

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM203869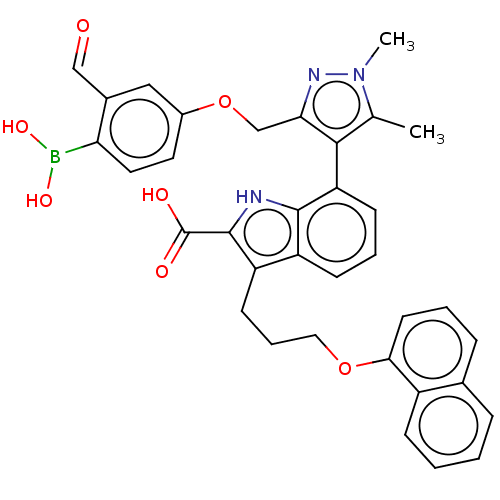 (7-(3-((4-Borono-3-formylphenoxy)methyl)-1,5-dimeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | -51.5 | 3.40 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit | Assay Description TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... | Nat Chem Biol 12: 931-936 (2016) Article DOI: 10.1038/nchembio.2174 BindingDB Entry DOI: 10.7270/Q2FN1513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM203875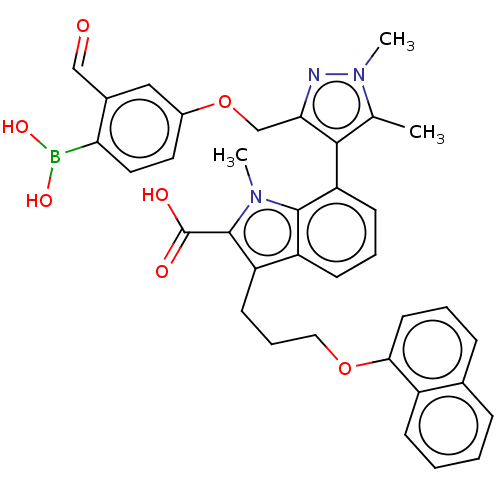 (7-(3-((4-Borono-3-formylphenoxy)methyl)-1,5-dimeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | -50.7 | 4.20 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit | Assay Description TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... | Nat Chem Biol 12: 931-936 (2016) Article DOI: 10.1038/nchembio.2174 BindingDB Entry DOI: 10.7270/Q2FN1513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM203870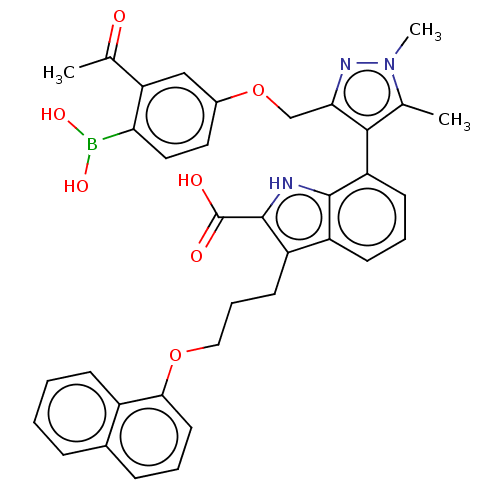 (7-(3-((3-Acetyl-4-boronophenoxy)methyl)-1,5-dimeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | -50.7 | 4.70 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit | Assay Description TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... | Nat Chem Biol 12: 931-936 (2016) Article DOI: 10.1038/nchembio.2174 BindingDB Entry DOI: 10.7270/Q2FN1513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM203876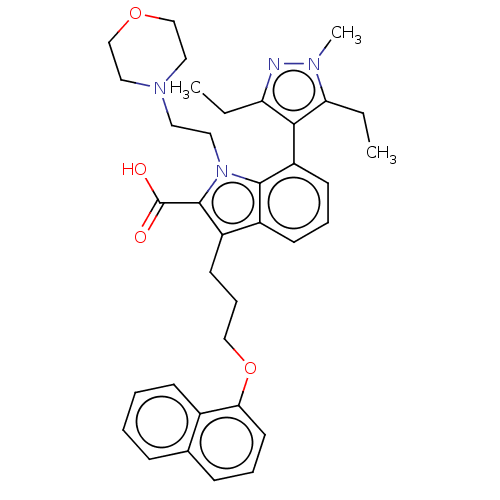 (Mcl-1 inhibitor 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | -50.0 | 5.96 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit | Assay Description TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... | Nat Chem Biol 12: 931-936 (2016) Article DOI: 10.1038/nchembio.2174 BindingDB Entry DOI: 10.7270/Q2FN1513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM203871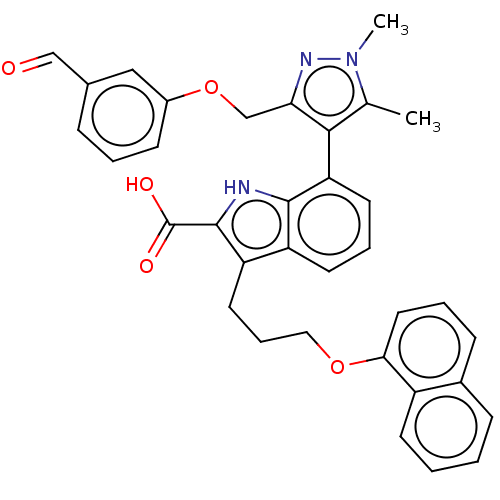 (7-(3-((3-formylphenoxy)methyl)-1,5-dimethyl-1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | -44.3 | 59.5 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit | Assay Description TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... | Nat Chem Biol 12: 931-936 (2016) Article DOI: 10.1038/nchembio.2174 BindingDB Entry DOI: 10.7270/Q2FN1513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM203873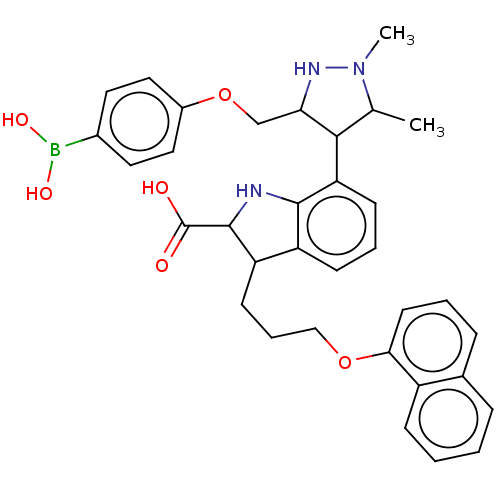 (7-(3-((4-Boronophenoxy)methyl)-1,5-dimethyl-1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 44 | -41.8 | 162 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit | Assay Description TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... | Nat Chem Biol 12: 931-936 (2016) Article DOI: 10.1038/nchembio.2174 BindingDB Entry DOI: 10.7270/Q2FN1513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM203872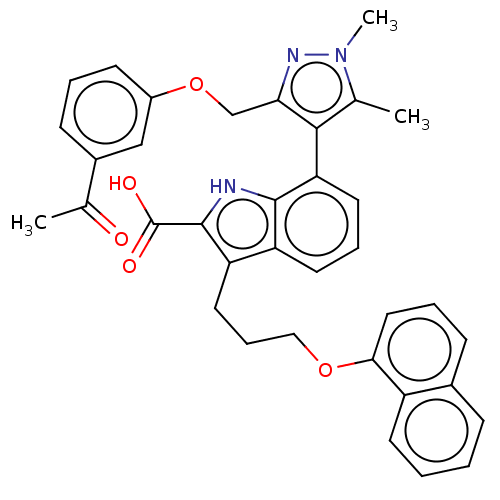 (7-(3-((3-Acetylphenoxy)methyl)-1,5-dimethyl-1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 64 | -40.9 | 237 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit | Assay Description TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... | Nat Chem