Initial = 'sl'
Found 796 hits with Last Name = 'gould' and Initial = 'sl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against specific binding of [125I]-MIP-1 alpha to human CCR5 receptor |
Bioorg Med Chem Lett 11: 265-70 (2001)
BindingDB Entry DOI: 10.7270/Q2668CFZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141931

((R)-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl-2...)Show SMILES CCn1nc(Cc2ccc(cc2)-c2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C2CCCCC2)C(O)=O)CC1 Show InChI InChI=1S/C42H51FN4O2/c1-2-47-40(26-38(44-47)24-30-16-18-32(19-17-30)31-10-5-3-6-11-31)33-20-22-45(23-21-33)27-36-28-46(29-39(36)35-14-9-15-37(43)25-35)41(42(48)49)34-12-7-4-8-13-34/h3,5-6,9-11,14-19,25-26,33-34,36,39,41H,2,4,7-8,12-13,20-24,27-29H2,1H3,(H,48,49)/t36-,39+,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141911

((R)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethyl-2H...)Show SMILES CCn1nc(Cc2ccc(cc2)C#N)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C35H44FN5O2/c1-5-41-32(19-30(38-41)17-24-9-11-25(20-37)12-10-24)26-13-15-39(16-14-26)21-28-22-40(33(34(42)43)35(2,3)4)23-31(28)27-7-6-8-29(36)18-27/h6-12,18-19,26,28,31,33H,5,13-17,21-23H2,1-4H3,(H,42,43)/t28-,31+,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141908

