 Found 179 hits with Last Name = 'greenfeder' and Initial = 's'
Found 179 hits with Last Name = 'greenfeder' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50116078
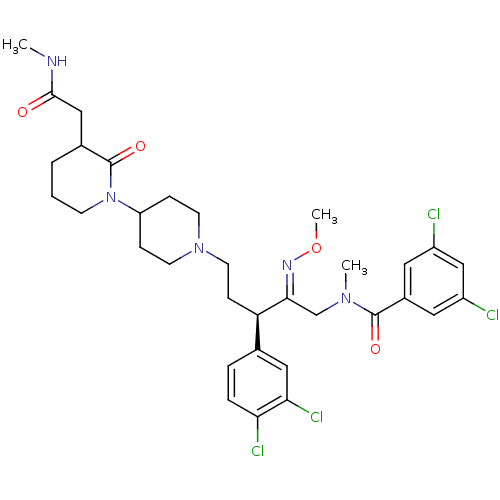
(3,5-Dichloro-N-[3-(3,4-dichloro-phenyl)-2-methoxyi...)Show SMILES CNC(=O)CC1CCCN(C2CCN(CC[C@@H](\C(CN(C)C(=O)c3cc(Cl)cc(Cl)c3)=N\OC)c3ccc(Cl)c(Cl)c3)CC2)C1=O Show InChI InChI=1S/C33H41Cl4N5O4/c1-38-31(43)18-22-5-4-11-42(33(22)45)26-8-12-41(13-9-26)14-10-27(21-6-7-28(36)29(37)17-21)30(39-46-3)20-40(2)32(44)23-15-24(34)19-25(35)16-23/h6-7,15-17,19,22,26-27H,4-5,8-14,18,20H2,1-3H3,(H,38,43)/b39-30+/t22?,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50116078

(3,5-Dichloro-N-[3-(3,4-dichloro-phenyl)-2-methoxyi...)Show SMILES CNC(=O)CC1CCCN(C2CCN(CC[C@@H](\C(CN(C)C(=O)c3cc(Cl)cc(Cl)c3)=N\OC)c3ccc(Cl)c(Cl)c3)CC2)C1=O Show InChI InChI=1S/C33H41Cl4N5O4/c1-38-31(43)18-22-5-4-11-42(33(22)45)26-8-12-41(13-9-26)14-10-27(21-6-7-28(36)29(37)17-21)30(39-46-3)20-40(2)32(44)23-15-24(34)19-25(35)16-23/h6-7,15-17,19,22,26-27H,4-5,8-14,18,20H2,1-3H3,(H,38,43)/b39-30+/t22?,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50071112
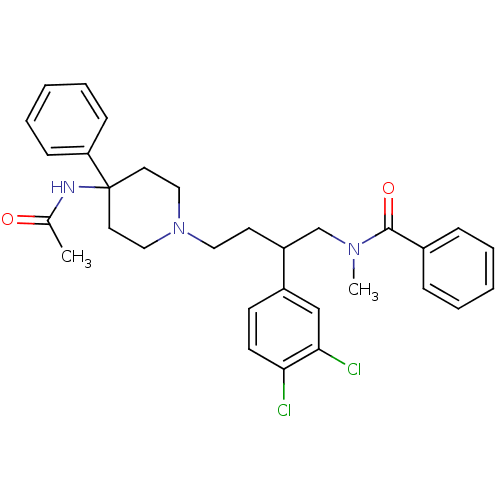
(CHEMBL56835 | N-[4-(4-Acetylamino-4-phenyl-piperid...)Show SMILES CN(CC(CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50116078

(3,5-Dichloro-N-[3-(3,4-dichloro-phenyl)-2-methoxyi...)Show SMILES CNC(=O)CC1CCCN(C2CCN(CC[C@@H](\C(CN(C)C(=O)c3cc(Cl)cc(Cl)c3)=N\OC)c3ccc(Cl)c(Cl)c3)CC2)C1=O Show InChI InChI=1S/C33H41Cl4N5O4/c1-38-31(43)18-22-5-4-11-42(33(22)45)26-8-12-41(13-9-26)14-10-27(21-6-7-28(36)29(37)17-21)30(39-46-3)20-40(2)32(44)23-15-24(34)19-25(35)16-23/h6-7,15-17,19,22,26-27H,4-5,8-14,18,20H2,1-3H3,(H,38,43)/b39-30+/t22?,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50051290

