 Found 475 hits with Last Name = 'graneto' and Initial = 'mj'
Found 475 hits with Last Name = 'graneto' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057581
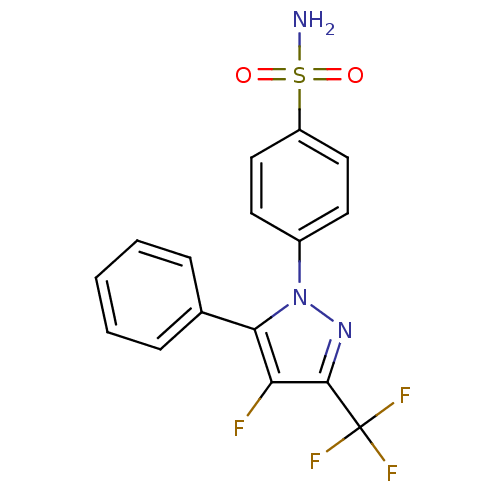
(4-(4-Fluoro-5-phenyl-3-trifluoromethyl-pyrazol-1-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(c(F)c1-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C16H11F4N3O2S/c17-13-14(10-4-2-1-3-5-10)23(22-15(13)16(18,19)20)11-6-8-12(9-7-11)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50332752

((2R)-6,8-dichloro-7-(2-methylpropoxy)-2-(trifluoro...)Show SMILES CC(C)COc1c(Cl)cc2C=C([C@@H](Oc2c1Cl)C(F)(F)F)C(O)=O |r,c:10| Show InChI InChI=1S/C15H13Cl2F3O4/c1-6(2)5-23-12-9(16)4-7-3-8(14(21)22)13(15(18,19)20)24-11(7)10(12)17/h3-4,6,13H,5H2,1-2H3,(H,21,22)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX1 |
Bioorg Med Chem Lett 20: 7159-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.054
BindingDB Entry DOI: 10.7270/Q23X86W1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50331873

(5,6-dichloro-2-(trifluoromethyl)-2H-chromene-3-car...)Show SMILES OC(=O)C1=Cc2c(OC1C(F)(F)F)ccc(Cl)c2Cl |t:3| Show InChI InChI=1S/C11H5Cl2F3O3/c12-6-1-2-7-4(8(6)13)3-5(10(17)18)9(19-7)11(14,15)16/h1-3,9H,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX-2 |
Bioorg Med Chem Lett 20: 7155-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.053
BindingDB Entry DOI: 10.7270/Q2513ZF2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049024

(4-[6-(4-Fluoro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C19H18FNO2S/c20-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)24(21,22)23/h1-8H,9-12H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle-Monsanto
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity against human Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 9: 1171-4 (1999)
BindingDB Entry DOI: 10.7270/Q2D21WTX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057618
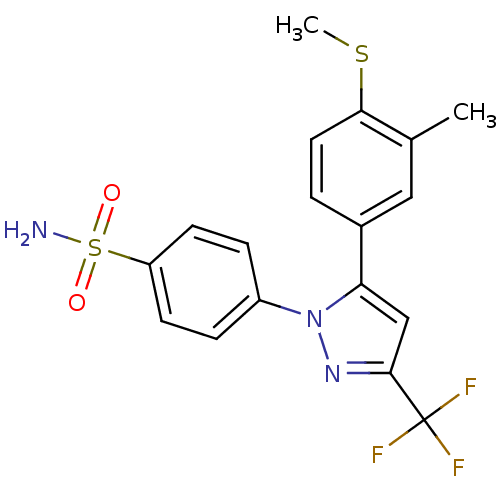
(4-[5-(3-Methyl-4-methylsulfanyl-phenyl)-3-trifluor...)Show SMILES CSc1ccc(cc1C)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H16F3N3O2S2/c1-11-9-12(3-8-16(11)27-2)15-10-17(18(19,20)21)23-24(15)13-4-6-14(7-5-13)28(22,25)26/h3-10H,1-2H3,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057551
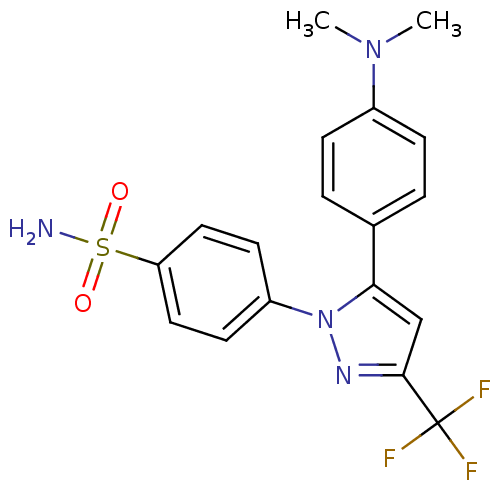
(4-[5-(4-Dimethylamino-phenyl)-3-trifluoromethyl-py...)Show SMILES CN(C)c1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H17F3N4O2S/c1-24(2)13-5-3-12(4-6-13)16-11-17(18(19,20)21)23-25(16)14-7-9-15(10-8-14)28(22,26)27/h3-11H,1-2H3,(H2,22,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In Vitro activity of compound against human recombinant Prostaglandin G/H synthase 2 |
J Med Chem 43: 775-7 (2000)
BindingDB Entry DOI: 10.7270/Q2NV9HG1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50332761

