 Found 298 hits with Last Name = 'green' and Initial = 'i'
Found 298 hits with Last Name = 'green' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50234418
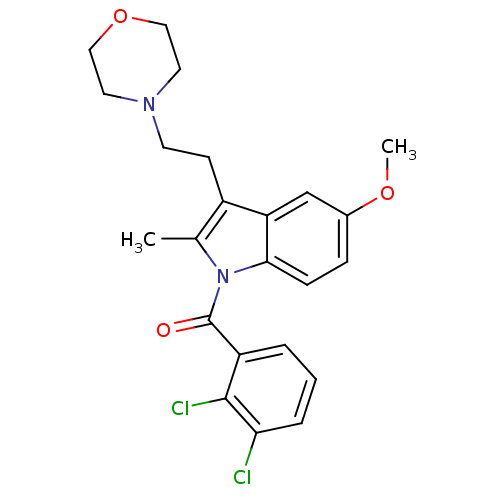
((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...)Show SMILES COc1ccc2n(C(=O)c3cccc(Cl)c3Cl)c(C)c(CCN3CCOCC3)c2c1 Show InChI InChI=1S/C23H24Cl2N2O3/c1-15-17(8-9-26-10-12-30-13-11-26)19-14-16(29-2)6-7-21(19)27(15)23(28)18-4-3-5-20(24)22(18)25/h3-7,14H,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50234418

((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...)Show SMILES COc1ccc2n(C(=O)c3cccc(Cl)c3Cl)c(C)c(CCN3CCOCC3)c2c1 Show InChI InChI=1S/C23H24Cl2N2O3/c1-15-17(8-9-26-10-12-30-13-11-26)19-14-16(29-2)6-7-21(19)27(15)23(28)18-4-3-5-20(24)22(18)25/h3-7,14H,8-13H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081221