Biol 12: 931-936 (2016) Article DOI: 10.1038/nchembio.2174 BindingDB Entry DOI: 10.7270/Q2FN1513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM203874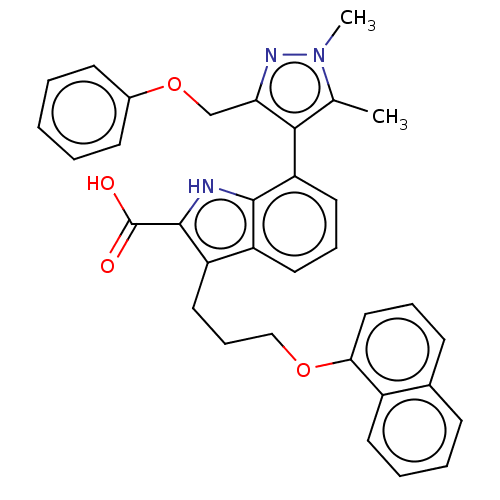 (Mcl-1 inhibitor 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 104 | -39.7 | 383 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit | Assay Description TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... | Nat Chem Biol 12: 931-936 (2016) Article DOI: 10.1038/nchembio.2174 BindingDB Entry DOI: 10.7270/Q2FN1513 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138200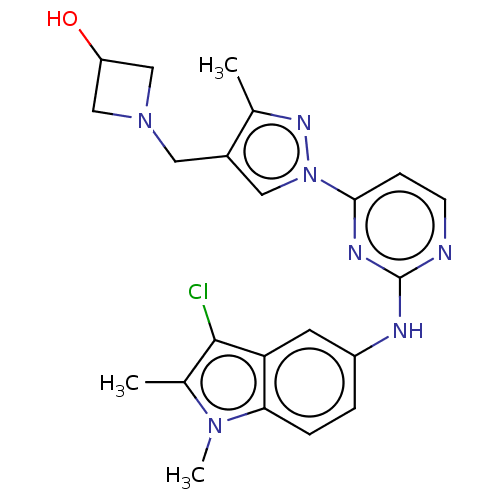 (US8871778, 141) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548361 (CHEMBL4744190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548350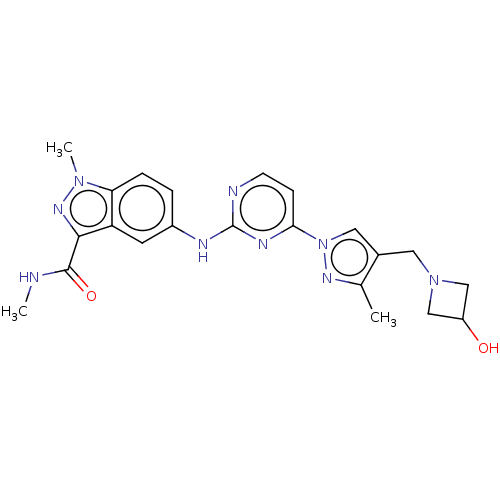 (CHEMBL4780257) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548354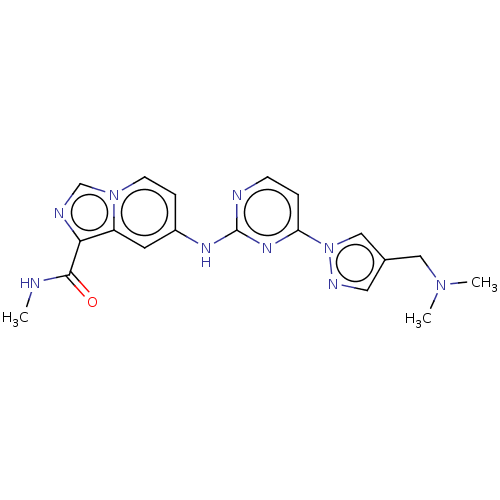 (CHEMBL4753469) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548358 (CHEMBL4753141) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548362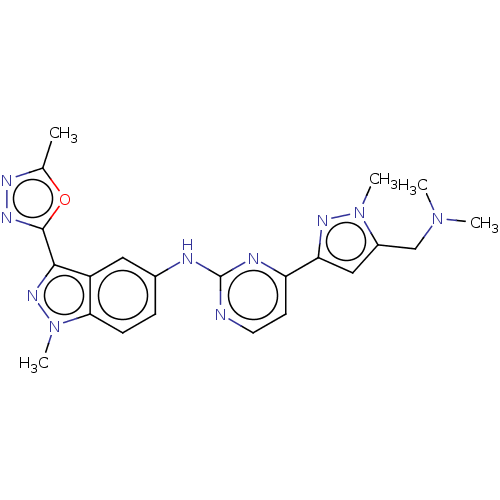 (CHEMBL4784088) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548357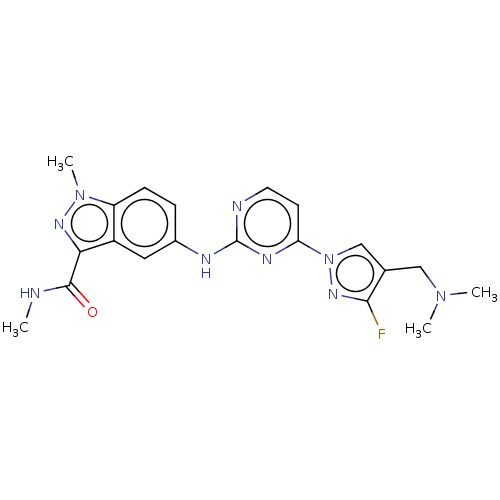 (CHEMBL4752008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548363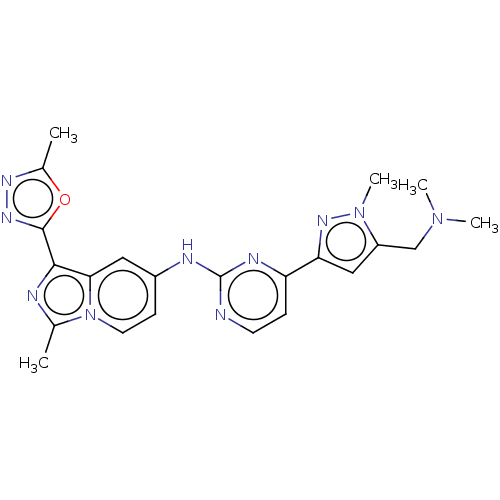 (CHEMBL4753164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548356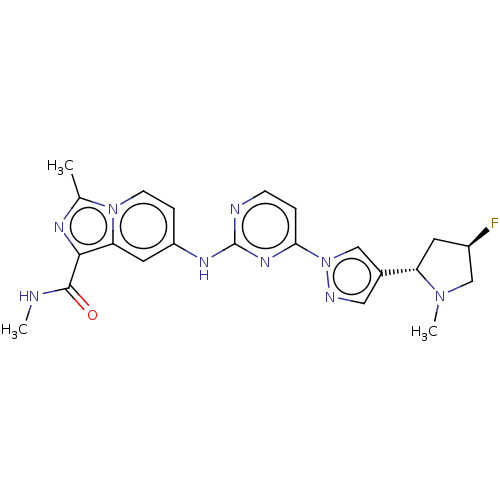 (CHEMBL4792651) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548351 (CHEMBL4786316) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548353 (CHEMBL4762290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548355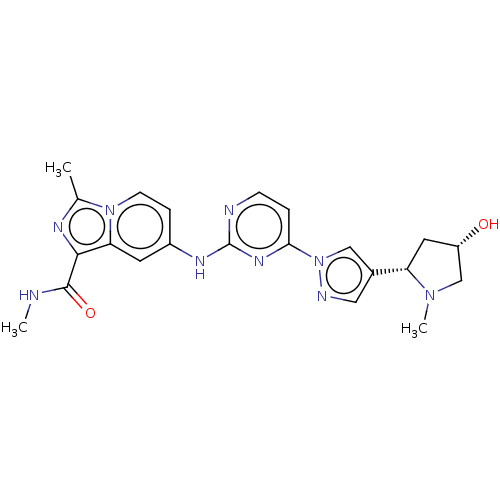 (CHEMBL4784783) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548360 (CHEMBL4790107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50546393 (CHEMBL4751083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548352 (CHEMBL4793844) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50548359 (CHEMBL4757235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length SYK (unknown origin) by biochemical Omnia assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127433 BindingDB Entry DOI: 10.7270/Q2C2511G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50270877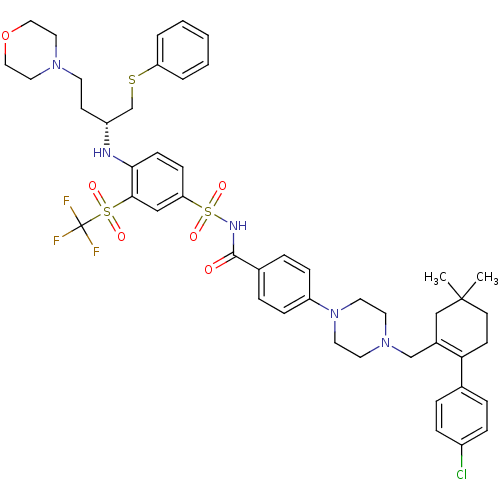 ((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal 6xHis-tagged Bcl-2 (M1 to F212 residues) expressed in Escherichia coli using HyLite Fluor 647-labeled BIM ... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126682 BindingDB Entry DOI: 10.7270/Q2P84GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264231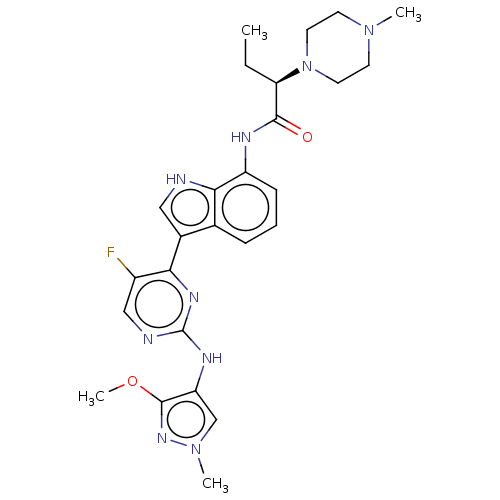 ((2R)-N-(3-{5-fluoro- 2-[(3-methoxy-1- methyl-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264233 ((2R)-N-(3-{2- [(1,3-dimethyl-1H- pyrazol-4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264233 ((2R)-N-(3-{2- [(1,3-dimethyl-1H- pyrazol-4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264231 ((2R)-N-(3-{5-fluoro- 