 Found 21306 hits with Last Name = 'gu' and Initial = 'x'
Found 21306 hits with Last Name = 'gu' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM82547
(SRIF-D-Trp8)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55+,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Research in Biomedicine (IRB Barcelona)
Curated by ChEMBL
| Assay Description
Displacement of [125I]-somatostatin from human SSTR2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 24: 103-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.065
BindingDB Entry DOI: 10.7270/Q24X5BR2 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767
(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Research in Biomedicine (IRB Barcelona)
Curated by ChEMBL
| Assay Description
Displacement of [125I]-somatostatin from human SSTR2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 24: 103-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.065
BindingDB Entry DOI: 10.7270/Q24X5BR2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50366764

(CHEMBL1790045 | MCL-117)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC#C)c2c1 Show InChI InChI=1S/C19H23NO/c1-2-10-20-11-9-19-8-4-3-5-16(19)18(20)12-14-6-7-15(21)13-17(14)19/h1,6-7,13,16,18,21H,3-5,8-12H2/t16-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards guinea pig Opioid receptor kappa 1 using radioligand [3H]U-69593 |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50366764

(CHEMBL1790045 | MCL-117)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC#C)c2c1 Show InChI InChI=1S/C19H23NO/c1-2-10-20-11-9-19-8-4-3-5-16(19)18(20)12-14-6-7-15(21)13-17(14)19/h1,6-7,13,16,18,21H,3-5,8-12H2/t16-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50451302

(CHEMBL2115245)Show SMILES [H][C@@]12Cc3cc(O)ccc3[C@]3(CCCC[C@@]13[H])CCN2C\C=C\I |THB:20:19:15:9.3.2| Show InChI InChI=1S/C19H24INO/c20-9-3-10-21-11-8-19-7-2-1-4-17(19)18(21)13-14-12-15(22)5-6-16(14)19/h3,5-6,9,12,17-18,22H,1-2,4,7-8,10-11,13H2/b9-3+/t17-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602306
(CHEMBL5208487)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295665
((S)-2-((2-((S)-4-(difluoromethyl)- 2-oxooxazolidin...)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C(N)=O |r| Show InChI InChI=1S/C18H19F2N5O4/c1-9(16(21)26)22-10-2-3-11-13(6-10)28-5-4-24-7-14(23-17(11)24)25-12(15(19)20)8-29-18(25)27/h2-3,6-7,9,12,15,22H,4-5,8H2,1H3,(H2,21,26)/t9-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50451302