(7-tert-butyl-6-chloro-2-(trifluoromethyl)-2H-chrom...)Show SMILES CC(C)(C)c1cc2OC(C(=Cc2cc1Cl)C(O)=O)C(F)(F)F |c:9| Show InChI InChI=1S/C15H14ClF3O3/c1-14(2,3)9-6-11-7(5-10(9)16)4-8(13(20)21)12(22-11)15(17,18)19/h4-6,12H,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 |
Bioorg Med Chem Lett 20: 7159-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.054
BindingDB Entry DOI: 10.7270/Q23X86W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50057554
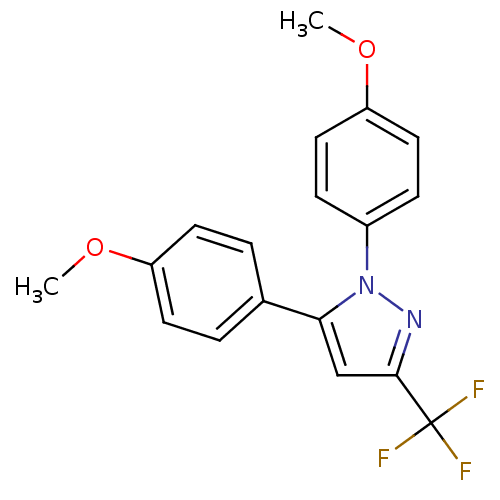
(1,5-Bis-(4-methoxy-phenyl)-3-trifluoromethyl-1H-py...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(OC)cc1)C(F)(F)F Show InChI InChI=1S/C18H15F3N2O2/c1-24-14-7-3-12(4-8-14)16-11-17(18(19,20)21)22-23(16)13-5-9-15(25-2)10-6-13/h3-11H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block recombinant human prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against recombinant human Prostaglandin G/H synthase 2 |
J Med Chem 43: 1661-3 (2000)
BindingDB Entry DOI: 10.7270/Q2RB73TJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50076888
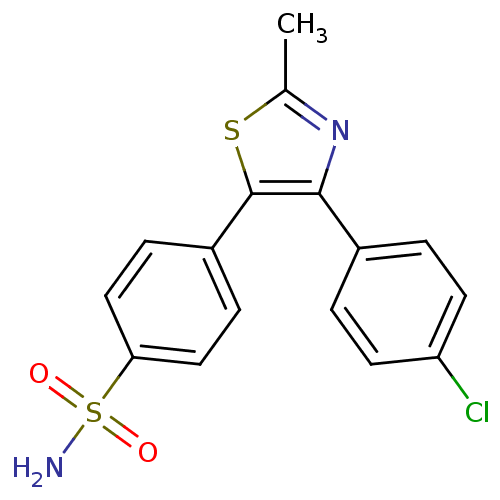
(4-[4-(4-Chloro-phenyl)-2-methyl-thiazol-5-yl]-benz...)Show SMILES Cc1nc(c(s1)-c1ccc(cc1)S(N)(=O)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C16H13ClN2O2S2/c1-10-19-15(11-2-6-13(17)7-3-11)16(22-10)12-4-8-14(9-5-12)23(18,20)21/h2-9H,1H3,(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle-Monsanto
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity against human Prostaglandin G/H synthase 2 (COX-2) |
Bioorg Med Chem Lett 9: 1171-4 (1999)
BindingDB Entry DOI: 10.7270/Q2D21WTX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50332769