(CHEMBL299377 | N-(1-{3-[1-Benzoyl-3-(3,4-dichloro-...)Show SMILES CN(C(C)=O)C1(CCN(CCCC2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50051290

(CHEMBL299377 | N-(1-{3-[1-Benzoyl-3-(3,4-dichloro-...)Show SMILES CN(C(C)=O)C1(CCN(CCCC2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50071112

(CHEMBL56835 | N-[4-(4-Acetylamino-4-phenyl-piperid...)Show SMILES CN(CC(CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50051290

(CHEMBL299377 | N-(1-{3-[1-Benzoyl-3-(3,4-dichloro-...)Show SMILES CN(C(C)=O)C1(CCN(CCCC2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 291 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50071112

(CHEMBL56835 | N-[4-(4-Acetylamino-4-phenyl-piperid...)Show SMILES CN(CC(CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50000041

((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...)Show InChI InChI=1S/C19H24N2O/c1-22-18-12-6-5-10-16(18)14-21-17-11-7-13-20-19(17)15-8-3-2-4-9-15/h2-6,8-10,12,17,19-21H,7,11,13-14H2,1H3/t17-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50000041

((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...)Show InChI InChI=1S/C19H24N2O/c1-22-18-12-6-5-10-16(18)14-21-17-11-7-13-20-19(17)15-8-3-2-4-9-15/h2-6,8-10,12,17,19-21H,7,11,13-14H2,1H3/t17-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 191-202 (2002)
Article DOI: 10.1016/s0014-2999(02)02124-6
BindingDB Entry DOI: 10.7270/Q2862F1R |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50337038
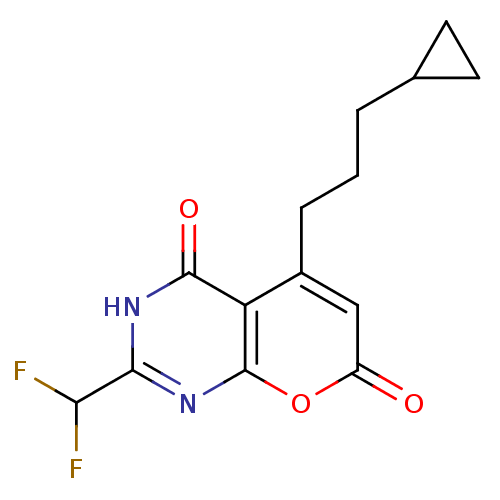
(5-(3-cyclopropylpropyl)-2-(difluoromethyl)-3H-pyra...)Show InChI InChI=1S/C14H14F2N2O3/c15-11(16)12-17-13(20)10-8(3-1-2-7-4-5-7)6-9(19)21-14(10)18-12/h6-7,11H,1-5H2,(H,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50384612
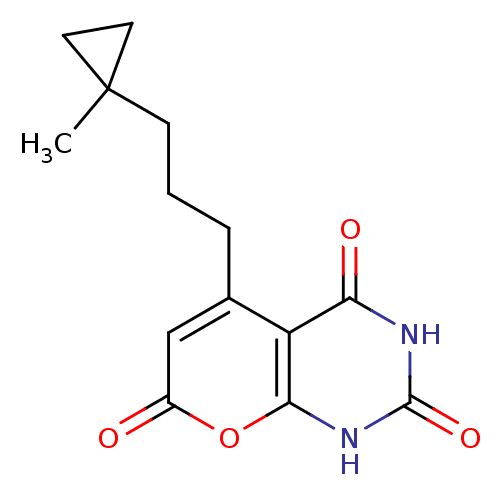
(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50384612