((R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butyl-benzyl)-2-eth...)Show SMILES CCn1nc(Cc2ccc(cc2)C(C)(C)C)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H53FN4O2/c1-8-43-34(22-32(40-43)20-26-12-14-30(15-13-26)37(2,3)4)27-16-18-41(19-17-27)23-29-24-42(35(36(44)45)38(5,6)7)25-33(29)28-10-9-11-31(39)21-28/h9-15,21-22,27,29,33,35H,8,16-20,23-25H2,1-7H3,(H,44,45)/t29-,33+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141905
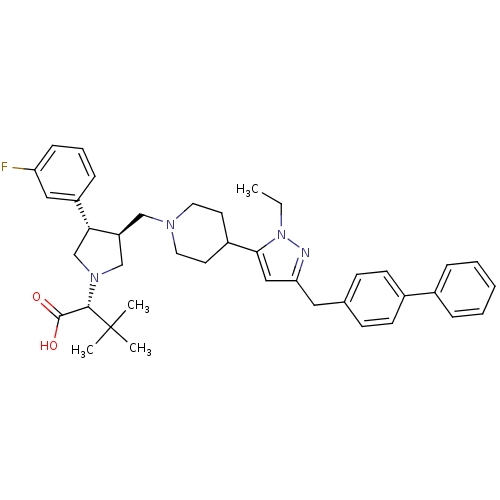
((R)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl...)Show SMILES CCn1nc(Cc2ccc(cc2)-c2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C40H49FN4O2/c1-5-45-37(24-35(42-45)22-28-14-16-30(17-15-28)29-10-7-6-8-11-29)31-18-20-43(21-19-31)25-33-26-44(38(39(46)47)40(2,3)4)27-36(33)32-12-9-13-34(41)23-32/h6-17,23-24,31,33,36,38H,5,18-22,25-27H2,1-4H3,(H,46,47)/t33-,36+,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141978
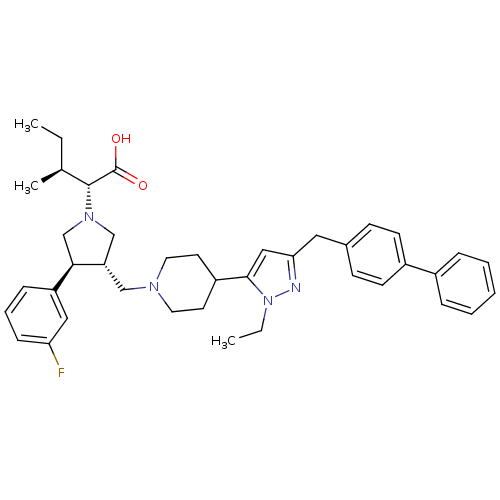
((2R,4S)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-e...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)-c3ccccc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C40H49FN4O2/c1-4-28(3)39(40(46)47)44-26-34(37(27-44)33-12-9-13-35(41)23-33)25-43-20-18-32(19-21-43)38-24-36(42-45(38)5-2)22-29-14-16-31(17-15-29)30-10-7-6-8-11-30/h6-17,23-24,28,32,34,37,39H,4-5,18-22,25-27H2,1-3H3,(H,46,47)/t28-,34-,37+,39+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141935
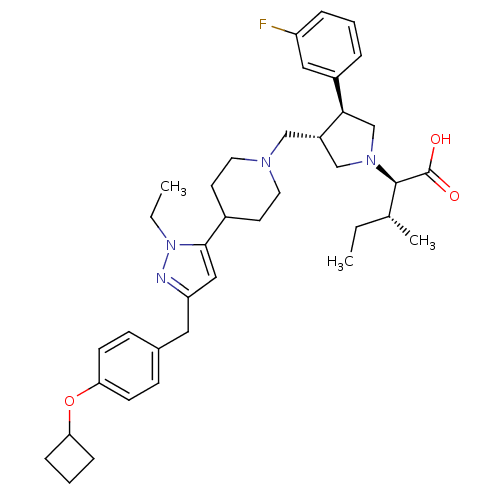
((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC4CCC4)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H51FN4O3/c1-4-26(3)37(38(44)45)42-24-30(35(25-42)29-8-6-9-31(39)21-29)23-41-18-16-28(17-19-41)36-22-32(40-43(36)5-2)20-27-12-14-34(15-13-27)46-33-10-7-11-33/h6,8-9,12-15,21-22,26,28,30,33,35,37H,4-5,7,10-11,16-20,23-25H2,1-3H3,(H,44,45)/t26-,30+,35-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141951
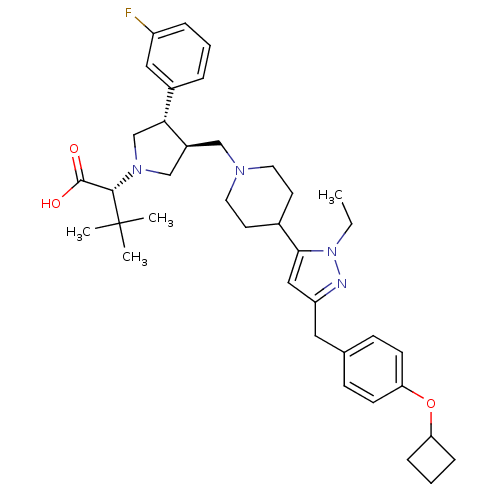
((R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-2-et...)Show SMILES CCn1nc(Cc2ccc(OC3CCC3)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H51FN4O3/c1-5-43-35(22-31(40-43)20-26-12-14-33(15-13-26)46-32-10-7-11-32)27-16-18-41(19-17-27)23-29-24-42(36(37(44)45)38(2,3)4)25-34(29)28-8-6-9-30(39)21-28/h6,8-9,12-15,21-22,27,29,32,34,36H,5,7,10-11,16-20,23-25H2,1-4H3,(H,44,45)/t29-,34+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141913
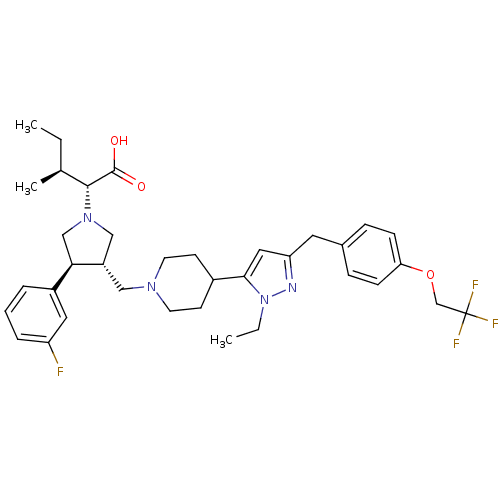
((2R,4S)-2-[(2S,3S)-3-(4-{2-Ethyl-5-[4-(2,2,2-trifl...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OCC(F)(F)F)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C36H46F4N4O3/c1-4-24(3)34(35(45)46)43-21-28(32(22-43)27-7-6-8-29(37)18-27)20-42-15-13-26(14-16-42)33-19-30(41-44(33)5-2)17-25-9-11-31(12-10-25)47-23-36(38,39)40/h6-12,18-19,24,26,28,32,34H,4-5,13-17,20-23H2,1-3H3,(H,45,46)/t24-,28-,32+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141941
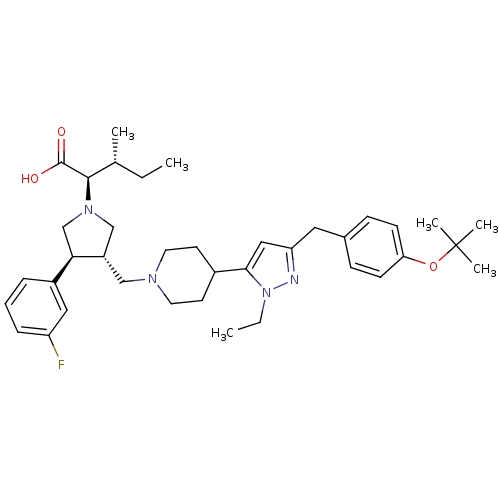
((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butoxy-benzyl)-...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC(C)(C)C)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H53FN4O3/c1-7-26(3)36(37(44)45)42-24-30(34(25-42)29-10-9-11-31(39)21-29)23-41-18-16-28(17-19-41)35-22-32(40-43(35)8-2)20-27-12-14-33(15-13-27)46-38(4,5)6/h9-15,21-22,26,28,30,34,36H,7-8,16-20,23-25H2,1-6H3,(H,44,45)/t26-,30+,34-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141906
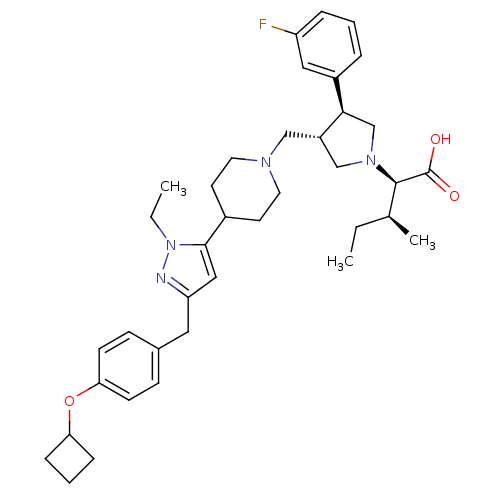
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC4CCC4)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H51FN4O3/c1-4-26(3)37(38(44)45)42-24-30(35(25-42)29-8-6-9-31(39)21-29)23-41-18-16-28(17-19-41)36-22-32(40-43(36)5-2)20-27-12-14-34(15-13-27)46-33-10-7-11-33/h6,8-9,12-15,21-22,26,28,30,33,35,37H,4-5,7,10-11,16-20,23-25H2,1-3H3,(H,44,45)/t26-,30-,35+,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141973
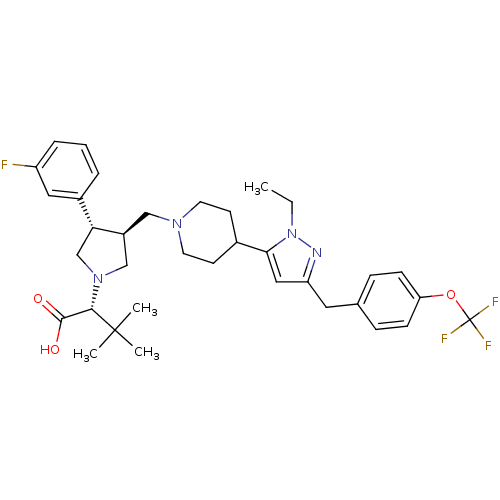
(2-[3-{4-[2-Ethyl-5-(4-trifluoromethoxy-benzyl)-2H-...)Show SMILES CCn1nc(Cc2ccc(OC(F)(F)F)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C35H44F4N4O3/c1-5-43-31(19-28(40-43)17-23-9-11-29(12-10-23)46-35(37,38)39)24-13-15-41(16-14-24)20-26-21-42(32(33(44)45)34(2,3)4)22-30(26)25-7-6-8-27(36)18-25/h6-12,18-19,24,26,30,32H,5,13-17,20-22H2,1-4H3,(H,44,45)/t26-,30+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141910
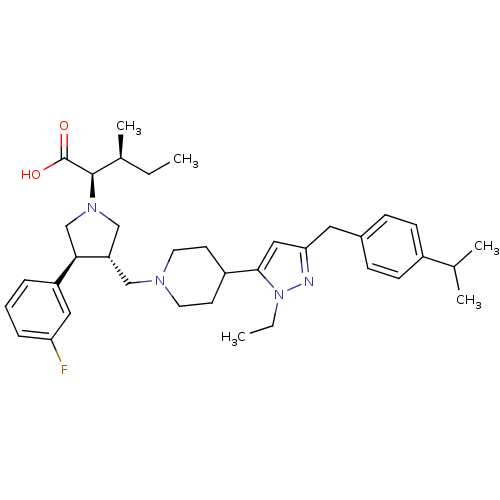
((2R,4S)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropyl-be...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)C(C)C)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C37H51FN4O2/c1-6-26(5)36(37(43)44)41-23-31(34(24-41)30-9-8-10-32(38)20-30)22-40-17-15-29(16-18-40)35-21-33(39-42(35)7-2)19-27-11-13-28(14-12-27)25(3)4/h8-14,20-21,25-26,29,31,34,36H,6-7,15-19,22-24H2,1-5H3,(H,43,44)/t26-,31-,34+,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141979
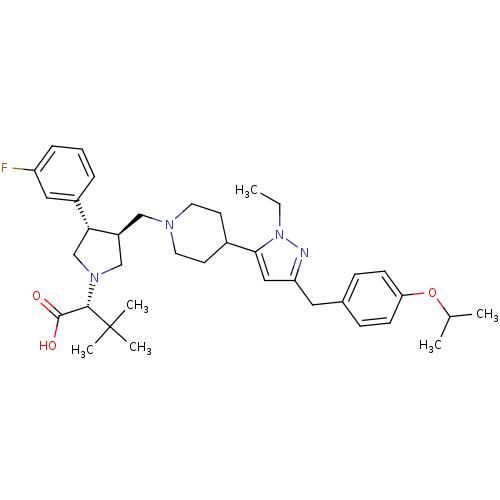
((R)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropoxy-benzy...)Show SMILES CCn1nc(Cc2ccc(OC(C)C)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C37H51FN4O3/c1-7-42-34(21-31(39-42)19-26-11-13-32(14-12-26)45-25(2)3)27-15-17-40(18-16-27)22-29-23-41(35(36(43)44)37(4,5)6)24-33(29)28-9-8-10-30(38)20-28/h8-14,20-21,25,27,29,33,35H,7,15-19,22-24H2,1-6H3,(H,43,44)/t29-,33+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141984