((R)-6-chloro-7-propyl-2-(trifluoromethyl)-2H-chrom...)Show SMILES CCCc1cc2O[C@H](C(=Cc2cc1Cl)C(O)=O)C(F)(F)F |r,c:8| Show InChI InChI=1S/C14H12ClF3O3/c1-2-3-7-6-11-8(5-10(7)15)4-9(13(19)20)12(21-11)14(16,17)18/h4-6,12H,2-3H2,1H3,(H,19,20)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 |
Bioorg Med Chem Lett 20: 7159-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.054
BindingDB Entry DOI: 10.7270/Q23X86W1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In Vitro activity of compound against human recombinant Prostaglandin G/H synthase 2 |
J Med Chem 43: 775-7 (2000)
BindingDB Entry DOI: 10.7270/Q2NV9HG1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057606
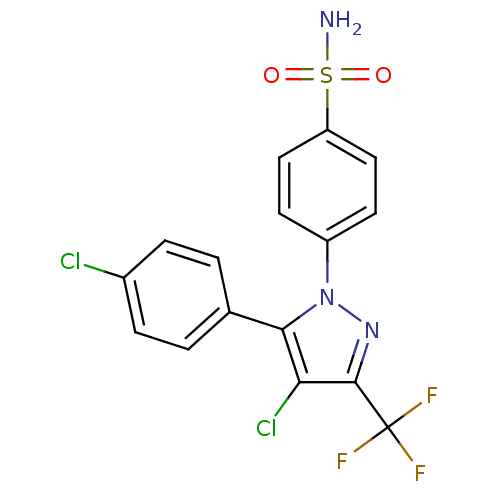
(4-[4-Chloro-5-(4-chloro-phenyl)-3-trifluoromethyl-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(c(Cl)c1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C16H10Cl2F3N3O2S/c17-10-3-1-9(2-4-10)14-13(18)15(16(19,20)21)23-24(14)11-5-7-12(8-6-11)27(22,25)26/h1-8H,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057575
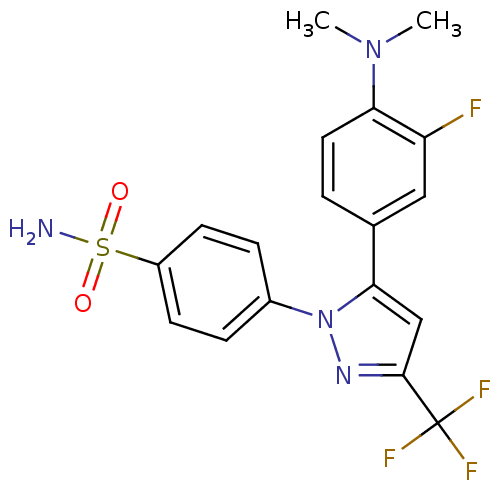
(4-[5-(4-Dimethylamino-3-fluoro-phenyl)-3-trifluoro...)Show SMILES CN(C)c1ccc(cc1F)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H16F4N4O2S/c1-25(2)15-8-3-11(9-14(15)19)16-10-17(18(20,21)22)24-26(16)12-4-6-13(7-5-12)29(23,27)28/h3-10H,1-2H3,(H2,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50026234
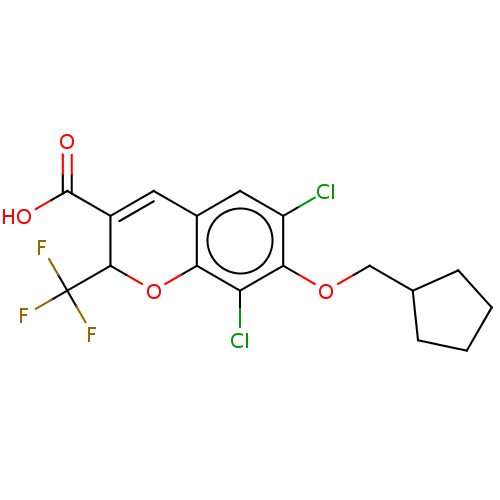
(CHEMBL1630239)Show SMILES OC(=O)C1=Cc2cc(Cl)c(OCC3CCCC3)c(Cl)c2OC1C(F)(F)F |t:3| Show InChI InChI=1S/C17H15Cl2F3O4/c18-11-6-9-5-10(16(23)24)15(17(20,21)22)26-13(9)12(19)14(11)25-7-8-3-1-2-4-8/h5-6,8,15H,1-4,7H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 |
Bioorg Med Chem Lett 20: 7159-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.054
BindingDB Entry DOI: 10.7270/Q23X86W1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50076878
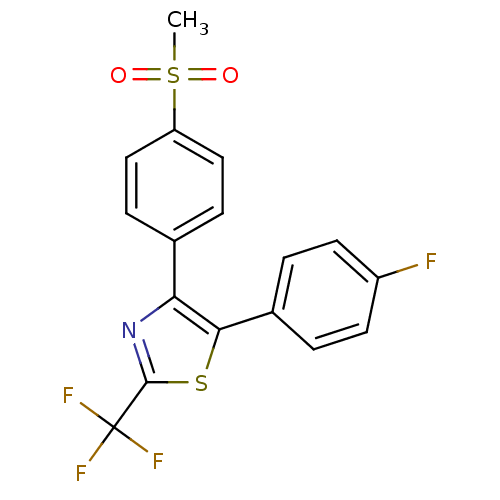
(5-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-2...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(sc1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C17H11F4NO2S2/c1-26(23,24)13-8-4-10(5-9-13)14-15(11-2-6-12(18)7-3-11)25-16(22-14)17(19,20)21/h2-9H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle-Monsanto
Curated by ChEMBL
| Assay Description
Inhibition of human cyclooxygenase-2 (hCOX-2) enzyme. |
Bioorg Med Chem Lett 9: 1167-70 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NHW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50331879

(5,6,7-trichloro-2-(trifluoromethyl)-2H-chromene-3-...)Show SMILES OC(=O)C1=Cc2c(OC1C(F)(F)F)cc(Cl)c(Cl)c2Cl |t:3| Show InChI InChI=1S/C11H4Cl3F3O3/c12-5-2-6-3(7(13)8(5)14)1-4(10(18)19)9(20-6)11(15,16)17/h1-2,9H,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX-2 |
Bioorg Med Chem Lett 20: 7155-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.053
BindingDB Entry DOI: 10.7270/Q2513ZF2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288331

(4-(7,8-Difluoro-3-trifluoromethyl-5H-isothiochrome...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(c2SCc3cc(F)c(F)cc3-c12)C(F)(F)F Show InChI InChI=1S/C17H10F5N3O2S2/c18-12-5-8-7-28-15-14(11(8)6-13(12)19)25(24-16(15)17(20,21)22)9-1-3-10(4-2-9)29(23,26)27/h1-6H,7H2,(H2,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 (COX-2) |
Bioorg Med Chem Lett 6: 2827-2830 (1996)
Article DOI: 10.1016/S0960-894X(96)00530-6
BindingDB Entry DOI: 10.7270/Q2GX4BJ3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057609
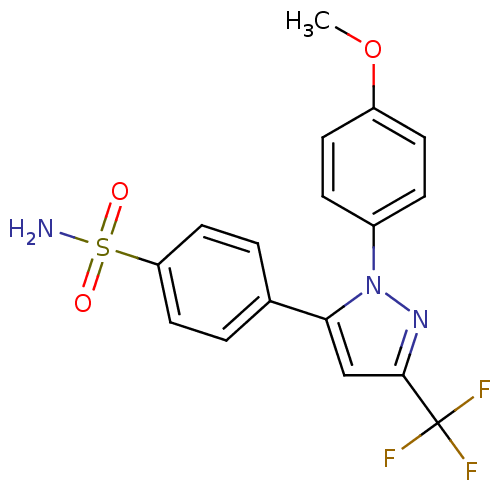
(4-[2-(4-Methoxy-phenyl)-5-trifluoromethyl-2H-pyraz...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O3S/c1-26-13-6-4-12(5-7-13)23-15(10-16(22-23)17(18,19)20)11-2-8-14(9-3-11)27(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50076863