(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50384612

(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50337038

(5-(3-cyclopropylpropyl)-2-(difluoromethyl)-3H-pyra...)Show InChI InChI=1S/C14H14F2N2O3/c15-11(16)12-17-13(20)10-8(3-1-2-7-4-5-7)6-9(19)21-14(10)18-12/h6-7,11H,1-5H2,(H,17,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50337038

(5-(3-cyclopropylpropyl)-2-(difluoromethyl)-3H-pyra...)Show InChI InChI=1S/C14H14F2N2O3/c15-11(16)12-17-13(20)10-8(3-1-2-7-4-5-7)6-9(19)21-14(10)18-12/h6-7,11H,1-5H2,(H,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50384612

(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50337038

(5-(3-cyclopropylpropyl)-2-(difluoromethyl)-3H-pyra...)Show InChI InChI=1S/C14H14F2N2O3/c15-11(16)12-17-13(20)10-8(3-1-2-7-4-5-7)6-9(19)21-14(10)18-12/h6-7,11H,1-5H2,(H,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50337024
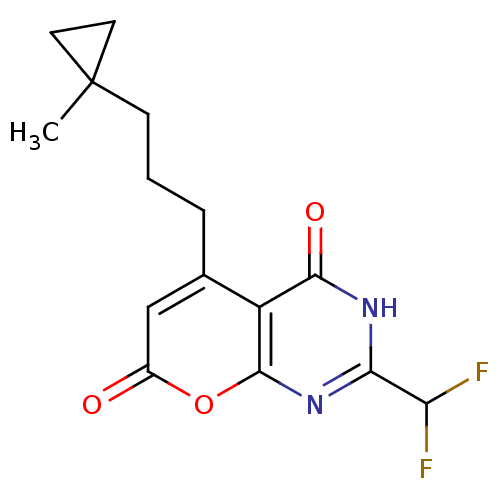
(2-(difluoromethyl)-5-(3-(1-methylcyclopropyl)propy...)Show SMILES CC1(CCCc2cc(=O)oc3nc([nH]c(=O)c23)C(F)F)CC1 Show InChI InChI=1S/C15H16F2N2O3/c1-15(5-6-15)4-2-3-8-7-9(20)22-14-10(8)13(21)18-12(19-14)11(16)17/h7,11H,2-6H2,1H3,(H,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50337038

(5-(3-cyclopropylpropyl)-2-(difluoromethyl)-3H-pyra...)Show InChI InChI=1S/C14H14F2N2O3/c15-11(16)12-17-13(20)10-8(3-1-2-7-4-5-7)6-9(19)21-14(10)18-12/h6-7,11H,1-5H2,(H,17,18,20) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50336998

(2-(difluoromethyl)-5-(3-(1-(hydroxymethyl)cyclopro...)Show SMILES OCC1(CCCc2cc(=O)oc3nc([nH]c(=O)c23)C(F)F)CC1 Show InChI InChI=1S/C15H16F2N2O4/c16-11(17)12-18-13(22)10-8(6-9(21)23-14(10)19-12)2-1-3-15(7-20)4-5-15/h6,11,20H,1-5,7H2,(H,18,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50336999

(5-(cyclopropylmethyl)-2-(difluoromethyl)-3H-pyrano...)Show InChI InChI=1S/C12H10F2N2O3/c13-9(14)10-15-11(18)8-6(3-5-1-2-5)4-7(17)19-12(8)16-10/h4-5,9H,1-3H2,(H,15,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337000