2-[(3-methoxy-1- methyl-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264225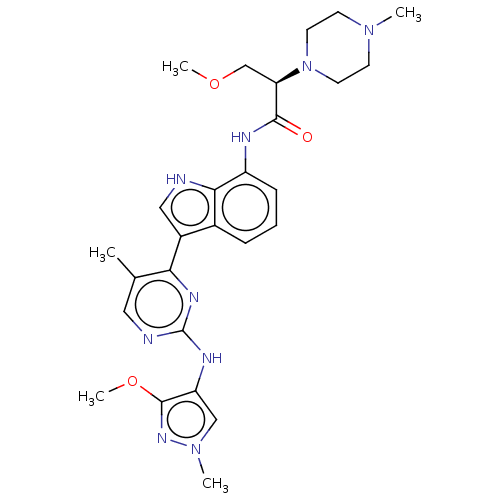 ((2R)-3-methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50288439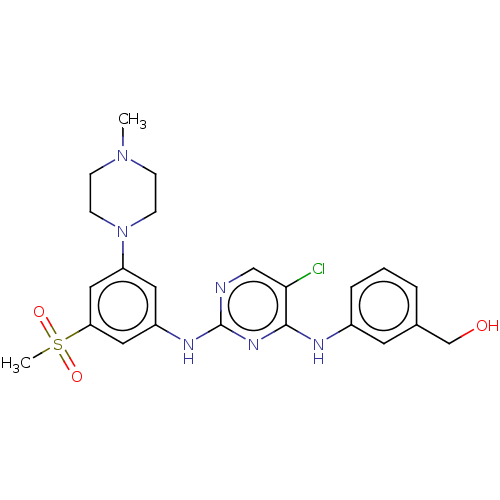 (CHEMBL4163817) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... | J Med Chem 61: 5235-5244 (2018) Article DOI: 10.1021/acs.jmedchem.8b00076 BindingDB Entry DOI: 10.7270/Q2JH3PP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50288372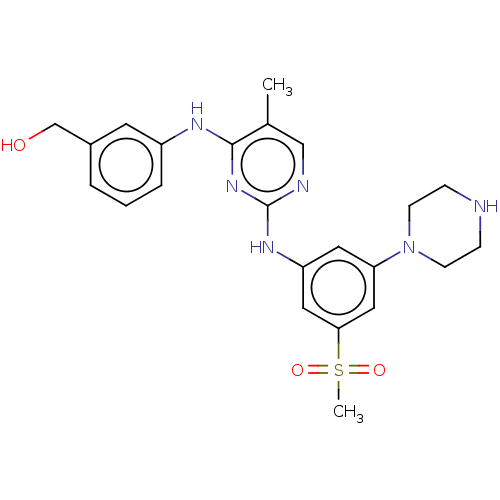 (CHEMBL4173429) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... | J Med Chem 61: 5235-5244 (2018) Article DOI: 10.1021/acs.jmedchem.8b00076 BindingDB Entry DOI: 10.7270/Q2JH3PP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50288378 (CHEMBL4162065) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description GRAC: human IMPase 1 inhibitor | J Med Chem 61: 5235-5244 (2018) Article DOI: 10.1021/acs.jmedchem.8b00076 BindingDB Entry DOI: 10.7270/Q2JH3PP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50288437 (CHEMBL4165758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description GRAC: human PDE2A selective inhibitor | J Med Chem 61: 5235-5244 (2018) Article DOI: 10.1021/acs.jmedchem.8b00076 BindingDB Entry DOI: 10.7270/Q2JH3PP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50288438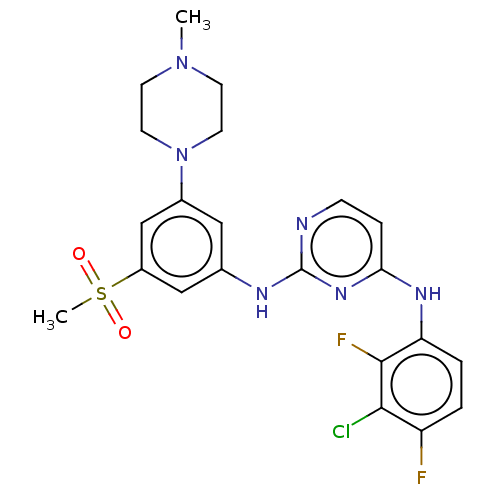 (CHEMBL4173676) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... | J Med Chem 61: 5235-5244 (2018) Article DOI: 10.1021/acs.jmedchem.8b00076 BindingDB Entry DOI: 