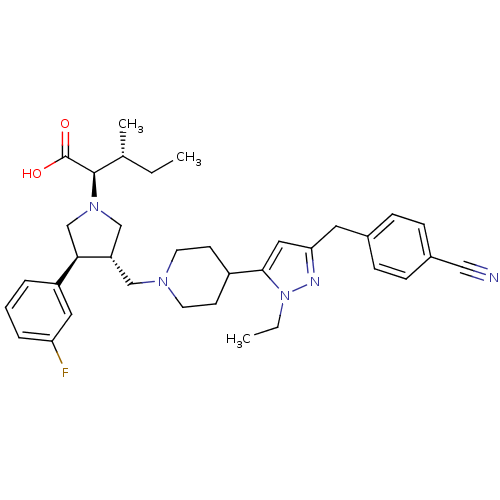
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethy...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)C#N)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C35H44FN5O2/c1-4-24(3)34(35(42)43)40-22-29(32(23-40)28-7-6-8-30(36)18-28)21-39-15-13-27(14-16-39)33-19-31(38-41(33)5-2)17-25-9-11-26(20-37)12-10-25/h6-12,18-19,24,27,29,32,34H,4-5,13-17,21-23H2,1-3H3,(H,42,43)/t24-,29+,32-,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141883
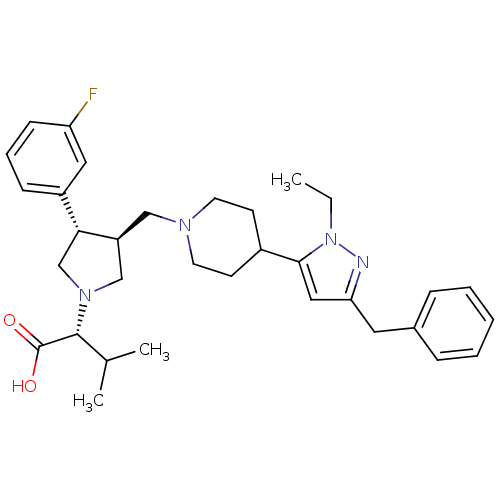
((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...)Show SMILES CCn1nc(Cc2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C(C)C)C(O)=O)CC1 |r| Show InChI InChI=1S/C33H43FN4O2/c1-4-38-31(19-29(35-38)17-24-9-6-5-7-10-24)25-13-15-36(16-14-25)20-27-21-37(32(23(2)3)33(39)40)22-30(27)26-11-8-12-28(34)18-26/h5-12,18-19,23,25,27,30,32H,4,13-17,20-22H2,1-3H3,(H,39,40)/t27-,30+,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141972
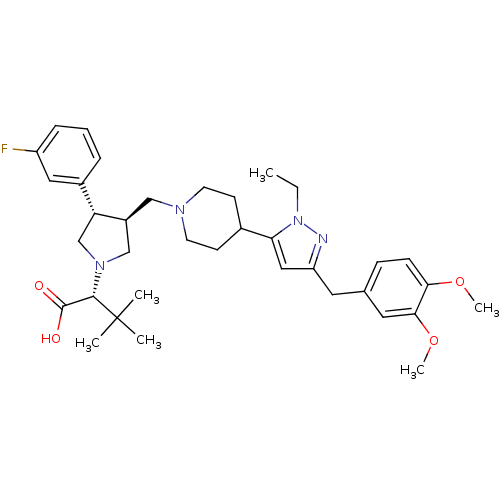
((R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-2-et...)Show SMILES CCn1nc(Cc2ccc(OC)c(OC)c2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C36H49FN4O4/c1-7-41-31(20-29(38-41)17-24-11-12-32(44-5)33(18-24)45-6)25-13-15-39(16-14-25)21-27-22-40(34(35(42)43)36(2,3)4)23-30(27)26-9-8-10-28(37)19-26/h8-12,18-20,25,27,30,34H,7,13-17,21-23H2,1-6H3,(H,42,43)/t27-,30+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141874
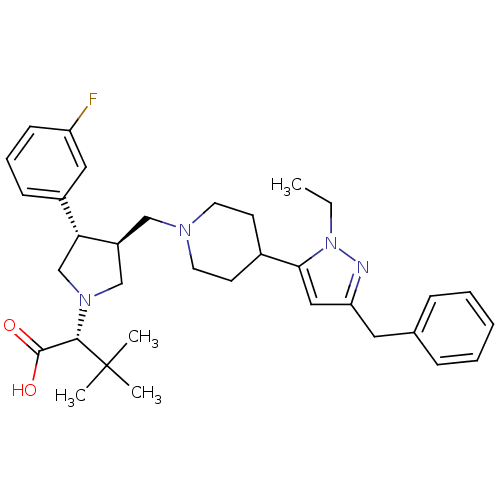
((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...)Show SMILES CCn1nc(Cc2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 |r| Show InChI InChI=1S/C34H45FN4O2/c1-5-39-31(20-29(36-39)18-24-10-7-6-8-11-24)25-14-16-37(17-15-25)21-27-22-38(32(33(40)41)34(2,3)4)23-30(27)26-12-9-13-28(35)19-26/h6-13,19-20,25,27,30,32H,5,14-18,21-23H2,1-4H3,(H,40,41)/t27-,30+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141945
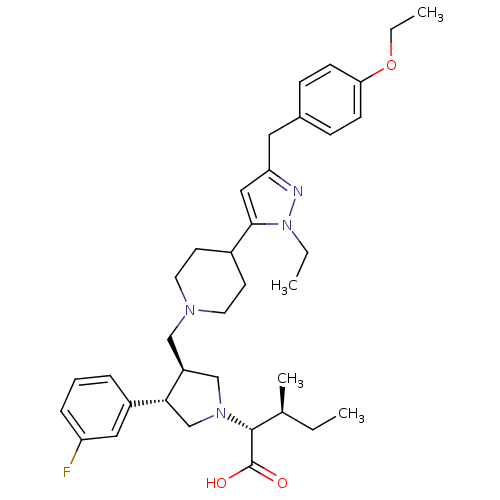
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Ethoxy-benzyl)-2-eth...)Show SMILES CCOc1ccc(Cc2cc(C3CCN(C[C@H]4CN(C[C@@H]4c4cccc(F)c4)[C@H]([C@@H](C)CC)C(O)=O)CC3)n(CC)n2)cc1 Show InChI InChI=1S/C36H49FN4O3/c1-5-25(4)35(36(42)43)40-23-29(33(24-40)28-9-8-10-30(37)20-28)22-39-17-15-27(16-18-39)34-21-31(38-41(34)6-2)19-26-11-13-32(14-12-26)44-7-3/h8-14,20-21,25,27,29,33,35H,5-7,15-19,22-24H2,1-4H3,(H,42,43)/t25-,29-,33+,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141914
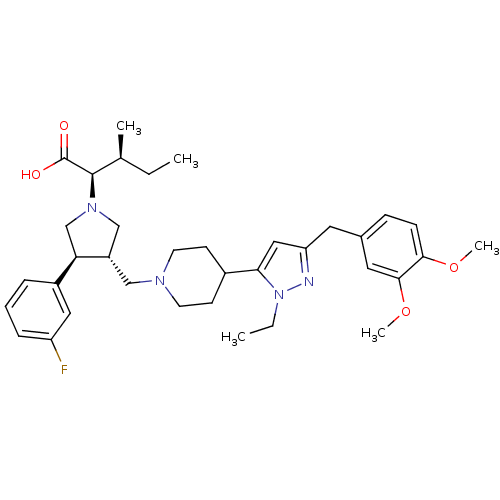
((2R,4S)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC)c(OC)c3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C36H49FN4O4/c1-6-24(3)35(36(42)43)40-22-28(31(23-40)27-9-8-10-29(37)19-27)21-39-15-13-26(14-16-39)32-20-30(38-41(32)7-2)17-25-11-12-33(44-4)34(18-25)45-5/h8-12,18-20,24,26,28,31,35H,6-7,13-17,21-23H2,1-5H3,(H,42,43)/t24-,28-,31+,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141875
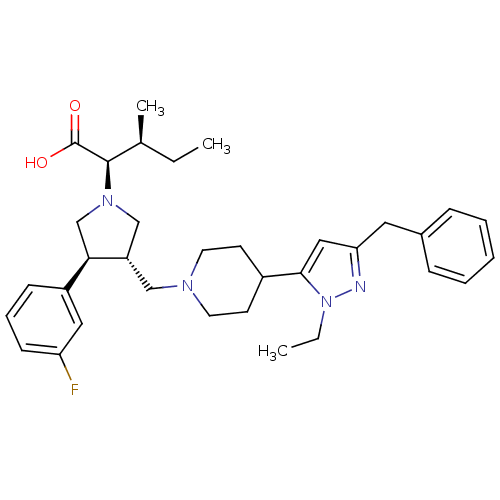
((2R,3S)-2-[(2S,3S)-3-[4-(5-Benzyl-2-ethyl-2H-pyraz...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccccc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C34H45FN4O2/c1-4-24(3)33(34(40)41)38-22-28(31(23-38)27-12-9-13-29(35)19-27)21-37-16-14-26(15-17-37)32-20-30(36-39(32)5-2)18-25-10-7-6-8-11-25/h6-13,19-20,24,26,28,31,33H,4-5,14-18,21-23H2,1-3H3,(H,40,41)/t24-,28-,31+,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141887
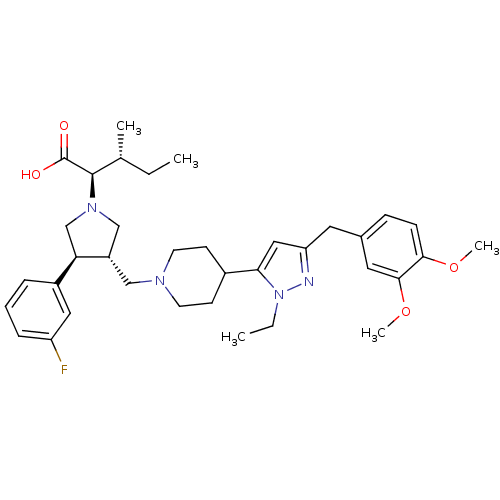
((2R,4R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC)c(OC)c3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C36H49FN4O4/c1-6-24(3)35(36(42)43)40-22-28(31(23-40)27-9-8-10-29(37)19-27)21-39-15-13-26(14-16-39)32-20-30(38-41(32)7-2)17-25-11-12-33(44-4)34(18-25)45-5/h8-12,18-20,24,26,28,31,35H,6-7,13-17,21-23H2,1-5H3,(H,42,43)/t24-,28+,31-,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50121838
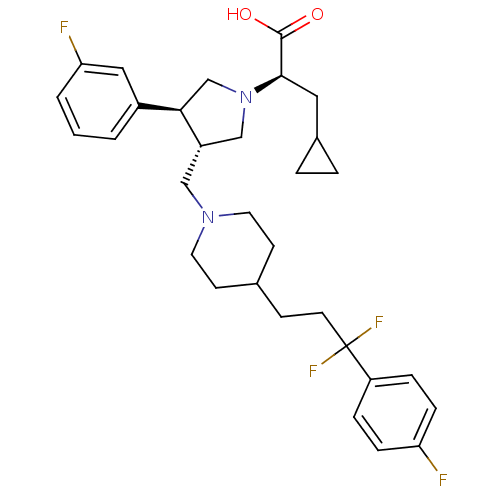
((R)-3-cyclopropyl-2-((3S,4S)-3-((4-(3,3-difluoro-3...)Show SMILES OC(=O)[C@@H](CC1CC1)N1C[C@H](CN2CCC(CCC(F)(F)c3ccc(F)cc3)CC2)[C@H](C1)c1cccc(F)c1 |r| Show InChI InChI=1S/C31H38F4N2O2/c32-26-8-6-25(7-9-26)31(34,35)13-10-21-11-14-36(15-12-21)18-24-19-37(29(30(38)39)16-22-4-5-22)20-28(24)23-2-1-3-27(33)17-23/h1-3,6-9,17,21-22,24,28-29H,4-5,10-16,18-20H2,(H,38,39)/t24-,28+,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane |
Bioorg Med Chem Lett 13: 119-23 (2002)
BindingDB Entry DOI: 10.7270/Q2CN7382 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119338
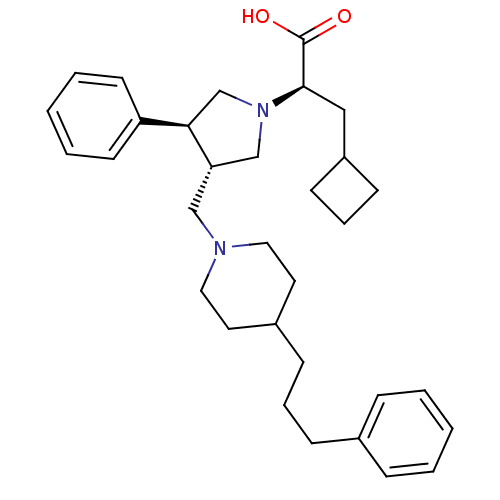
((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...)Show SMILES OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CCCc3ccccc3)CC2)[C@H](C1)c1ccccc1 Show InChI InChI=1S/C32H44N2O2/c35-32(36)31(21-27-13-8-14-27)34-23-29(30(24-34)28-15-5-2-6-16-28)22-33-19-17-26(18-20-33)12-7-11-25-9-3-1-4-10-25/h1-6,9-10,15-16,26-27,29-31H,7-8,11-14,17-24H2,(H,35,36)/t29-,30+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane |
Bioorg Med Chem Lett 13: 119-23 (2002)
BindingDB Entry DOI: 10.7270/Q2CN7382 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119338