(4-{5-[(S)-2-Butyl-4-(3-chloro-phenyl)-5-oxo-pipera...)Show SMILES CCCC[C@H]1CN(C(=O)CN1Cc1cncn1Cc1ccc(cc1)C#N)c1cccc(Cl)c1 Show InChI InChI=1S/C26H28ClN5O/c1-2-3-6-24-17-32(23-7-4-5-22(27)12-23)26(33)18-30(24)16-25-14-29-19-31(25)15-21-10-8-20(13-28)9-11-21/h4-5,7-12,14,19,24H,2-3,6,15-18H2,1H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081212
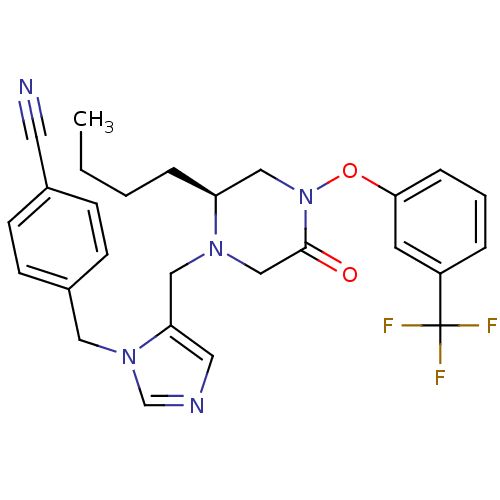
(4-{5-[(S)-2-Butyl-5-oxo-4-(3-trifluoromethyl-pheno...)Show SMILES CCCC[C@H]1CN(Oc2cccc(c2)C(F)(F)F)C(=O)CN1Cc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C27H28F3N5O2/c1-2-3-6-23-17-35(37-25-7-4-5-22(12-25)27(28,29)30)26(36)18-33(23)16-24-14-32-19-34(24)15-21-10-8-20(13-31)9-11-21/h4-5,7-12,14,19,23H,2-3,6,15-18H2,1H3/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081215
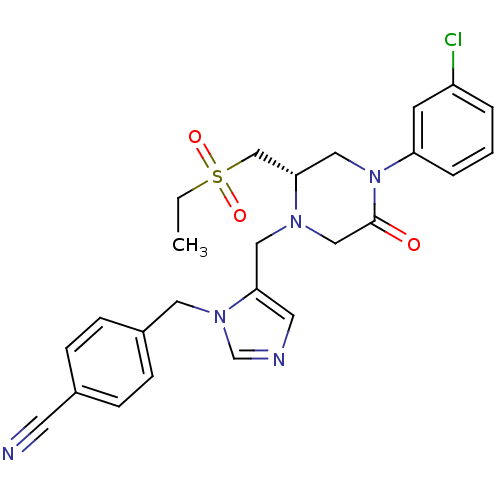
(4-{5-[(S)-4-(3-Chloro-phenyl)-2-ethanesulfonylmeth...)Show SMILES CCS(=O)(=O)C[C@@H]1CN(C(=O)CN1Cc1cncn1Cc1ccc(cc1)C#N)c1cccc(Cl)c1 Show InChI InChI=1S/C25H26ClN5O3S/c1-2-35(33,34)17-24-15-31(22-5-3-4-21(26)10-22)25(32)16-29(24)14-23-12-28-18-30(23)13-20-8-6-19(11-27)7-9-20/h3-10,12,18,24H,2,13-17H2,1H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340312
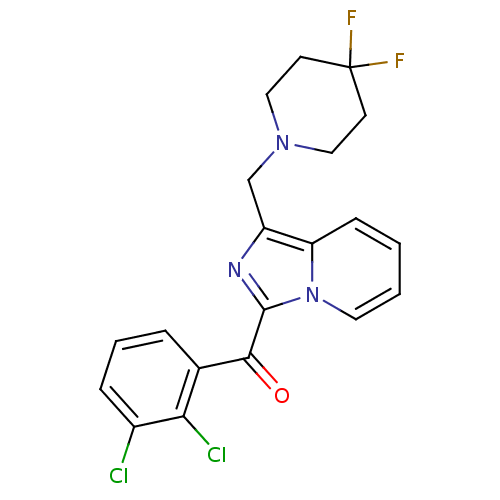
((2,3-dichlorophenyl)(1-((4,4-difluoropiperidin-1-y...)Show SMILES FC1(F)CCN(Cc2nc(C(=O)c3cccc(Cl)c3Cl)n3ccccc23)CC1 Show InChI InChI=1S/C20H17Cl2F2N3O/c21-14-5-3-4-13(17(14)22)18(28)19-25-15(16-6-1-2-9-27(16)19)12-26-10-7-20(23,24)8-11-26/h1-6,9H,7-8,10-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081217
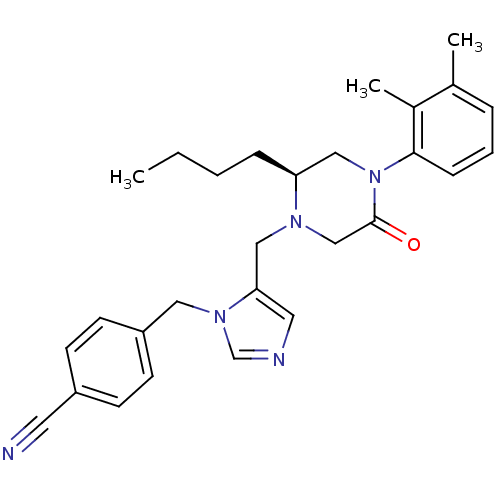
(4-{5-[(S)-2-Butyl-4-(2,3-dimethyl-phenyl)-5-oxo-pi...)Show SMILES CCCC[C@H]1CN(C(=O)CN1Cc1cncn1Cc1ccc(cc1)C#N)c1cccc(C)c1C Show InChI InChI=1S/C28H33N5O/c1-4-5-8-25-18-33(27-9-6-7-21(2)22(27)3)28(34)19-31(25)17-26-15-30-20-32(26)16-24-12-10-23(14-29)11-13-24/h6-7,9-13,15,20,25H,4-5,8,16-19H2,1-3H3/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340311
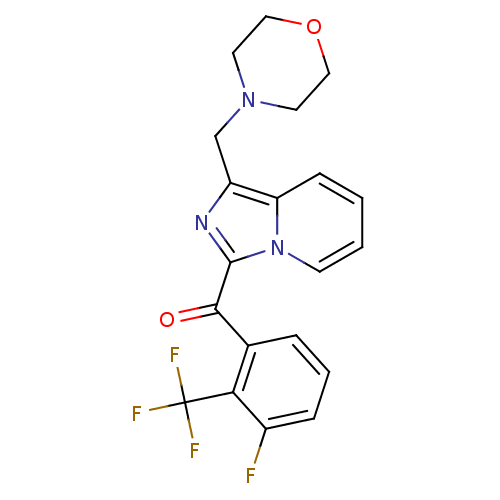
((3-fluoro-2-(trifluoromethyl)phenyl)(1-(morpholino...)Show SMILES Fc1cccc(C(=O)c2nc(CN3CCOCC3)c3ccccn23)c1C(F)(F)F Show InChI InChI=1S/C20H17F4N3O2/c21-14-5-3-4-13(17(14)20(22,23)24)18(28)19-25-15(12-26-8-10-29-11-9-26)16-6-1-2-7-27(16)19/h1-7H,8-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329079
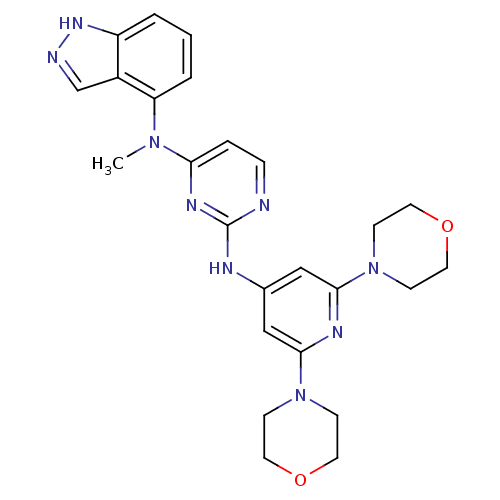
(CHEMBL1270280 | N2-(2,6-dimorpholinopyridin-4-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C25H29N9O2/c1-32(21-4-2-3-20-19(21)17-27-31-20)22-5-6-26-25(30-22)28-18-15-23(33-7-11-35-12-8-33)29-24(16-18)34-9-13-36-14-10-34/h2-6,15-17H,7-14H2,1H3,(H,27,31)(H,26,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081214

(CHEMBL340493 | [(S)-4-((S)-2-Amino-2-mercapto-ethy...)Show SMILES CCCC[C@H]1CN(CCN1C[C@@H](N)S)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C21H29N3OS/c1-2-3-9-17-14-24(13-12-23(17)15-20(22)26)21(25)19-11-6-8-16-7-4-5-10-18(16)19/h4-8,10-11,17,20,26H,2-3,9,12-15,22H2,1H3/t17-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329070
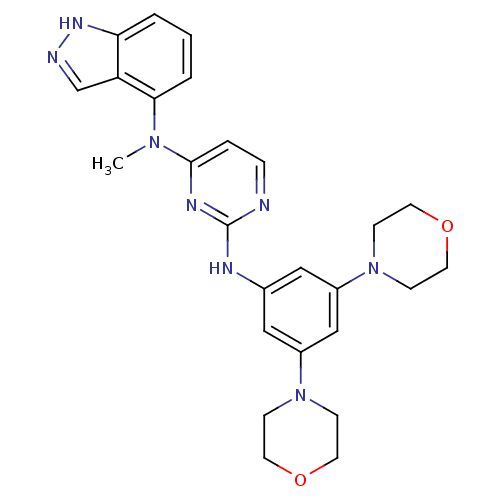
(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340313