10.7270/Q2JH3PP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50288511 (CHEMBL4171692) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... | J Med Chem 61: 5235-5244 (2018) Article DOI: 10.1021/acs.jmedchem.8b00076 BindingDB Entry DOI: 10.7270/Q2JH3PP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264225 ((2R)-3-methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50288443 (CHEMBL4166989) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... | J Med Chem 61: 5235-5244 (2018) Article DOI: 10.1021/acs.jmedchem.8b00076 BindingDB Entry DOI: 10.7270/Q2JH3PP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264240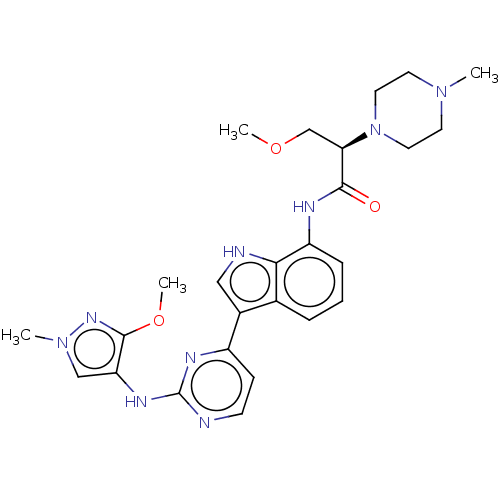 ((2R)-3-Methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264229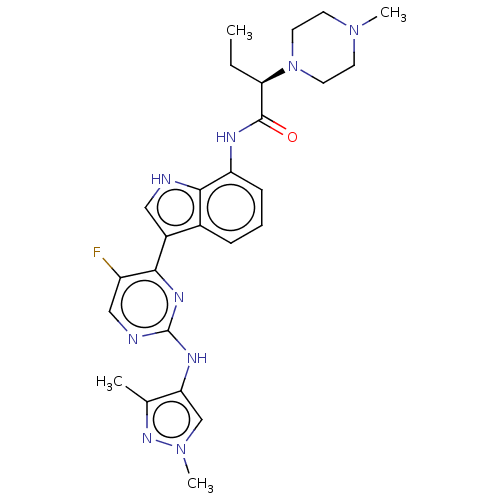 ((2R)-N-(3-{2-[(1,3- dimethyl-1H-pyrazol- 4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264224 ((2R)-N-(3-{2-[(3- Methoxy-1-methyl- 1H-pyrazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50288437 (CHEMBL4165758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description GRAC: human H3 selective antagonist | J Med Chem 61: 5235-5244 (2018) Article DOI: 10.1021/acs.jmedchem.8b00076 BindingDB Entry DOI: 10.7270/Q2JH3PP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264240 ((2R)-3-Methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264229 ((2R)-N-(3-{2-[(1,3- dimethyl-1H-pyrazol- 4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527916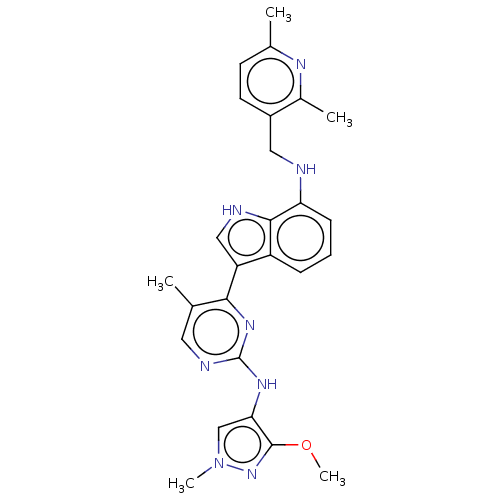 (CHEMBL4446962) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged JAK1 (866 to 1154 residues) expressed in insect cells using FITC-labeled C6-KKHTDDGYMPMSPGVA-NH... | J Med Chem 63: 4517-4527 (2020) Article DOI: 10.1021/acs.jmedchem.9b01392 BindingDB Entry DOI: 10.7270/Q21V5JDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264224 ((2R)-N-(3-{2-[(3- Methoxy-1-methyl- 1H-pyrazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264221 ((2R)-N-(3-{2-[(1,3- dimethyl-1H-pyrazol- 4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM313401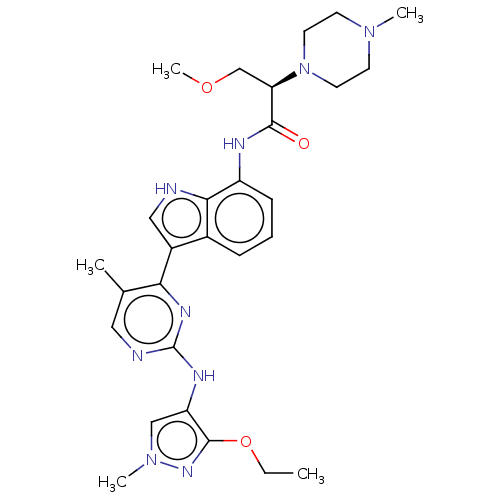 ((2R)-N-(3-{2-[(3-ethoxy-1-methyl-1H- pyrazol-4-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dizal (Jiangsu) Pharmaceutical Co., Ltd. US Patent | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acid... | US Patent US10654835 (2020) BindingDB Entry DOI: 10.7270/Q29C71F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50288442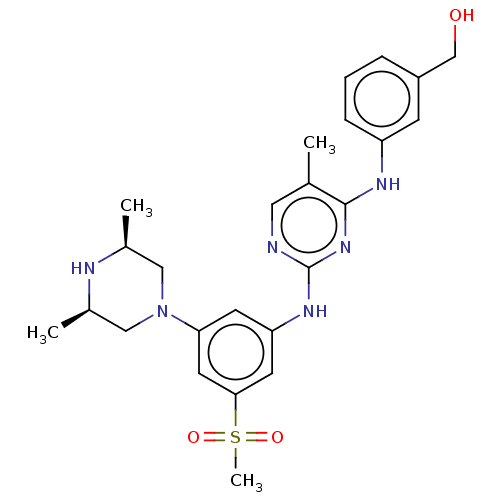 (CHEMBL4173835) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cytoplasmic recombinant human GST-tagged JAK1 (886 to 1154 residues) expressed in insect cells using FITC-C6-KKHTDDGYMPMSPGVA-NH as sub... | J Med Chem 61: 5235-5244 (2018) Article DOI: 10.1021/acs.jmedchem.8b00076 BindingDB Entry DOI: 10.7270/Q2JH3PP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM264221 ((2R)-N-(3-{2-[(1,3- dimethyl-1H-pyrazol- 4-yl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... | Citation and Details BindingDB Entry DOI: 10.7270/Q29G5R1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 657 total ) | Next | Last >> |