(5-cyclopropyl-2-(difluoromethyl)-3H-pyrano[2,3-d]p...)Show InChI InChI=1S/C11H8F2N2O3/c12-8(13)9-14-10(17)7-5(4-1-2-4)3-6(16)18-11(7)15-9/h3-4,8H,1-2H2,(H,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337001
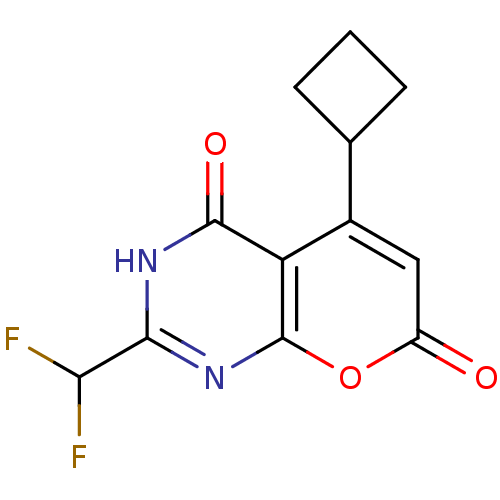
(5-cyclobutyl-2-(difluoromethyl)-3H-pyrano[2,3-d]py...)Show InChI InChI=1S/C12H10F2N2O3/c13-9(14)10-15-11(18)8-6(5-2-1-3-5)4-7(17)19-12(8)16-10/h4-5,9H,1-3H2,(H,15,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337002
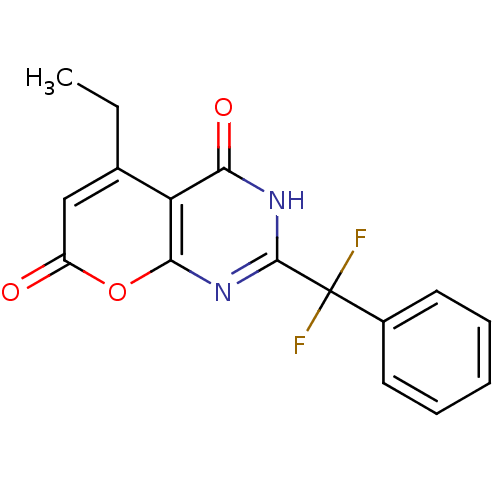
(2-(difluoro(phenyl)methyl)-5-ethyl-3H-pyrano[2,3-d...)Show SMILES CCc1cc(=O)oc2nc([nH]c(=O)c12)C(F)(F)c1ccccc1 Show InChI InChI=1S/C16H12F2N2O3/c1-2-9-8-11(21)23-14-12(9)13(22)19-15(20-14)16(17,18)10-6-4-3-5-7-10/h3-8H,2H2,1H3,(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337003

(5-ethyl-2-(phenylsulfonylmethyl)-3H-pyrano[2,3-d]p...)Show SMILES CCc1cc(=O)oc2nc(CS(=O)(=O)c3ccccc3)[nH]c(=O)c12 Show InChI InChI=1S/C16H14N2O5S/c1-2-10-8-13(19)23-16-14(10)15(20)17-12(18-16)9-24(21,22)11-6-4-3-5-7-11/h3-8H,2,9H2,1H3,(H,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337004

(5-ethyl-2-(piperidin-1-ylmethyl)-3H-pyrano[2,3-d]p...)Show InChI InChI=1S/C15H19N3O3/c1-2-10-8-12(19)21-15-13(10)14(20)16-11(17-15)9-18-6-4-3-5-7-18/h8H,2-7,9H2,1H3,(H,16,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337005
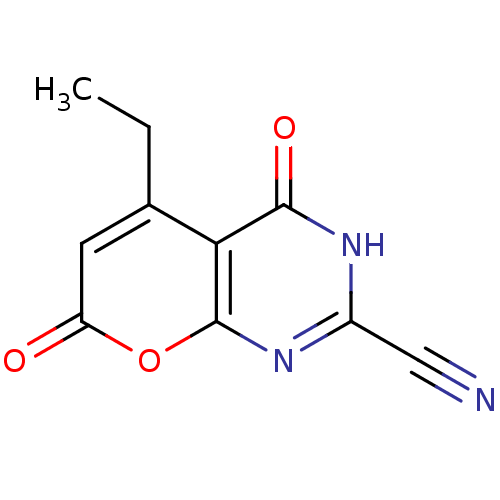
(5-ethyl-4,7-dioxo-4,7-dihydro-3H-pyrano[2,3-d]pyri...)Show InChI InChI=1S/C10H7N3O3/c1-2-5-3-7(14)16-10-8(5)9(15)12-6(4-11)13-10/h3H,2H2,1H3,(H,12,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337006