((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...)Show SMILES OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CCCc3ccccc3)CC2)[C@H](C1)c1ccccc1 Show InChI InChI=1S/C32H44N2O2/c35-32(36)31(21-27-13-8-14-27)34-23-29(30(24-34)28-15-5-2-6-16-28)22-33-19-17-26(18-20-33)12-7-11-25-9-3-1-4-10-25/h1-6,9-10,15-16,26-27,29-31H,7-8,11-14,17-24H2,(H,35,36)/t29-,30+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranes |
Bioorg Med Chem Lett 15: 2129-34 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.030
BindingDB Entry DOI: 10.7270/Q21C1WD7 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119338

((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...)Show SMILES OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CCCc3ccccc3)CC2)[C@H](C1)c1ccccc1 Show InChI InChI=1S/C32H44N2O2/c35-32(36)31(21-27-13-8-14-27)34-23-29(30(24-34)28-15-5-2-6-16-28)22-33-19-17-26(18-20-33)12-7-11-25-9-3-1-4-10-25/h1-6,9-10,15-16,26-27,29-31H,7-8,11-14,17-24H2,(H,35,36)/t29-,30+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. |
Bioorg Med Chem Lett 12: 3001-4 (2002)
BindingDB Entry DOI: 10.7270/Q2MK6C71 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50105517
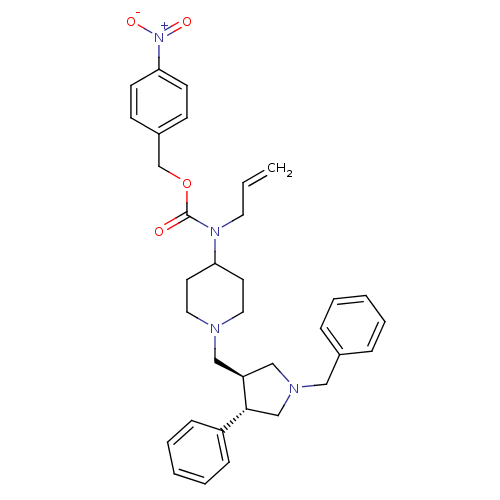
(Allyl-[1-((3S,4S)-1-benzyl-4-phenyl-pyrrolidin-3-y...)Show SMILES [O-][N+](=O)c1ccc(COC(=O)N(CC=C)C2CCN(C[C@H]3CN(Cc4ccccc4)C[C@@H]3c3ccccc3)CC2)cc1 Show InChI InChI=1S/C34H40N4O4/c1-2-19-37(34(39)42-26-28-13-15-32(16-14-28)38(40)41)31-17-20-35(21-18-31)23-30-24-36(22-27-9-5-3-6-10-27)25-33(30)29-11-7-4-8-12-29/h2-16,30-31,33H,1,17-26H2/t30-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells |
Bioorg Med Chem Lett 11: 2741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NFC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50105510