(1-((4,4-difluoropiperidin-1-yl)methyl)-N-(6-(trifl...)Show SMILES FC(F)(F)c1cccc(NC(=O)c2nc(CN3CCC(F)(F)CC3)c3ccccn23)n1 Show InChI InChI=1S/C20H18F5N5O/c21-19(22)7-10-29(11-8-19)12-13-14-4-1-2-9-30(14)17(26-13)18(31)28-16-6-3-5-15(27-16)20(23,24)25/h1-6,9H,7-8,10-12H2,(H,27,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329080

(CHEMBL1270378 | N2-(2,6-dimorpholinopyrimidin-4-yl...)Show SMILES CN(c1ccnc(Nc2cc(nc(n2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C24H28N10O2/c1-32(19-4-2-3-18-17(19)16-26-31-18)21-5-6-25-23(29-21)27-20-15-22(33-7-11-35-12-8-33)30-24(28-20)34-9-13-36-14-10-34/h2-6,15-16H,7-14H2,1H3,(H,26,31)(H,25,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50335201
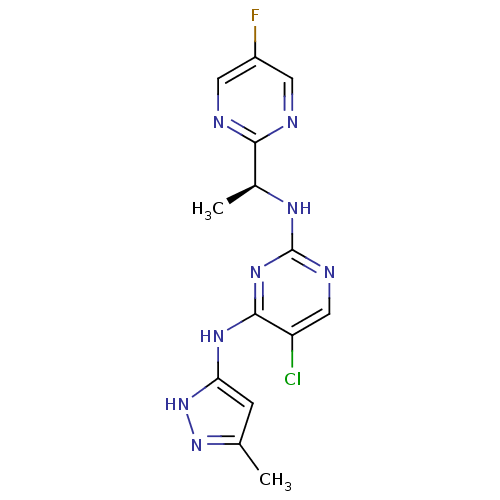
(5-Chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]...)Show SMILES C[C@H](Nc1ncc(Cl)c(Nc2cc(C)n[nH]2)n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C14H14ClFN8/c1-7-3-11(24-23-7)21-13-10(15)6-19-14(22-13)20-8(2)12-17-4-9(16)5-18-12/h3-6,8H,1-2H3,(H3,19,20,21,22,23,24)/t8-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of TrkA |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335202
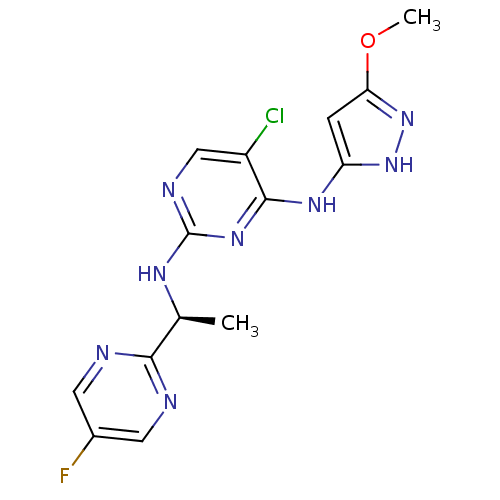
(5-Chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]...)Show SMILES COc1cc(Nc2nc(N[C@@H](C)c3ncc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C14H14ClFN8O/c1-7(12-17-4-8(16)5-18-12)20-14-19-6-9(15)13(22-14)21-10-3-11(25-2)24-23-10/h3-7H,1-2H3,(H3,19,20,21,22,23,24)/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using Km ATP concentration |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335203
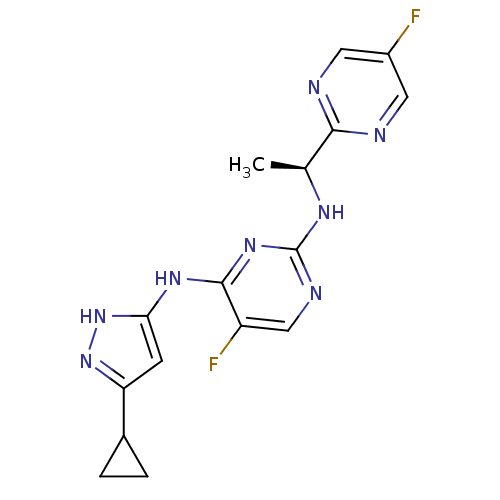
(CHEMBL1650698 | N4-(5-Cyclopropyl-1H-pyrazol-3-yl)...)Show SMILES C[C@H](Nc1ncc(F)c(Nc2cc(n[nH]2)C2CC2)n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C16H16F2N8/c1-8(14-19-5-10(17)6-20-14)22-16-21-7-11(18)15(24-16)23-13-4-12(25-26-13)9-2-3-9/h4-9H,2-3H2,1H3,(H3,21,22,23,24,25,26)/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using Km ATP concentration |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340315
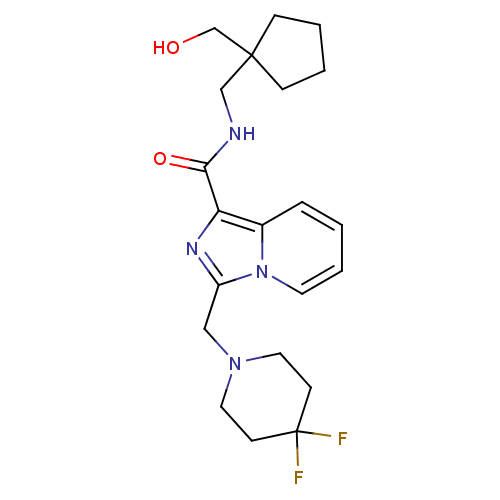
(3-((4,4-difluoropiperidin-1-yl)methyl)-N-((1-(hydr...)Show SMILES OCC1(CNC(=O)c2nc(CN3CCC(F)(F)CC3)n3ccccc23)CCCC1 Show InChI InChI=1S/C21H28F2N4O2/c22-21(23)8-11-26(12-9-21)13-17-25-18(16-5-1-4-10-27(16)17)19(29)24-14-20(15-28)6-2-3-7-20/h1,4-5,10,28H,2-3,6-9,11-15H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50097071
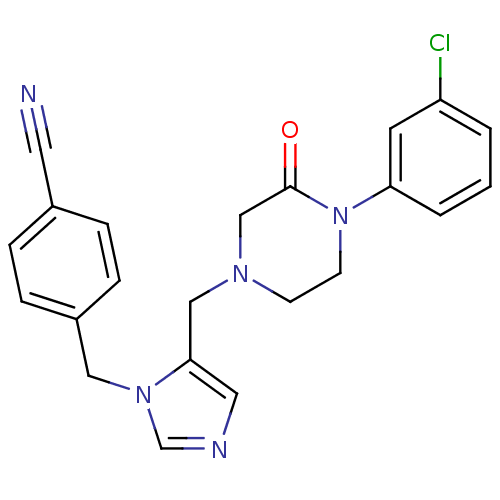
(4-[(5-{[4-(3-CHLOROPHENYL)-3-OXOPIPERAZIN-1-YL]MET...)Show SMILES Clc1cccc(c1)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1=O Show InChI InChI=1S/C22H20ClN5O/c23-19-2-1-3-20(10-19)28-9-8-26(15-22(28)29)14-21-12-25-16-27(21)13-18-6-4-17(11-24)5-7-18/h1-7,10,12,16H,8-9,13-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a radiolabelled farnesyltransferase inhibitor from FPTase in cultured Ha-Ras transformed RAT1 cells |
Bioorg Med Chem Lett 11: 1411-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52J0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335209