(2-[3-(4-Bromo-phenyl)-propyl]-4-(4-fluoro-phenyl)-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1sc(CCCc2ccc(Br)cc2)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H21BrFNO2S2/c1-32(29,30)22-15-9-19(10-16-22)25-24(18-7-13-21(27)14-8-18)28-23(31-25)4-2-3-17-5-11-20(26)12-6-17/h5-16H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle-Monsanto
Curated by ChEMBL
| Assay Description
Inhibition of human cyclooxygenase-2 (hCOX-2) enzyme. |
Bioorg Med Chem Lett 9: 1167-70 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NHW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM13065

(5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C17H12ClF3N2O/c1-24-14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block recombinant human prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50332771

((R)-6-chloro-7-isobutyl-2-(trifluoromethyl)-2H-chr...)Show SMILES CC(C)Cc1cc2O[C@H](C(=Cc2cc1Cl)C(O)=O)C(F)(F)F |r,c:9| Show InChI InChI=1S/C15H14ClF3O3/c1-7(2)3-8-6-12-9(5-11(8)16)4-10(14(20)21)13(22-12)15(17,18)19/h4-7,13H,3H2,1-2H3,(H,20,21)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 |
Bioorg Med Chem Lett 20: 7159-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.054
BindingDB Entry DOI: 10.7270/Q23X86W1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50286050
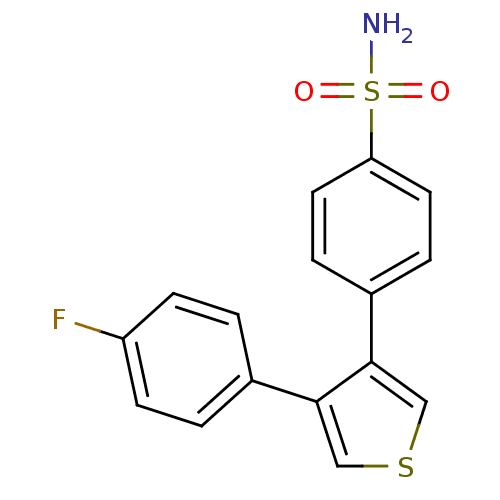
(4-[4-(4-Fluoro-phenyl)-thiophen-3-yl]-benzenesulfo...)Show InChI InChI=1S/C16H12FNO2S2/c17-13-5-1-11(2-6-13)15-9-21-10-16(15)12-3-7-14(8-4-12)22(18,19)20/h1-10H,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 5: 2919-2922 (1995)
Article DOI: 10.1016/0960-894X(95)00512-R
BindingDB Entry DOI: 10.7270/Q2HM58D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057589
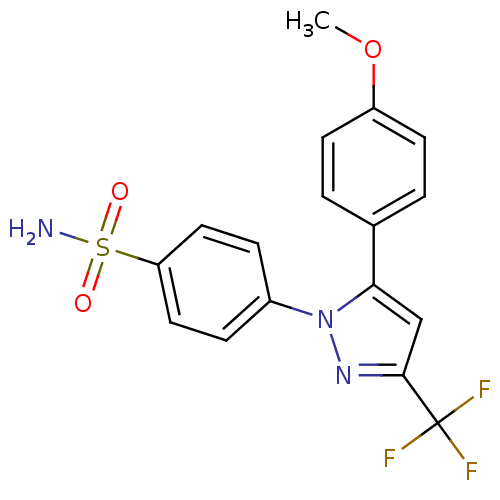
(4-[5-(4-Methoxy-phenyl)-3-trifluoromethyl-pyrazol-...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O3S/c1-26-13-6-2-11(3-7-13)15-10-16(17(18,19)20)22-23(15)12-4-8-14(9-5-12)27(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50332773
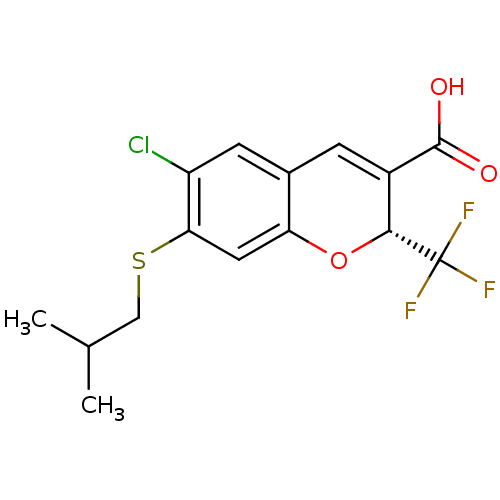
((R)-6-chloro-7-(isobutylthio)-2-(trifluoromethyl)-...)Show SMILES CC(C)CSc1cc2O[C@H](C(=Cc2cc1Cl)C(O)=O)C(F)(F)F |r,c:10| Show InChI InChI=1S/C15H14ClF3O3S/c1-7(2)6-23-12-5-11-8(4-10(12)16)3-9(14(20)21)13(22-11)15(17,18)19/h3-5,7,13H,6H2,1-2H3,(H,20,21)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 |
Bioorg Med Chem Lett 20: 7159-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.054
BindingDB Entry DOI: 10.7270/Q23X86W1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288322