(Allyl-{1-[(S)-4-(benzenesulfonyl-methyl-amino)-3-m...)Show SMILES CN(C[C@@](C)(CCN1CCC(CC1)N(CC=C)C(=O)OCc1ccc(cc1)[N+]([O-])=O)c1ccccc1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C34H42N4O6S/c1-4-22-37(33(39)44-26-28-15-17-31(18-16-28)38(40)41)30-19-23-36(24-20-30)25-21-34(2,29-11-7-5-8-12-29)27-35(3)45(42,43)32-13-9-6-10-14-32/h4-18,30H,1,19-27H2,2-3H3/t34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells |
Bioorg Med Chem Lett 11: 2741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NFC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119349
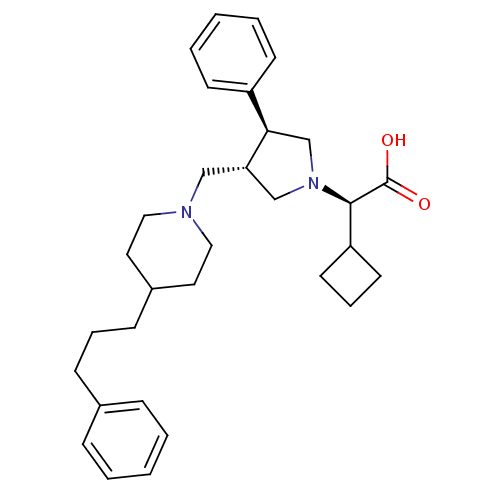
((R)-2-cyclobutyl-2-((3S,4S)-3-phenyl-4-((4-(3-phen...)Show SMILES OC(=O)[C@@H](C1CCC1)N1C[C@H](CN2CCC(CCCc3ccccc3)CC2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C31H42N2O2/c34-31(35)30(27-15-8-16-27)33-22-28(29(23-33)26-13-5-2-6-14-26)21-32-19-17-25(18-20-32)12-7-11-24-9-3-1-4-10-24/h1-6,9-10,13-14,25,27-30H,7-8,11-12,15-23H2,(H,34,35)/t28-,29+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. |
Bioorg Med Chem Lett 12: 3001-4 (2002)
BindingDB Entry DOI: 10.7270/Q2MK6C71 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119341
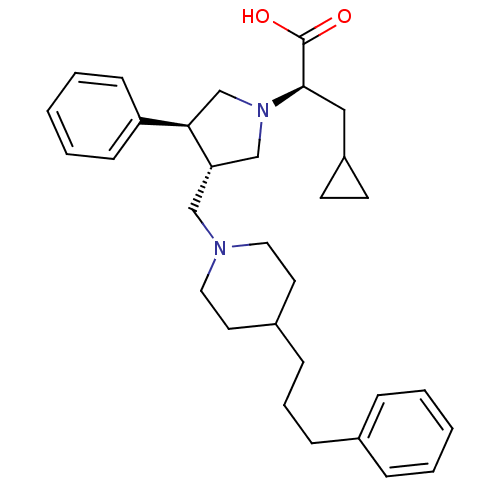
((R)-3-cyclopropyl-2-((3S,4S)-3-phenyl-4-((4-(3-phe...)Show SMILES OC(=O)[C@@H](CC1CC1)N1C[C@H](CN2CCC(CCCc3ccccc3)CC2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C31H42N2O2/c34-31(35)30(20-26-14-15-26)33-22-28(29(23-33)27-12-5-2-6-13-27)21-32-18-16-25(17-19-32)11-7-10-24-8-3-1-4-9-24/h1-6,8-9,12-13,25-26,28-30H,7,10-11,14-23H2,(H,34,35)/t28-,29+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. |
Bioorg Med Chem Lett 12: 3001-4 (2002)
BindingDB Entry DOI: 10.7270/Q2MK6C71 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119336