(CHEMBL1672763 | methyl 5-ethyl-4,7-dioxo-4,7-dihyd...)Show InChI InChI=1S/C11H10N2O5/c1-3-5-4-6(14)18-10-7(5)9(15)12-8(13-10)11(16)17-2/h4H,3H2,1-2H3,(H,12,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337007

(5-ethyl-2-(4-(trifluoromethyl)phenyl)-3H-pyrano[2,...)Show SMILES CCc1cc(=O)oc2nc([nH]c(=O)c12)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C16H11F3N2O3/c1-2-8-7-11(22)24-15-12(8)14(23)20-13(21-15)9-3-5-10(6-4-9)16(17,18)19/h3-7H,2H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337008

(5-ethyl-2-(prop-1-en-2-yl)-3H-pyrano[2,3-d]pyrimid...)Show InChI InChI=1S/C12H12N2O3/c1-4-7-5-8(15)17-12-9(7)11(16)13-10(14-12)6(2)3/h5H,2,4H2,1,3H3,(H,13,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337009

(2-cyclopropyl-5-ethyl-3H-pyrano[2,3-d]pyrimidine-4...)Show InChI InChI=1S/C12H12N2O3/c1-2-6-5-8(15)17-12-9(6)11(16)13-10(14-12)7-3-4-7/h5,7H,2-4H2,1H3,(H,13,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337010

(5-ethyl-2-phenethyl-3H-pyrano[2,3-d]pyrimidine-4,7...)Show InChI InChI=1S/C17H16N2O3/c1-2-12-10-14(20)22-17-15(12)16(21)18-13(19-17)9-8-11-6-4-3-5-7-11/h3-7,10H,2,8-9H2,1H3,(H,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337011
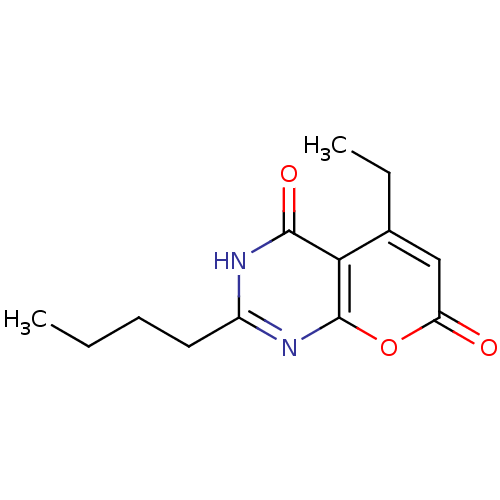
(2-butyl-5-ethyl-3H-pyrano[2,3-d]pyrimidine-4,7-dio...)Show InChI InChI=1S/C13H16N2O3/c1-3-5-6-9-14-12(17)11-8(4-2)7-10(16)18-13(11)15-9/h7H,3-6H2,1-2H3,(H,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337012

(5-ethyl-2-isopropyl-3H-pyrano[2,3-d]pyrimidine-4,7...)Show InChI InChI=1S/C12H14N2O3/c1-4-7-5-8(15)17-12-9(7)11(16)13-10(14-12)6(2)3/h5-6H,4H2,1-3H3,(H,13,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337013

(5-ethyl-3H-pyrano[2,3-d]pyrimidine-4,7-dione | CHE...)Show InChI InChI=1S/C9H8N2O3/c1-2-5-3-6(12)14-9-7(5)8(13)10-4-11-9/h3-4H,2H2,1H3,(H,10,11,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337014
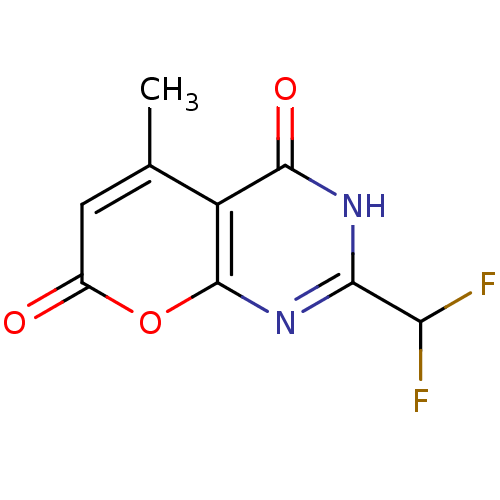
(2-(difluoromethyl)-5-methyl-3H-pyrano[2,3-d]pyrimi...)Show InChI InChI=1S/C9H6F2N2O3/c1-3-2-4(14)16-9-5(3)8(15)12-7(13-9)6(10)11/h2,6H,1H3,(H,12,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.58E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337015