(5-fluoro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]...)Show SMILES COc1cc(Nc2nc(N[C@@H](C)c3ncc(F)cn3)nc(N3CCOCC3)c2F)[nH]n1 |r| Show InChI InChI=1S/C18H21F2N9O2/c1-10(15-21-8-11(19)9-22-15)23-18-25-16(24-12-7-13(30-2)28-27-12)14(20)17(26-18)29-3-5-31-6-4-29/h7-10H,3-6H2,1-2H3,(H3,23,24,25,26,27,28)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using Km ATP concentration |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335205

(5-bromo-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-...)Show SMILES COc1cc(Nc2nc(N[C@@H](C)c3ncc(F)cn3)ncc2Br)[nH]n1 |r| Show InChI InChI=1S/C14H14BrFN8O/c1-7(12-17-4-8(16)5-18-12)20-14-19-6-9(15)13(22-14)21-10-3-11(25-2)24-23-10/h3-7H,1-2H3,(H3,19,20,21,22,23,24)/t7-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using Km ATP concentration |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335212

(CHEMBL1650725 | N2-[(1S)-1-(5-fluoropyrimidin-2-yl...)Show SMILES C[C@H](Nc1ncc(C)c(Nc2cc(C)n[nH]2)n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C15H17FN8/c1-8-5-19-15(20-10(3)14-17-6-11(16)7-18-14)22-13(8)21-12-4-9(2)23-24-12/h4-7,10H,1-3H3,(H3,19,20,21,22,23,24)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using 5 mM of ATP |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293247
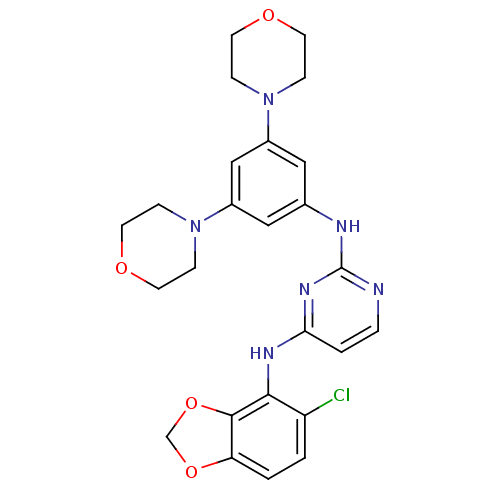
(CHEMBL468970 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES Clc1ccc2OCOc2c1Nc1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H27ClN6O4/c26-20-1-2-21-24(36-16-35-21)23(20)29-22-3-4-27-25(30-22)28-17-13-18(31-5-9-33-10-6-31)15-19(14-17)32-7-11-34-12-8-32/h1-4,13-15H,5-12,16H2,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335210

(5-chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]...)Show SMILES COc1cc(Nc2nc(N[C@@H](C)c3ncc(F)cn3)nc(N3CCOCC3)c2Cl)[nH]n1 |r| Show InChI InChI=1S/C18H21ClFN9O2/c1-10(15-21-8-11(20)9-22-15)23-18-25-16(24-12-7-13(30-2)28-27-12)14(19)17(26-18)29-3-5-31-6-4-29/h7-10H,3-6H2,1-2H3,(H3,23,24,25,26,27,28)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using Km ATP concentration |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50387299
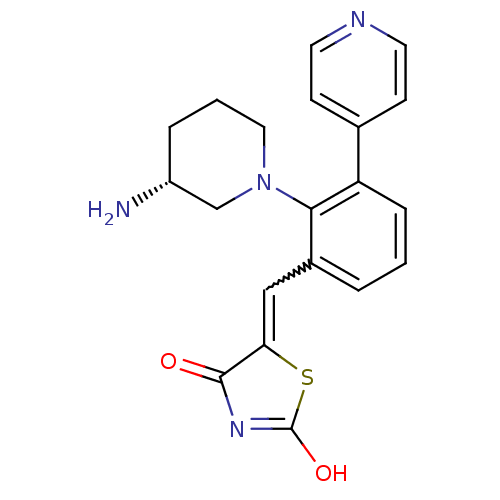
(CHEMBL2048873)Show SMILES N[C@@H]1CCCN(C1)c1c(C=C2SC(O)=NC2=O)cccc1-c1ccncc1 |r,w:9.9,c:14| Show InChI InChI=1S/C20H20N4O2S/c21-15-4-2-10-24(12-15)18-14(11-17-19(25)23-20(26)27-17)3-1-5-16(18)13-6-8-22-9-7-13/h1,3,5-9,11,15H,2,4,10,12,21H2,(H,23,25,26)/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 50 uM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50387299