(2-cyclohexyl-2-{3-phenyl-4-[4-(3-phenylpropyl)hexa...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CCCc3ccccc3)CC2)[C@H](C1)c1ccccc1 Show InChI InChI=1S/C33H46N2O2/c36-33(37)32(29-17-8-3-9-18-29)35-24-30(31(25-35)28-15-6-2-7-16-28)23-34-21-19-27(20-22-34)14-10-13-26-11-4-1-5-12-26/h1-2,4-7,11-12,15-16,27,29-32H,3,8-10,13-14,17-25H2,(H,36,37)/t30-,31+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. |
Bioorg Med Chem Lett 12: 3001-4 (2002)
BindingDB Entry DOI: 10.7270/Q2MK6C71 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50121836
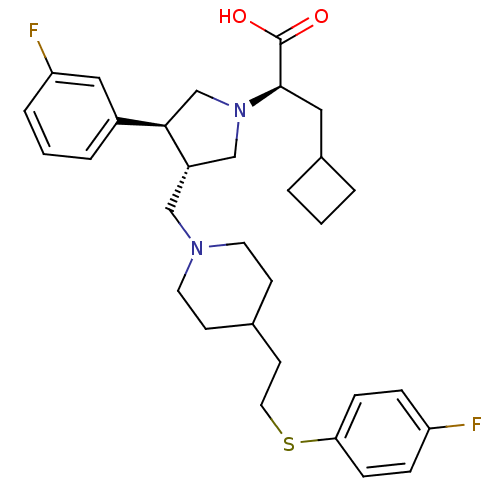
((R)-3-Cyclobutyl-2-((S)-3-(3-fluoro-phenyl)-4-{4-[...)Show SMILES OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CCSc3ccc(F)cc3)CC2)[C@H](C1)c1cccc(F)c1 |r| Show InChI InChI=1S/C31H40F2N2O2S/c32-26-7-9-28(10-8-26)38-16-13-22-11-14-34(15-12-22)19-25-20-35(30(31(36)37)17-23-3-1-4-23)21-29(25)24-5-2-6-27(33)18-24/h2,5-10,18,22-23,25,29-30H,1,3-4,11-17,19-21H2,(H,36,37)/t25-,29+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane |
Bioorg Med Chem Lett 13: 119-23 (2002)
BindingDB Entry DOI: 10.7270/Q2CN7382 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50110088
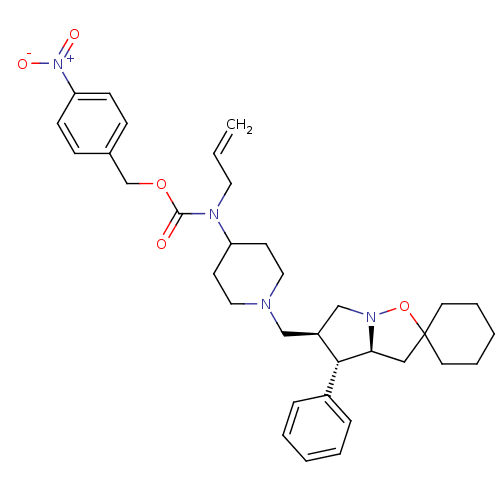
(4N-allyl-4N-[4-nitrobenzyloxycarboyl]-1-[4'-phenyl...)Show SMILES [O-][N+](=O)c1ccc(COC(=O)N(CC=C)C2CCN(C[C@H]3CN4OC5(C[C@H]4[C@@H]3c3ccccc3)CCCCC5)CC2)cc1 Show InChI InChI=1S/C34H44N4O5/c1-2-19-36(33(39)42-25-26-11-13-30(14-12-26)38(40)41)29-15-20-35(21-16-29)23-28-24-37-31(32(28)27-9-5-3-6-10-27)22-34(43-37)17-7-4-8-18-34/h2-3,5-6,9-14,28-29,31-32H,1,4,7-8,15-25H2/t28-,31-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranes |
Bioorg Med Chem Lett 12: 677-9 (2002)
BindingDB Entry DOI: 10.7270/Q2DF6QHH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119321
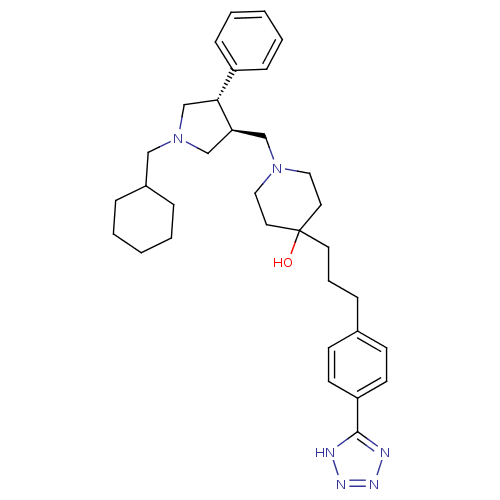
(1-(1-Cyclohexylmethyl-4-phenyl-pyrrolidin-3-ylmeth...)Show SMILES OC1(CCCc2ccc(cc2)-c2nnn[nH]2)CCN(C[C@H]2CN(CC3CCCCC3)C[C@@H]2c2ccccc2)CC1 |r| Show InChI InChI=1S/C33H46N6O/c40-33(17-7-10-26-13-15-29(16-14-26)32-34-36-37-35-32)18-20-38(21-19-33)23-30-24-39(22-27-8-3-1-4-9-27)25-31(30)28-11-5-2-6-12-28/h2,5-6,11-16,27,30-31,40H,1,3-4,7-10,17-25H2,(H,34,35,36,37)/t30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. |
Bioorg Med Chem Lett 12: 3001-4 (2002)
BindingDB Entry DOI: 10.7270/Q2MK6C71 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119336

(2-cyclohexyl-2-{3-phenyl-4-[4-(3-phenylpropyl)hexa...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CCCc3ccccc3)CC2)[C@H](C1)c1ccccc1 Show InChI InChI=1S/C33H46N2O2/c36-33(37)32(29-17-8-3-9-18-29)35-24-30(31(25-35)28-15-6-2-7-16-28)23-34-21-19-27(20-22-34)14-10-13-26-11-4-1-5-12-26/h1-2,4-7,11-12,15-16,27,29-32H,3,8-10,13-14,17-25H2,(H,36,37)/t30-,31+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CX3C chemokine receptor 5 from GP120-membrane-based assay |
Bioorg Med Chem Lett 14: 935-9 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.004
BindingDB Entry DOI: 10.7270/Q2PK0FKP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141904
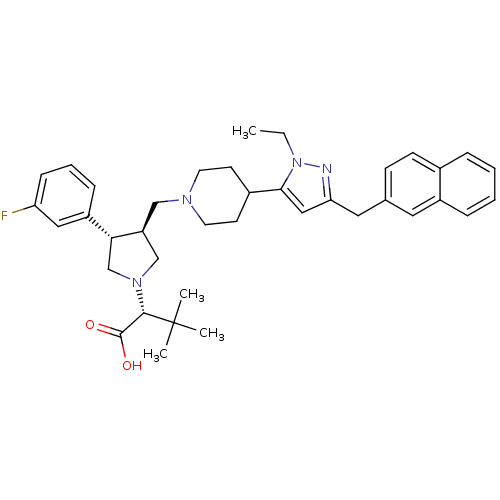
((R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-2-ylmeth...)Show SMILES CCn1nc(Cc2ccc3ccccc3c2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H47FN4O2/c1-5-43-35(22-33(40-43)20-26-13-14-27-9-6-7-10-29(27)19-26)28-15-17-41(18-16-28)23-31-24-42(36(37(44)45)38(2,3)4)25-34(31)30-11-8-12-32(39)21-30/h6-14,19,21-22,28,31,34,36H,5,15-18,20,23-25H2,1-4H3,(H,44,45)/t31-,34+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50105521