(4-(8-Fluoro-7-methoxy-3-trifluoromethyl-5H-isothio...)Show SMILES COc1cc2CSc3c(nn(c3-c2cc1F)-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H13F4N3O3S2/c1-28-14-6-9-8-29-16-15(12(9)7-13(14)19)25(24-17(16)18(20,21)22)10-2-4-11(5-3-10)30(23,26)27/h2-7H,8H2,1H3,(H2,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 (COX-2) |
Bioorg Med Chem Lett 6: 2827-2830 (1996)
Article DOI: 10.1016/S0960-894X(96)00530-6
BindingDB Entry DOI: 10.7270/Q2GX4BJ3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50286047

(4-[4-(3-Fluoro-4-methoxy-phenyl)-thiophen-3-yl]-be...)Show InChI InChI=1S/C17H14FNO3S2/c1-22-17-7-4-12(8-16(17)18)15-10-23-9-14(15)11-2-5-13(6-3-11)24(19,20)21/h2-10H,1H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 5: 2919-2922 (1995)
Article DOI: 10.1016/0960-894X(95)00512-R
BindingDB Entry DOI: 10.7270/Q2HM58D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057591
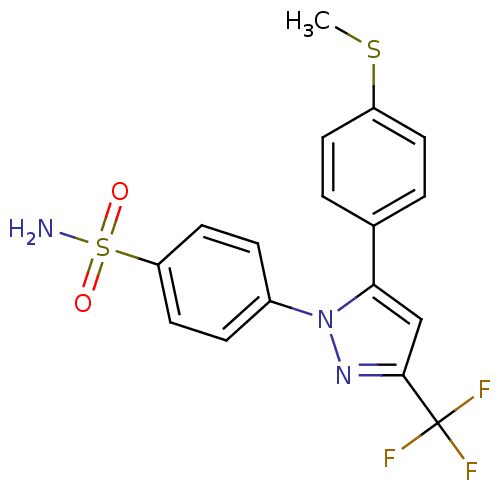
(4-[5-(4-Methylsulfanyl-phenyl)-3-trifluoromethyl-p...)Show SMILES CSc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S2/c1-26-13-6-2-11(3-7-13)15-10-16(17(18,19)20)22-23(15)12-4-8-14(9-5-12)27(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50076880
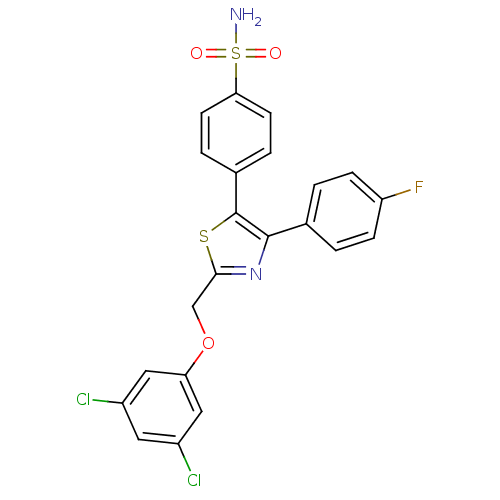
(4-[2-(3,5-Dichloro-phenoxymethyl)-4-(4-fluoro-phen...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1sc(COc2cc(Cl)cc(Cl)c2)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C22H15Cl2FN2O3S2/c23-15-9-16(24)11-18(10-15)30-12-20-27-21(13-1-5-17(25)6-2-13)22(31-20)14-3-7-19(8-4-14)32(26,28)29/h1-11H,12H2,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle-Monsanto
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity against human Prostaglandin G/H synthase 2 (COX-2) |
Bioorg Med Chem Lett 9: 1171-4 (1999)
BindingDB Entry DOI: 10.7270/Q2D21WTX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057620
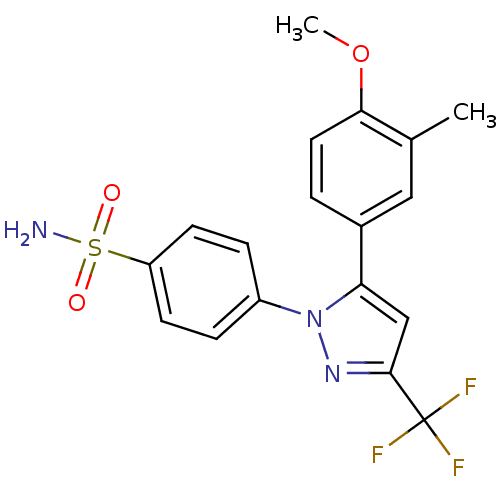
(4-[5-(4-Methoxy-3-methyl-phenyl)-3-trifluoromethyl...)Show SMILES COc1ccc(cc1C)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H16F3N3O3S/c1-11-9-12(3-8-16(11)27-2)15-10-17(18(19,20)21)23-24(15)13-4-6-14(7-5-13)28(22,25)26/h3-10H,1-2H3,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50331887