(CHEMBL2048873)Show SMILES N[C@@H]1CCCN(C1)c1c(C=C2SC(O)=NC2=O)cccc1-c1ccncc1 |r,w:9.9,c:14| Show InChI InChI=1S/C20H20N4O2S/c21-15-4-2-10-24(12-15)18-14(11-17-19(25)23-20(26)27-17)3-1-5-16(18)13-6-8-22-9-7-13/h1,3,5-9,11,15H,2,4,10,12,21H2,(H,23,25,26)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 5 mM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50387295
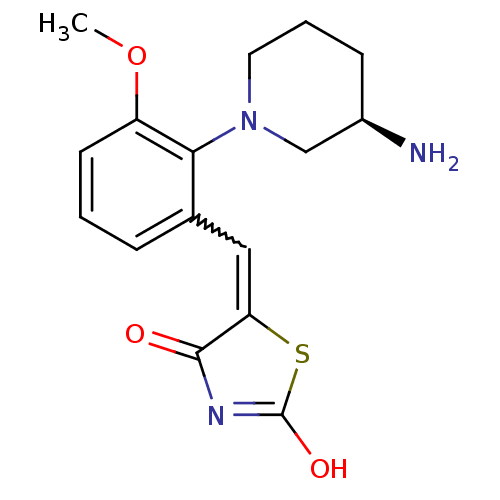
(CHEMBL2048868)Show SMILES COc1cccc(C=C2SC(O)=NC2=O)c1N1CCC[C@@H](N)C1 |r,w:7.6,c:11| Show InChI InChI=1S/C16H19N3O3S/c1-22-12-6-2-4-10(8-13-15(20)18-16(21)23-13)14(12)19-7-3-5-11(17)9-19/h2,4,6,8,11H,3,5,7,9,17H2,1H3,(H,18,20,21)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM2 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 50 uM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50387298
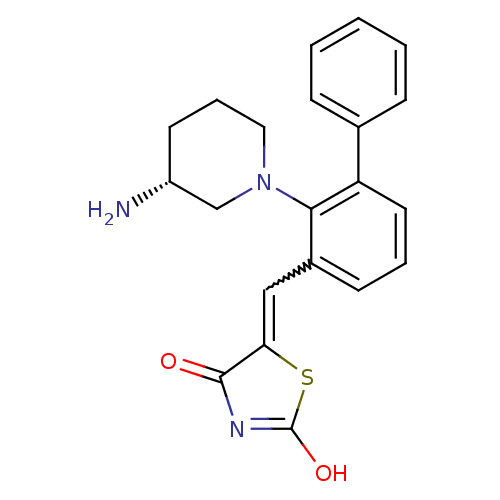
(CHEMBL2048872)Show SMILES N[C@@H]1CCCN(C1)c1c(C=C2SC(O)=NC2=O)cccc1-c1ccccc1 |r,w:9.9,c:14| Show InChI InChI=1S/C21H21N3O2S/c22-16-9-5-11-24(13-16)19-15(12-18-20(25)23-21(26)27-18)8-4-10-17(19)14-6-2-1-3-7-14/h1-4,6-8,10,12,16H,5,9,11,13,22H2,(H,23,25,26)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM2 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 50 uM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50387305
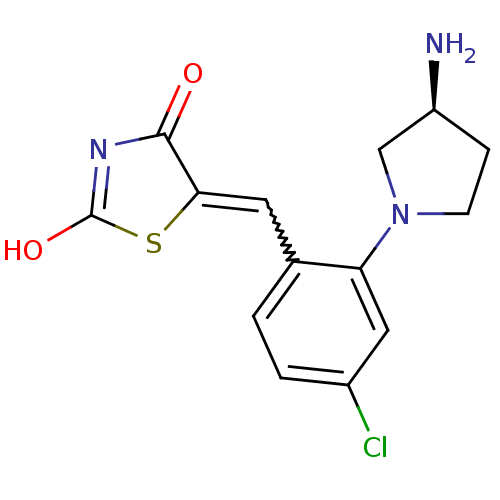
(CHEMBL2046468)Show SMILES N[C@H]1CCN(C1)c1cc(Cl)ccc1C=C1SC(O)=NC1=O |r,w:13.14,c:19| Show InChI InChI=1S/C14H14ClN3O2S/c15-9-2-1-8(5-12-13(19)17-14(20)21-12)11(6-9)18-4-3-10(16)7-18/h1-2,5-6,10H,3-4,7,16H2,(H,17,19,20)/t10-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 50 uM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329079