(Allyl-[1-((3S,4S)-1-cyclopentanecarbonyl-4-phenyl-...)Show SMILES [O-][N+](=O)c1ccc(COC(=O)N(CC=C)C2CCN(C[C@H]3CN(C[C@@H]3c3ccccc3)C(=O)C3CCCC3)CC2)cc1 Show InChI InChI=1S/C33H42N4O5/c1-2-18-36(33(39)42-24-25-12-14-30(15-13-25)37(40)41)29-16-19-34(20-17-29)21-28-22-35(32(38)27-10-6-7-11-27)23-31(28)26-8-4-3-5-9-26/h2-5,8-9,12-15,27-29,31H,1,6-7,10-11,16-24H2/t28-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells |
Bioorg Med Chem Lett 11: 2741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NFC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119342

((R)-2-cyclopentyl-2-((3S,4S)-3-phenyl-4-((4-(3-phe...)Show SMILES OC(=O)[C@@H](C1CCCC1)N1C[C@H](CN2CCC(CCCc3ccccc3)CC2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C32H44N2O2/c35-32(36)31(28-16-7-8-17-28)34-23-29(30(24-34)27-14-5-2-6-15-27)22-33-20-18-26(19-21-33)13-9-12-25-10-3-1-4-11-25/h1-6,10-11,14-15,26,28-31H,7-9,12-13,16-24H2,(H,35,36)/t29-,30+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. |
Bioorg Med Chem Lett 12: 3001-4 (2002)
BindingDB Entry DOI: 10.7270/Q2MK6C71 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141990

((R)-Cyclohexyl-[(2S,3S)-3-{4-[5-(4-cyclopropoxy-be...)Show SMILES CCn1nc(Cc2ccc(OC3CC3)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C2CCCCC2)C(O)=O)CC1 Show InChI InChI=1S/C39H51FN4O3/c1-2-44-37(23-33(41-44)21-27-11-13-34(14-12-27)47-35-15-16-35)28-17-19-42(20-18-28)24-31-25-43(26-36(31)30-9-6-10-32(40)22-30)38(39(45)46)29-7-4-3-5-8-29/h6,9-14,22-23,28-29,31,35-36,38H,2-5,7-8,15-21,24-26H2,1H3,(H,45,46)/t31-,36+,38+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50121828
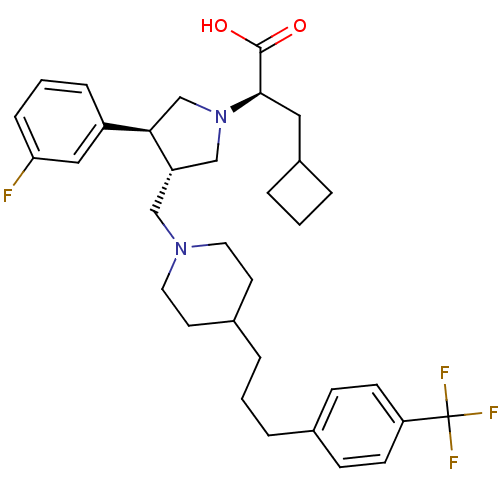
((R)-3-Cyclobutyl-2-((S)-3-(3-fluoro-phenyl)-4-{4-[...)Show SMILES OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CCCc3ccc(cc3)C(F)(F)F)CC2)[C@H](C1)c1cccc(F)c1 |r| Show InChI InChI=1S/C33H42F4N2O2/c34-29-9-3-8-26(19-29)30-22-39(31(32(40)41)18-25-6-2-7-25)21-27(30)20-38-16-14-24(15-17-38)5-1-4-23-10-12-28(13-11-23)33(35,36)37/h3,8-13,19,24-25,27,30-31H,1-2,4-7,14-18,20-22H2,(H,40,41)/t27-,30+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane |
Bioorg Med Chem Lett 13: 119-23 (2002)
BindingDB Entry DOI: 10.7270/Q2CN7382 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141937
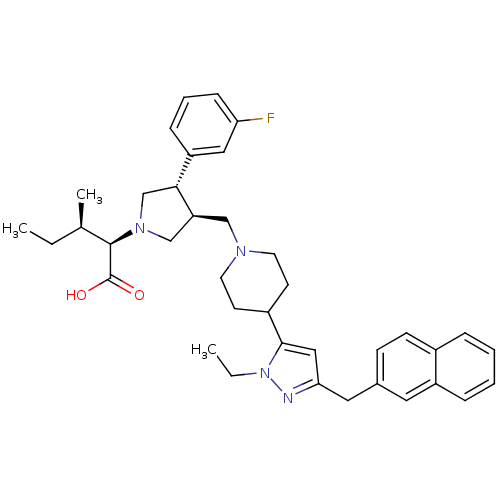
((2R,4R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-2-yl...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc4ccccc4c3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H47FN4O2/c1-4-26(3)37(38(44)45)42-24-32(35(25-42)31-11-8-12-33(39)21-31)23-41-17-15-29(16-18-41)36-22-34(40-43(36)5-2)20-27-13-14-28-9-6-7-10-30(28)19-27/h6-14,19,21-22,26,29,32,35,37H,4-5,15-18,20,23-25H2,1-3H3,(H,44,45)/t26-,32+,35-,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50121833
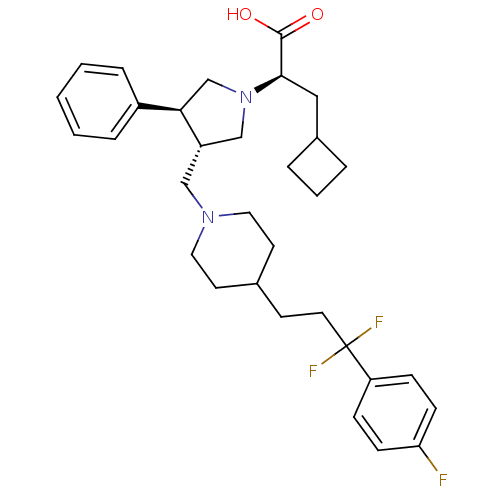
((R)-3-cyclobutyl-2-((3S,4S)-3-((4-(3,3-difluoro-3-...)Show SMILES OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CCC(F)(F)c3ccc(F)cc3)CC2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C32H41F3N2O2/c33-28-11-9-27(10-12-28)32(34,35)16-13-23-14-17-36(18-15-23)20-26-21-37(22-29(26)25-7-2-1-3-8-25)30(31(38)39)19-24-5-4-6-24/h1-3,7-12,23-24,26,29-30H,4-6,13-22H2,(H,38,39)/t26-,29+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane |
Bioorg Med Chem Lett 13: 119-23 (2002)
BindingDB Entry DOI: 10.7270/Q2CN7382 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119316
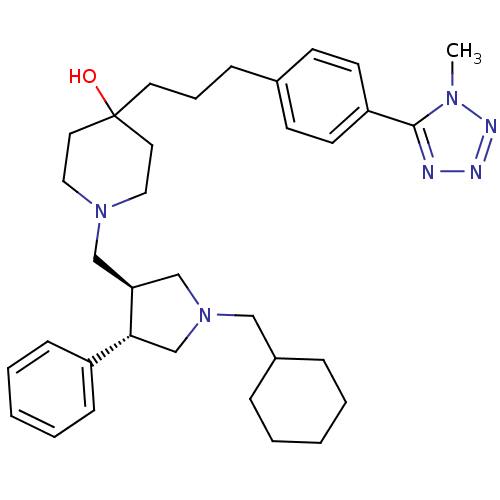
(1-(1-Cyclohexylmethyl-4-phenyl-pyrrolidin-3-ylmeth...)Show SMILES Cn1nnnc1-c1ccc(CCCC2(O)CCN(C[C@H]3CN(CC4CCCCC4)C[C@@H]3c3ccccc3)CC2)cc1 Show InChI InChI=1S/C34H48N6O/c1-38-33(35-36-37-38)30-16-14-27(15-17-30)11-8-18-34(41)19-21-39(22-20-34)24-31-25-40(23-28-9-4-2-5-10-28)26-32(31)29-12-6-3-7-13-29/h3,6-7,12-17,28,31-32,41H,2,4-5,8-11,18-26H2,1H3/t31-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranes |
Bioorg Med Chem Lett 12: 2997-3000 (2002)
BindingDB Entry DOI: 10.7270/Q2RB73ZB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141946