(5-ethyl-2-(1-fluoroethyl)-3H-pyrano[2,3-d]pyrimidi...)Show InChI InChI=1S/C11H11FN2O3/c1-3-6-4-7(15)17-11-8(6)10(16)13-9(14-11)5(2)12/h4-5H,3H2,1-2H3,(H,13,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337016

(2-(difluoromethyl)-5-propyl-3H-pyrano[2,3-d]pyrimi...)Show InChI InChI=1S/C11H10F2N2O3/c1-2-3-5-4-6(16)18-11-7(5)10(17)14-9(15-11)8(12)13/h4,8H,2-3H2,1H3,(H,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.44E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337017
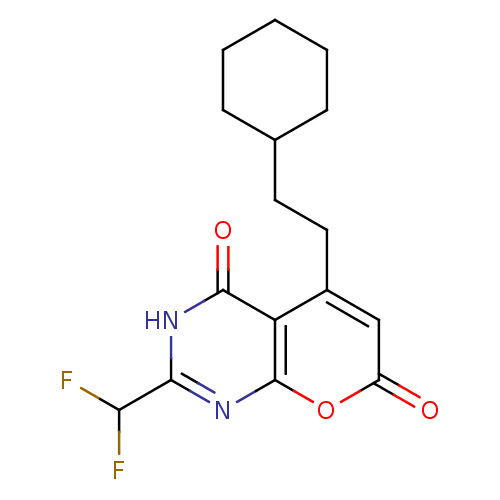
(5-(2-cyclohexylethyl)-2-(difluoromethyl)-3H-pyrano...)Show InChI InChI=1S/C16H18F2N2O3/c17-13(18)14-19-15(22)12-10(8-11(21)23-16(12)20-14)7-6-9-4-2-1-3-5-9/h8-9,13H,1-7H2,(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337018

(5-ethyl-2-(methoxymethyl)-3H-pyrano[2,3-d]pyrimidi...)Show InChI InChI=1S/C11H12N2O4/c1-3-6-4-8(14)17-11-9(6)10(15)12-7(13-11)5-16-2/h4H,3,5H2,1-2H3,(H,12,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337019
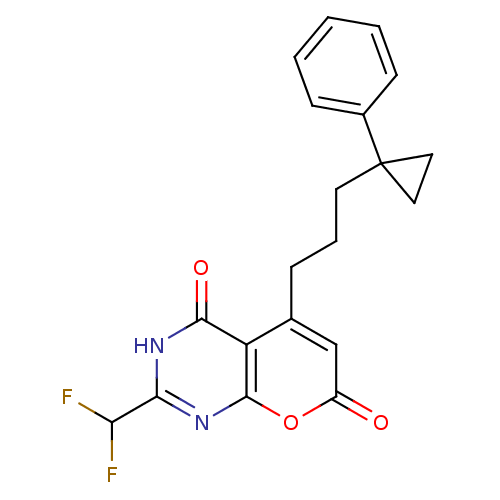
(2-(difluoromethyl)-5-(3-(1-phenylcyclopropyl)propy...)Show SMILES FC(F)c1nc2oc(=O)cc(CCCC3(CC3)c3ccccc3)c2c(=O)[nH]1 Show InChI InChI=1S/C20H18F2N2O3/c21-16(22)17-23-18(26)15-12(11-14(25)27-19(15)24-17)5-4-8-20(9-10-20)13-6-2-1-3-7-13/h1-3,6-7,11,16H,4-5,8-10H2,(H,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337020