(CHEMBL1270280 | N2-(2,6-dimorpholinopyridin-4-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C25H29N9O2/c1-32(21-4-2-3-20-19(21)17-27-31-20)22-5-6-26-25(30-22)28-18-15-23(33-7-11-35-12-8-33)29-24(16-18)34-9-13-36-14-10-34/h2-6,15-17H,7-14H2,1H3,(H,27,31)(H,26,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340310
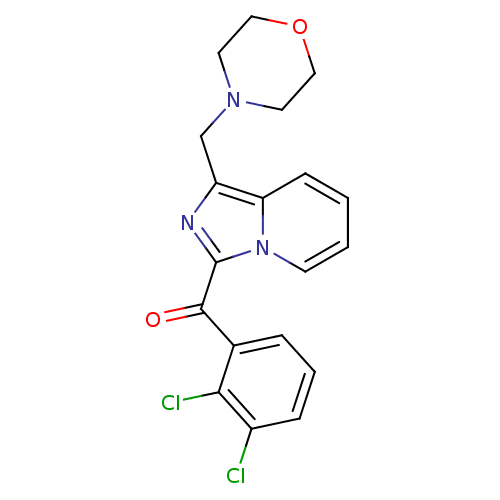
((2,3-dichlorophenyl)(1-(morpholinomethyl)imidazo[1...)Show SMILES Clc1cccc(C(=O)c2nc(CN3CCOCC3)c3ccccn23)c1Cl Show InChI InChI=1S/C19H17Cl2N3O2/c20-14-5-3-4-13(17(14)21)18(25)19-22-15(12-23-8-10-26-11-9-23)16-6-1-2-7-24(16)19/h1-7H,8-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335211
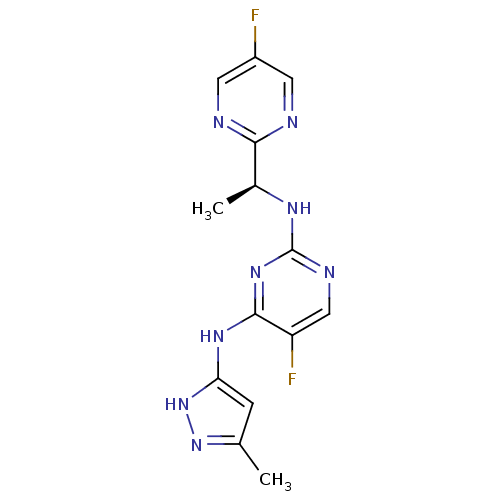
(5-Fluoro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]...)Show SMILES C[C@H](Nc1ncc(F)c(Nc2cc(C)n[nH]2)n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C14H14F2N8/c1-7-3-11(24-23-7)21-13-10(16)6-19-14(22-13)20-8(2)12-17-4-9(15)5-18-12/h3-6,8H,1-2H3,(H3,19,20,21,22,23,24)/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using 5 mM of ATP |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50387308

(CHEMBL2048866)Show SMILES N[C@@H]1CCCN(C1)c1c(Cl)cccc1C=C1SC(O)=NC1=O |r,w:14.15,c:20| Show InChI InChI=1S/C15H16ClN3O2S/c16-11-5-1-3-9(7-12-14(20)18-15(21)22-12)13(11)19-6-2-4-10(17)8-19/h1,3,5,7,10H,2,4,6,8,17H2,(H,18,20,21)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM2 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 50 uM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50387294
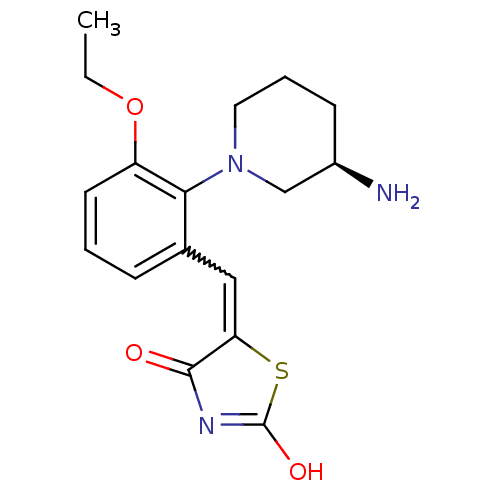
(CHEMBL2048869)Show SMILES CCOc1cccc(C=C2SC(O)=NC2=O)c1N1CCC[C@@H](N)C1 |r,w:8.7,c:12| Show InChI InChI=1S/C17H21N3O3S/c1-2-23-13-7-3-5-11(9-14-16(21)19-17(22)24-14)15(13)20-8-4-6-12(18)10-20/h3,5,7,9,12H,2,4,6,8,10,18H2,1H3,(H,19,21,22)/t12-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 5 mM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50340316
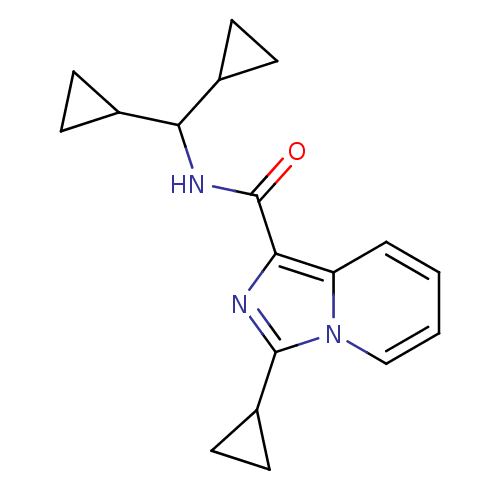
(3-cyclopropyl-N-(dicyclopropylmethyl)imidazo[1,5-a...)Show InChI InChI=1S/C18H21N3O/c22-18(20-15(11-4-5-11)12-6-7-12)16-14-3-1-2-10-21(14)17(19-16)13-8-9-13/h1-3,10-13,15H,4-9H2,(H,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level |
Bioorg Med Chem Lett 21: 2354-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.082
BindingDB Entry DOI: 10.7270/Q2C829MB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50387296
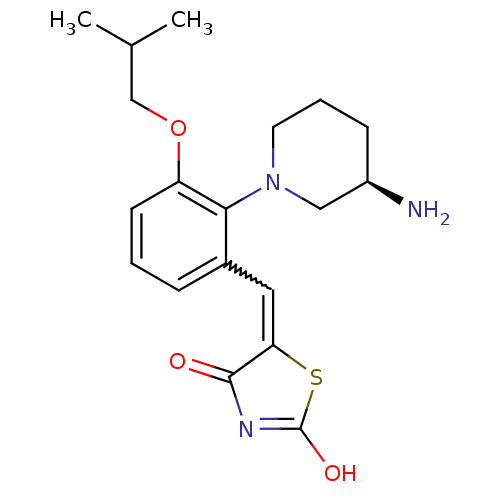
(CHEMBL2048870)Show SMILES CC(C)COc1cccc(C=C2SC(O)=NC2=O)c1N1CCC[C@@H](N)C1 |r,w:10.9,c:14| Show InChI InChI=1S/C19H25N3O3S/c1-12(2)11-25-15-7-3-5-13(9-16-18(23)21-19(24)26-16)17(15)22-8-4-6-14(20)10-22/h3,5,7,9,12,14H,4,6,8,10-11,20H2,1-2H3,(H,21,23,24)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 5 mM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335209