((2R,4R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-1-yl...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3cccc4ccccc34)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H47FN4O2/c1-4-26(3)37(38(44)45)42-24-31(35(25-42)29-13-9-14-32(39)20-29)23-41-18-16-28(17-19-41)36-22-33(40-43(36)5-2)21-30-12-8-11-27-10-6-7-15-34(27)30/h6-15,20,22,26,28,31,35,37H,4-5,16-19,21,23-25H2,1-3H3,(H,44,45)/t26-,31+,35-,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141948

((R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-1-ylmeth...)Show SMILES CCn1nc(Cc2cccc3ccccc23)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H47FN4O2/c1-5-43-35(22-32(40-43)21-29-12-8-11-26-10-6-7-15-33(26)29)27-16-18-41(19-17-27)23-30-24-42(36(37(44)45)38(2,3)4)25-34(30)28-13-9-14-31(39)20-28/h6-15,20,22,27,30,34,36H,5,16-19,21,23-25H2,1-4H3,(H,44,45)/t30-,34+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50119321

(1-(1-Cyclohexylmethyl-4-phenyl-pyrrolidin-3-ylmeth...)Show SMILES OC1(CCCc2ccc(cc2)-c2nnn[nH]2)CCN(C[C@H]2CN(CC3CCCCC3)C[C@@H]2c2ccccc2)CC1 |r| Show InChI InChI=1S/C33H46N6O/c40-33(17-7-10-26-13-15-29(16-14-26)32-34-36-37-35-32)18-20-38(21-19-33)23-30-24-39(22-27-8-3-1-4-9-27)25-31(30)28-11-5-2-6-12-28/h2,5-6,11-16,27,30-31,40H,1,3-4,7-10,17-25H2,(H,34,35,36,37)/t30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranes |
Bioorg Med Chem Lett 12: 2997-3000 (2002)
BindingDB Entry DOI: 10.7270/Q2RB73ZB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50105503
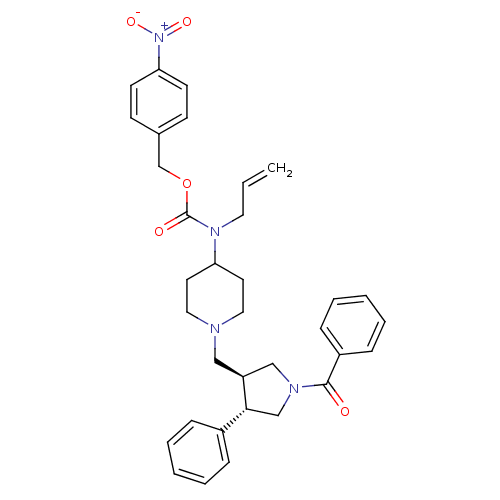
(Allyl-[1-((3S,4S)-1-benzoyl-4-phenyl-pyrrolidin-3-...)Show SMILES [O-][N+](=O)c1ccc(COC(=O)N(CC=C)C2CCN(C[C@H]3CN(C[C@@H]3c3ccccc3)C(=O)c3ccccc3)CC2)cc1 Show InChI InChI=1S/C34H38N4O5/c1-2-19-37(34(40)43-25-26-13-15-31(16-14-26)38(41)42)30-17-20-35(21-18-30)22-29-23-36(33(39)28-11-7-4-8-12-28)24-32(29)27-9-5-3-6-10-27/h2-16,29-30,32H,1,17-25H2/t29-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells |
Bioorg Med Chem Lett 11: 2741-5 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NFC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50148285

(2-{(S)-3-[4-(2-Benzyl-thiazol-5-yl)-piperidin-1-yl...)Show SMILES OC(=O)C(CC1CCC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)s2)C(C1)c1ccccc1 Show InChI InChI=1S/C33H41N3O2S/c37-33(38)30(18-24-10-7-11-24)36-22-28(29(23-36)26-12-5-2-6-13-26)21-35-16-14-27(15-17-35)31-20-34-32(39-31)19-25-8-3-1-4-9-25/h1-6,8-9,12-13,20,24,27-30H,7,10-11,14-19,21-23H2,(H,37,38)/t28-,29?,30?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50148286
(2-{(S)-3-[4-(2-Benzyl-thiazol-5-yl)-piperidin-1-yl...)Show SMILES OC(=O)C(CC1CC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)s2)C(C1)c1ccccc1 Show InChI InChI=1S/C32H39N3O2S/c36-32(37)29(17-24-11-12-24)35-21-27(28(22-35)25-9-5-2-6-10-25)20-34-15-13-26(14-16-34)30-19-33-31(38-30)18-23-7-3-1-4-8-23/h1-10,19,24,26-29H,11-18,20-22H2,(H,36,37)/t27-,28?,29?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249465

((R)-2-((3S,4S)-3-((4-(2-benzylthiazol-5-yl)piperid...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)s2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O2S/c38-34(39)33(28-14-8-3-9-15-28)37-23-29(30(24-37)26-12-6-2-7-13-26)22-36-18-16-27(17-19-36)31-21-35-32(40-31)20-25-10-4-1-5-11-25/h1-2,4-7,10-13,21,27-30,33H,3,8-9,14-20,22-24H2,(H,38,39)/t29-,30+,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data