((2S)-6,8-dichloro-2-(trifluoromethyl)-2H-chromene-...)Show SMILES OC(=O)C1=Cc2cc(Cl)cc(Cl)c2O[C@@H]1C(F)(F)F |r,t:3| Show InChI InChI=1S/C11H5Cl2F3O3/c12-5-1-4-2-6(10(17)18)9(11(14,15)16)19-8(4)7(13)3-5/h1-3,9H,(H,17,18)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX-2 |
Bioorg Med Chem Lett 20: 7155-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.053
BindingDB Entry DOI: 10.7270/Q2513ZF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50331887

((2S)-6,8-dichloro-2-(trifluoromethyl)-2H-chromene-...)Show SMILES OC(=O)C1=Cc2cc(Cl)cc(Cl)c2O[C@@H]1C(F)(F)F |r,t:3| Show InChI InChI=1S/C11H5Cl2F3O3/c12-5-1-4-2-6(10(17)18)9(11(14,15)16)19-8(4)7(13)3-5/h1-3,9H,(H,17,18)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 |
Bioorg Med Chem Lett 20: 7159-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.054
BindingDB Entry DOI: 10.7270/Q23X86W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50331873

(5,6-dichloro-2-(trifluoromethyl)-2H-chromene-3-car...)Show SMILES OC(=O)C1=Cc2c(OC1C(F)(F)F)ccc(Cl)c2Cl |t:3| Show InChI InChI=1S/C11H5Cl2F3O3/c12-6-1-2-7-4(8(6)13)3-5(10(17)18)9(19-7)11(14,15)16/h1-3,9H,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 |
Bioorg Med Chem Lett 20: 7159-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.054
BindingDB Entry DOI: 10.7270/Q23X86W1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057610

(4-[5-(4-Chloro-phenyl)-3-difluoromethyl-pyrazol-1-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)F Show InChI InChI=1S/C16H12ClF2N3O2S/c17-11-3-1-10(2-4-11)15-9-14(16(18)19)21-22(15)12-5-7-13(8-6-12)25(20,23)24/h1-9,16H,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057602
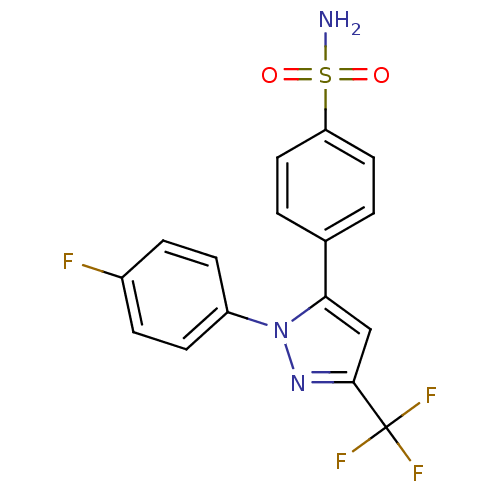
(4-[2-(4-Fluoro-phenyl)-5-trifluoromethyl-2H-pyrazo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(nn1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C16H11F4N3O2S/c17-11-3-5-12(6-4-11)23-14(9-15(22-23)16(18,19)20)10-1-7-13(8-2-10)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50076866
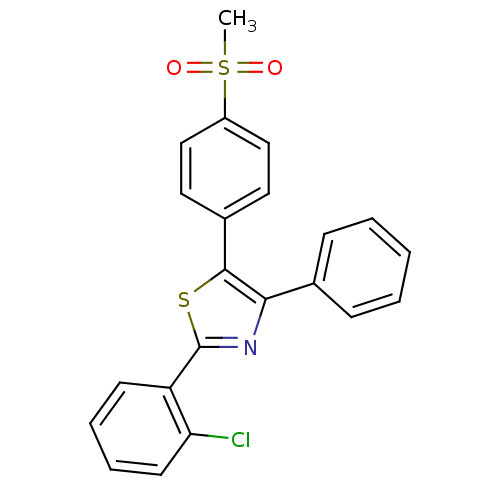
(2-(2-Chloro-phenyl)-5-(4-methanesulfonyl-phenyl)-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1sc(nc1-c1ccccc1)-c1ccccc1Cl Show InChI InChI=1S/C22H16ClNO2S2/c1-28(25,26)17-13-11-16(12-14-17)21-20(15-7-3-2-4-8-15)24-22(27-21)18-9-5-6-10-19(18)23/h2-14H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle-Monsanto
Curated by ChEMBL
| Assay Description
Inhibition of human cyclooxygenase-2 (hCOX-2) enzyme. |
Bioorg Med Chem Lett 9: 1167-70 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NHW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057527
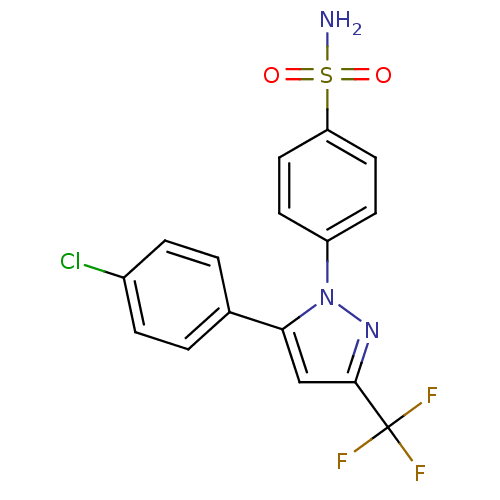
(4-(5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C16H11ClF3N3O2S/c17-11-3-1-10(2-4-11)14-9-15(16(18,19)20)22-23(14)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057572
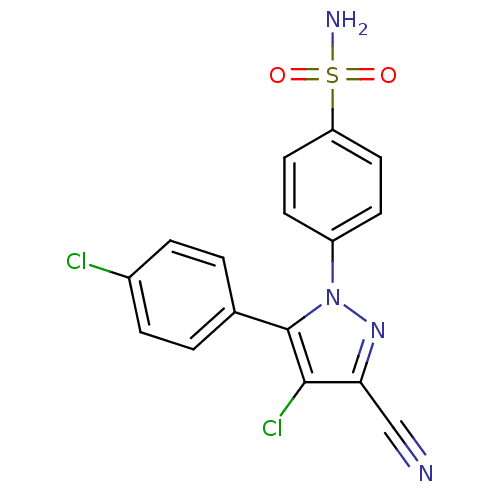
(4-[4-Chloro-5-(4-chloro-phenyl)-3-cyano-pyrazol-1-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(C#N)c(Cl)c1-c1ccc(Cl)cc1 Show InChI InChI=1S/C16H10Cl2N4O2S/c17-11-3-1-10(2-4-11)16-15(18)14(9-19)21-22(16)12-5-7-13(8-6-12)25(20,23)24/h1-8H,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50076868