(5-fluoro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]...)Show SMILES COc1cc(Nc2nc(N[C@@H](C)c3ncc(F)cn3)nc(N3CCOCC3)c2F)[nH]n1 |r| Show InChI InChI=1S/C18H21F2N9O2/c1-10(15-21-8-11(19)9-22-15)23-18-25-16(24-12-7-13(30-2)28-27-12)14(20)17(26-18)29-3-5-31-6-4-29/h7-10H,3-6H2,1-2H3,(H3,23,24,25,26,27,28)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using 5 mM of ATP |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50100004

(4-{5-[4-(3-Chloro-phenyl)-3-oxo-piperazin-1-ylmeth...)Show SMILES OCCOc1cccc(Oc2cc(Cn3cncc3CN3CCN(C(=O)C3)c3cccc(Cl)c3)ccc2C#N)c1 Show InChI InChI=1S/C30H28ClN5O4/c31-24-3-1-4-25(14-24)36-10-9-34(20-30(36)38)19-26-17-33-21-35(26)18-22-7-8-23(16-32)29(13-22)40-28-6-2-5-27(15-28)39-12-11-37/h1-8,13-15,17,21,37H,9-12,18-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a radiolabelled farnesyltransferase inhibitor from FPTase in cultured Ha-Ras transformed RAT1 cells |
Bioorg Med Chem Lett 11: 1411-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52J0 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329070

(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50387309
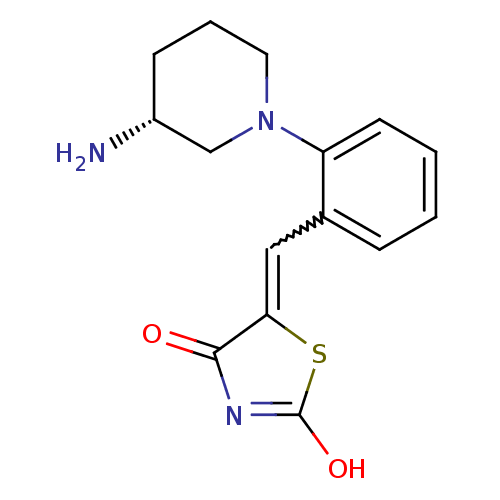
(CHEMBL2048865)Show SMILES N[C@@H]1CCCN(C1)c1ccccc1C=C1SC(O)=NC1=O |r,w:13.14,c:19| Show InChI InChI=1S/C15H17N3O2S/c16-11-5-3-7-18(9-11)12-6-2-1-4-10(12)8-13-14(19)17-15(20)21-13/h1-2,4,6,8,11H,3,5,7,9,16H2,(H,17,19,20)/t11-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 50 uM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335206

(5-chloro-N4-[5-(dimethylamino)-1H-pyrazol-3-yl]-N2...)Show SMILES C[C@H](Nc1ncc(Cl)c(Nc2cc(n[nH]2)N(C)C)n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C15H17ClFN9/c1-8(13-18-5-9(17)6-19-13)21-15-20-7-10(16)14(23-15)22-11-4-12(25-24-11)26(2)3/h4-8H,1-3H3,(H3,20,21,22,23,24,25)/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using Km ATP concentration |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081213

(4-{5-[(S)-4-(3-Chloro-phenyl)-2-methyl-5-oxo-piper...)Show SMILES C[C@H]1CN(C(=O)CN1Cc1cncn1Cc1ccc(cc1)C#N)c1cccc(Cl)c1 Show InChI InChI=1S/C23H22ClN5O/c1-17-12-29(21-4-2-3-20(24)9-21)23(30)15-27(17)14-22-11-26-16-28(22)13-19-7-5-18(10-25)6-8-19/h2-9,11,16-17H,12-15H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50100001
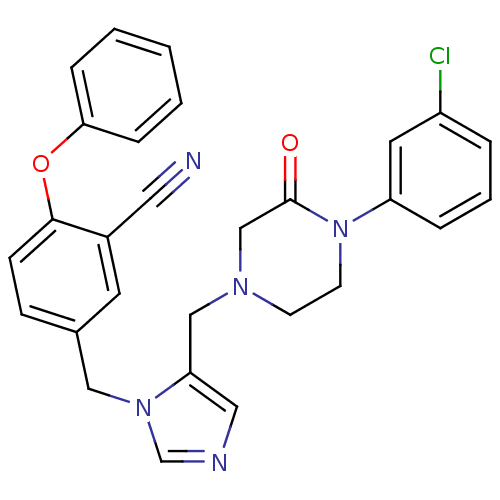
(5-{5-[4-(3-Chloro-phenyl)-3-oxo-piperazin-1-ylmeth...)Show SMILES Clc1cccc(c1)N1CCN(Cc2cncn2Cc2ccc(Oc3ccccc3)c(c2)C#N)CC1=O Show InChI InChI=1S/C28H24ClN5O2/c29-23-5-4-6-24(14-23)34-12-11-32(19-28(34)35)18-25-16-31-20-33(25)17-21-9-10-27(22(13-21)15-30)36-26-7-2-1-3-8-26/h1-10,13-14,16,20H,11-12,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition against human Farnesyltransferase catalyzed by incorporation of [3H]- FPP into recombinant Ras-CVIM |
Bioorg Med Chem Lett 11: 1411-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MP52J0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50387298