(5-ethyl-2-(phenoxymethyl)-3H-pyrano[2,3-d]pyrimidi...)Show InChI InChI=1S/C16H14N2O4/c1-2-10-8-13(19)22-16-14(10)15(20)17-12(18-16)9-21-11-6-4-3-5-7-11/h3-8H,2,9H2,1H3,(H,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337021

(2-(chlorofluoromethyl)-5-ethyl-3H-pyrano[2,3-d]pyr...)Show InChI InChI=1S/C10H8ClFN2O3/c1-2-4-3-5(15)17-10-6(4)9(16)13-8(14-10)7(11)12/h3,7H,2H2,1H3,(H,13,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337022
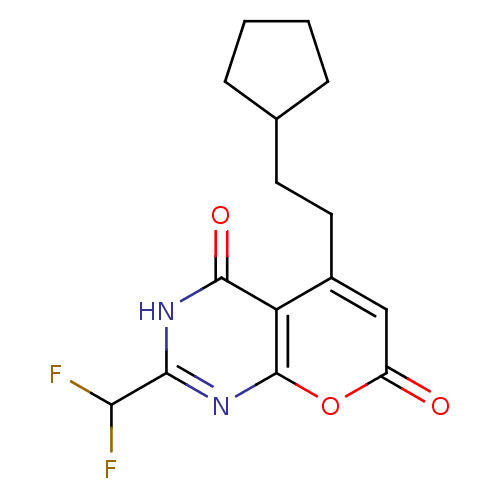
(5-(2-cyclopentylethyl)-2-(difluoromethyl)-3H-pyran...)Show InChI InChI=1S/C15H16F2N2O3/c16-12(17)13-18-14(21)11-9(6-5-8-3-1-2-4-8)7-10(20)22-15(11)19-13/h7-8,12H,1-6H2,(H,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337023

(5-ethyl-2-(trifluoromethyl)-3H-pyrano[2,3-d]pyrimi...)Show InChI InChI=1S/C10H7F3N2O3/c1-2-4-3-5(16)18-8-6(4)7(17)14-9(15-8)10(11,12)13/h3H,2H2,1H3,(H,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337025

(5-ethyl-2-(hydroxymethyl)-3H-pyrano[2,3-d]pyrimidi...)Show InChI InChI=1S/C10H10N2O4/c1-2-5-3-7(14)16-10-8(5)9(15)11-6(4-13)12-10/h3,13H,2,4H2,1H3,(H,11,12,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50337026
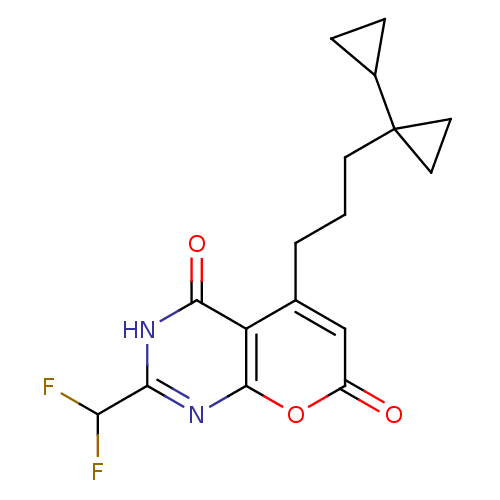
(5-(3-bi(cycloprop)ylpropyl)-2-(difluoromethyl)-3H-...)Show SMILES FC(F)c1nc2oc(=O)cc(CCCC3(CC3)C3CC3)c2c(=O)[nH]1 Show InChI InChI=1S/C17H18F2N2O3/c18-13(19)14-20-15(23)12-9(8-11(22)24-16(12)21-14)2-1-5-17(6-7-17)10-3-4-10/h8,10,13H,1-7H2,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation |
ACS Med Chem Lett 2: 171-176 (2011)
Article DOI: 10.1021/ml100251u
BindingDB Entry DOI: 10.7270/Q2DZ08KX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data