(2-(2-Chloro-phenyl)-4-(2-fluoro-phenyl)-5-(4-metha...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1sc(nc1-c1ccccc1F)-c1ccccc1Cl Show InChI InChI=1S/C22H15ClFNO2S2/c1-29(26,27)15-12-10-14(11-13-15)21-20(17-7-3-5-9-19(17)24)25-22(28-21)16-6-2-4-8-18(16)23/h2-13H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle-Monsanto
Curated by ChEMBL
| Assay Description
Inhibition of human cyclooxygenase-2 (hCOX-2) enzyme. |
Bioorg Med Chem Lett 9: 1167-70 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NHW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50076883

(4-[2-Benzylamino-4-(3-fluoro-4-methoxy-phenyl)-thi...)Show SMILES COc1ccc(cc1F)-c1nc(NCc2ccccc2)sc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C23H20FN3O3S2/c1-30-20-12-9-17(13-19(20)24)21-22(16-7-10-18(11-8-16)32(25,28)29)31-23(27-21)26-14-15-5-3-2-4-6-15/h2-13H,14H2,1H3,(H,26,27)(H2,25,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle-Monsanto
Curated by ChEMBL
| Assay Description
Inhibition of human Prostaglandin G/H synthase 1 |
Bioorg Med Chem Lett 9: 1171-4 (1999)
BindingDB Entry DOI: 10.7270/Q2D21WTX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057552