(CHEMBL2048872)Show SMILES N[C@@H]1CCCN(C1)c1c(C=C2SC(O)=NC2=O)cccc1-c1ccccc1 |r,w:9.9,c:14| Show InChI InChI=1S/C21H21N3O2S/c22-16-9-5-11-24(13-16)19-15(12-18-20(25)23-21(26)27-18)8-4-10-17(19)14-6-2-1-3-7-14/h1-4,6-8,10,12,16H,5,9,11,13,22H2,(H,23,25,26)/t16-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 5 mM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335215

((S)-5-chloro-N2-(1-(5-fluoropyrimidin-2-yl)ethyl)-...)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)c(Cl)c(n1)N1CCOCC1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C18H21ClFN9O/c1-10-7-13(28-27-10)24-16-14(19)17(29-3-5-30-6-4-29)26-18(25-16)23-11(2)15-21-8-12(20)9-22-15/h7-9,11H,3-6H2,1-2H3,(H3,23,24,25,26,27,28)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using 5 mM of ATP |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50387309

(CHEMBL2048865)Show SMILES N[C@@H]1CCCN(C1)c1ccccc1C=C1SC(O)=NC1=O |r,w:13.14,c:19| Show InChI InChI=1S/C15H17N3O2S/c16-11-5-3-7-18(9-11)12-6-2-1-4-10(12)8-13-14(19)17-15(20)21-13/h1-2,4,6,8,11H,3,5,7,9,16H2,(H,17,19,20)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 50 uM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50387295

(CHEMBL2048868)Show SMILES COc1cccc(C=C2SC(O)=NC2=O)c1N1CCC[C@@H](N)C1 |r,w:7.6,c:11| Show InChI InChI=1S/C16H19N3O3S/c1-22-12-6-2-4-10(8-13-15(20)18-16(21)23-13)14(12)19-7-3-5-11(17)9-19/h2,4,6,8,11H,3,5,7,9,17H2,1H3,(H,18,20,21)/t11-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 5 mM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50387310
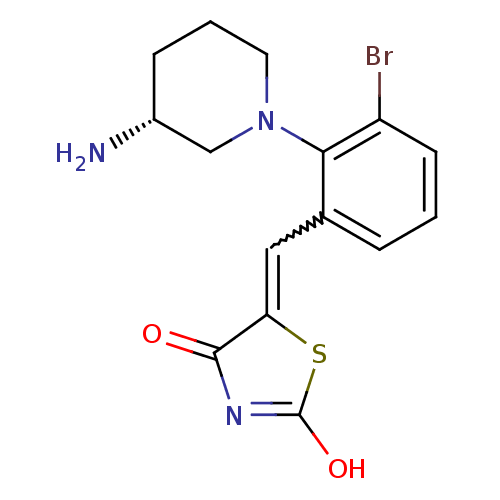
(CHEMBL2048867)Show SMILES N[C@@H]1CCCN(C1)c1c(Br)cccc1C=C1SC(O)=NC1=O |r,w:14.15,c:20| Show InChI InChI=1S/C15H16BrN3O2S/c16-11-5-1-3-9(7-12-14(20)18-15(21)22-12)13(11)19-6-2-4-10(17)8-19/h1,3,5,7,10H,2,4,6,8,17H2,(H,18,20,21)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 5 mM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50335214
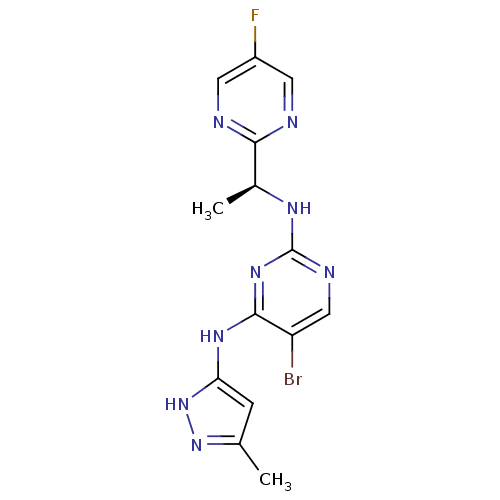
(5-bromo-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-...)Show SMILES C[C@H](Nc1ncc(Br)c(Nc2cc(C)n[nH]2)n1)c1ncc(F)cn1 |r| Show InChI InChI=1S/C14H14BrFN8/c1-7-3-11(24-23-7)21-13-10(15)6-19-14(22-13)20-8(2)12-17-4-9(16)5-18-12/h3-6,8H,1-2H3,(H3,19,20,21,22,23,24)/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 using 5 mM of ATP |
J Med Chem 54: 262-76 (2011)
Article DOI: 10.1021/jm1011319
BindingDB Entry DOI: 10.7270/Q2M32WRM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50387294

(CHEMBL2048869)Show SMILES CCOc1cccc(C=C2SC(O)=NC2=O)c1N1CCC[C@@H](N)C1 |r,w:8.7,c:12| Show InChI InChI=1S/C17H21N3O3S/c1-2-23-13-7-3-5-11(9-14-16(21)19-17(22)24-14)15(13)20-8-4-6-12(18)10-20/h3,5,7,9,12H,2,4,6,8,10,18H2,1H3,(H,19,21,22)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 5 mM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50387310

(CHEMBL2048867)Show SMILES N[C@@H]1CCCN(C1)c1c(Br)cccc1C=C1SC(O)=NC1=O |r,w:14.15,c:20| Show InChI InChI=1S/C15H16BrN3O2S/c16-11-5-1-3-9(7-12-14(20)18-15(21)22-12)13(11)19-6-2-4-10(17)8-19/h1,3,5,7,10H,2,4,6,8,17H2,(H,18,20,21)/t10-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 5 mM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data