(4-[5-(5-Bromo-thiophen-2-yl)-3-trifluoromethyl-pyr...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Br)s1)C(F)(F)F Show InChI InChI=1S/C14H9BrF3N3O2S2/c15-13-6-5-11(24-13)10-7-12(14(16,17)18)20-21(10)8-1-3-9(4-2-8)25(19,22)23/h1-7H,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM19440
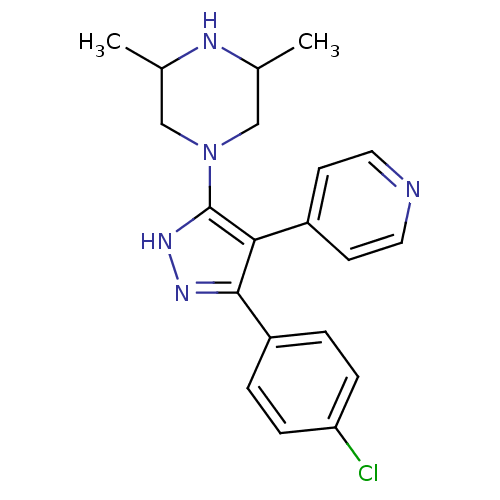
(1-[3-(4-chlorophenyl)-4-(pyridin-4-yl)-1H-pyrazol-...)Show SMILES CC1CN(CC(C)N1)c1[nH]nc(c1-c1ccncc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H22ClN5/c1-13-11-26(12-14(2)23-13)20-18(15-7-9-22-10-8-15)19(24-25-20)16-3-5-17(21)6-4-16/h3-10,13-14,23H,11-12H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
Kinase activity was assayed in reaction buffer containing substrate, enzyme, and inhibitor in the presence of 50 uM ATP/[gamma-33P] ATP. 33P incorpor... |
J Med Chem 50: 5712-9 (2007)
Article DOI: 10.1021/jm0611915
BindingDB Entry DOI: 10.7270/Q20G3HD5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057608
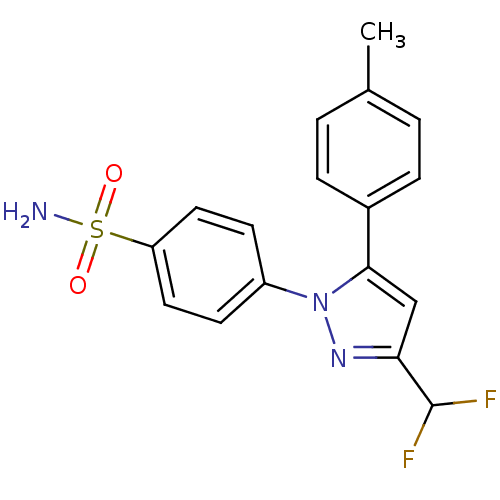
(4-(3-Difluoromethyl-5-p-tolyl-pyrazol-1-yl)-benzen...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)F Show InChI InChI=1S/C17H15F2N3O2S/c1-11-2-4-12(5-3-11)16-10-15(17(18)19)21-22(16)13-6-8-14(9-7-13)25(20,23)24/h2-10,17H,1H3,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50076884
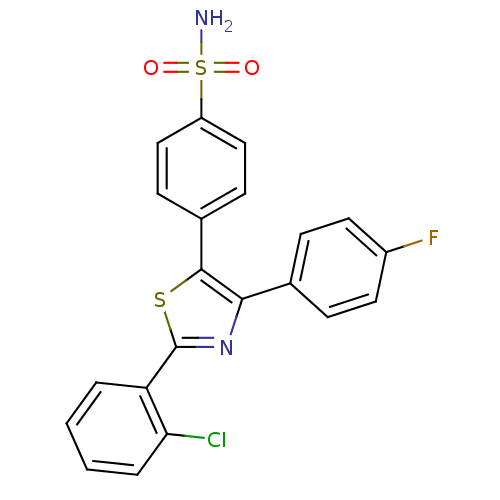
(4-[2-(2-Chloro-phenyl)-4-(4-fluoro-phenyl)-thiazol...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1sc(nc1-c1ccc(F)cc1)-c1ccccc1Cl Show InChI InChI=1S/C21H14ClFN2O2S2/c22-18-4-2-1-3-17(18)21-25-19(13-5-9-15(23)10-6-13)20(28-21)14-7-11-16(12-8-14)29(24,26)27/h1-12H,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle-Monsanto
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity against human Prostaglandin G/H synthase 2 (COX-2) |
Bioorg Med Chem Lett 9: 1171-4 (1999)
BindingDB Entry DOI: 10.7270/Q2D21WTX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288325
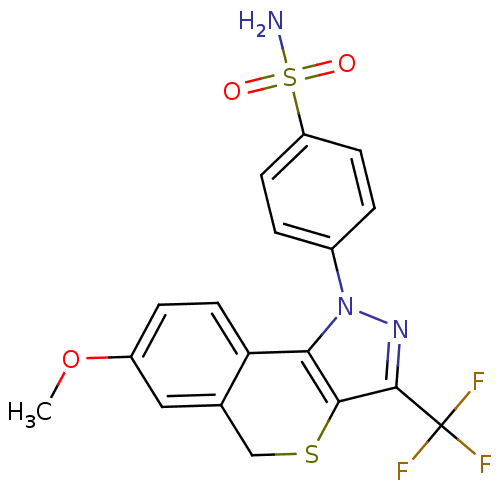
(4-(7-Methoxy-3-trifluoromethyl-5H-isothiochromeno[...)Show SMILES COc1ccc2-c3c(SCc2c1)c(nn3-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H14F3N3O3S2/c1-27-12-4-7-14-10(8-12)9-28-16-15(14)24(23-17(16)18(19,20)21)11-2-5-13(6-3-11)29(22,25)26/h2-8H,9H2,1H3,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 (COX-2) |
Bioorg Med Chem Lett 6: 2827-2830 (1996)
Article DOI: 10.1016/S0960-894X(96)00530-6
BindingDB Entry DOI: 10.7270/Q2GX4BJ3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029600
(5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(Br)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-16(18)22-17(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 5: 2919-2922 (1995)
Article DOI: 10.1016/0960-894X(95)00512-R
BindingDB Entry DOI: 10.7270/Q2HM58D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50286048
(2-Bromo-3-(4-fluoro-phenyl)-4-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1csc(Br)c1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-22-17(18)16(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 5: 2919-2922 (1995)
Article DOI: 10.1016/0960-894X(95)00512-R
BindingDB Entry DOI: 10.7270/Q2HM58D2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057560
(4-[5-(3,4-Dichloro-phenyl)-3-trifluoromethyl-pyraz...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)c(Cl)c1)C(F)(F)F Show InChI InChI=1S/C16H10Cl2F3N3O2S/c17-12-6-1-9(7-13(12)18)14-8-15(16(19,20)21)23-24(14)10-2-4-11(5-3-10)27(22,25)26/h1-8H,(H2,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057562

(4-[3-Difluoromethyl-5-(4-methoxy-phenyl)-pyrazol-1...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)F Show InChI InChI=1S/C17H15F2N3O3S/c1-25-13-6-2-11(3-7-13)16-10-15(17(18)19)21-22(16)12-4-8-14(9-5-12)26(20,23)24/h2-10,17H,1H3,(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required to block human recombinant prostaglandin G/H synthase 2 (COX-2) |
J Med Chem 40: 1347-65 (1997)
Article DOI: 10.1021/jm960803q
BindingDB Entry DOI: 10.7270/Q2Z89BHB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data