(CHEMBL2115245)Show SMILES [H][C@@]12Cc3cc(O)ccc3[C@]3(CCCC[C@@]13[H])CCN2C\C=C\I |THB:20:19:15:9.3.2| Show InChI InChI=1S/C19H24INO/c20-9-3-10-21-11-8-19-7-2-1-4-17(19)18(21)13-14-12-15(22)5-6-16(14)19/h3,5-6,9,12,17-18,22H,1-2,4,7-8,10-11,13H2/b9-3+/t17-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards guinea pig Opioid receptor kappa 1 using radioligand [3H]U-69593 |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602320
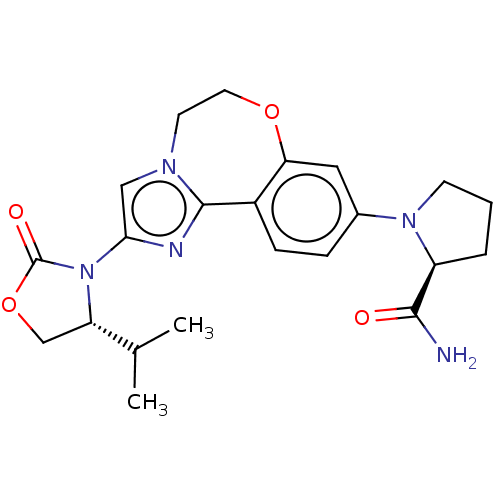
(CHEMBL5199631)Show SMILES CC(C)[C@@H]1COC(=O)N1c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602324
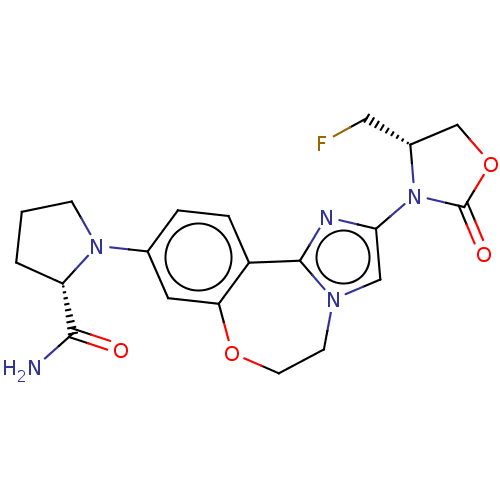
(CHEMBL5198796)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@H](CF)COC1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50362366
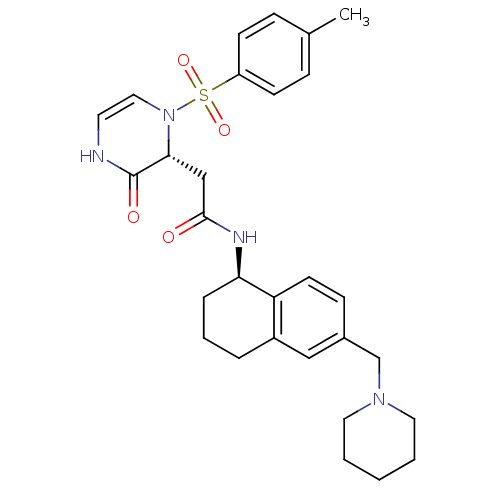
(CHEMBL1939755)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1C=CNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r,c:12| Show InChI InChI=1S/C29H36N4O4S/c1-21-8-11-24(12-9-21)38(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-23-18-22(10-13-25(23)26)20-32-15-3-2-4-16-32/h8-14,17-18,26-27H,2-7,15-16,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1061-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.112
BindingDB Entry DOI: 10.7270/Q22J6CB2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50331515
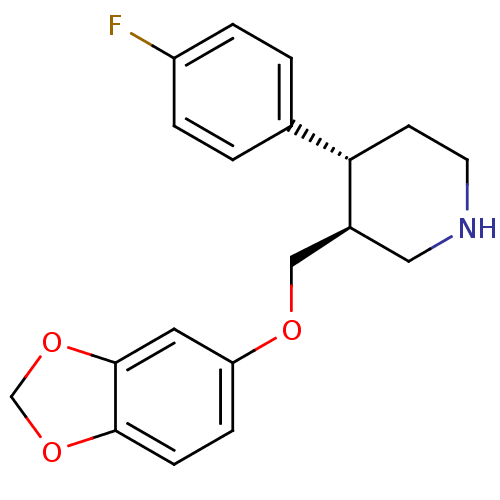
(CHEMBL1708 | PAROXETINE | Paroxetine hydrochloride...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from serotonin transporter in Sprague-Dawley rat brain after 1 hr by liquid scintillation counting |
Bioorg Med Chem 18: 8356-64 (2010)
Article DOI: 10.1016/j.bmc.2010.09.060
BindingDB Entry DOI: 10.7270/Q2CN744P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135806
(CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...)Show SMILES O=C(CCCCCCCCC(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 Show InChI InChI=1S/C52H72N2O4/c55-49(57-41-23-21-39-31-47-43-17-7-9-25-51(43,45(39)33-41)27-29-53(47)35-37-13-11-14-37)19-5-3-1-2-4-6-20-50(56)58-42-24-22-40-32-48-44-18-8-10-26-52(44,46(40)34-42)28-30-54(48)36-38-15-12-16-38/h21-24,33-34,37-38,43-44,47-48H,1-20,25-32,35-36H2/t43-,44-,47+,48+,51+,52+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295669
((S)-2-cyclopropyl-2-((2-((S)-4- (fluoromethyl)-2-o...)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@H](CF)COC1=O)C1CC1 |r| Show InChI InChI=1S/C20H22FN5O4/c21-8-13-10-30-20(28)26(13)16-9-25-5-6-29-15-7-12(3-4-14(15)19(25)24-16)23-17(18(22)27)11-1-2-11/h3-4,7,9,11,13,17,23H,1-2,5-6,8,10H2,(H2,22,27)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50192018

(CHEMBL3350037)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H](CO)[C@@H](C)O)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O |r| Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29-,34-,36+,37+,38-,39+,40+,41+,42+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Research in Biomedicine (IRB Barcelona)
Curated by ChEMBL
| Assay Description
Displacement of [125I]-somatostatin from human SSTR2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 24: 103-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.065
BindingDB Entry DOI: 10.7270/Q24X5BR2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602323
(CHEMBL5209168)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor kappa 1 in guinea pig brain membranes using [3H]U-69593 as radioligand |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM475607
(US10851091, Compound 103)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C1CC1 |r| Show InChI InChI=1S/C20H21F2N5O4/c21-17(22)13-9-31-20(29)27(13)15-8-26-5-6-30-14-7-11(3-4-12(14)19(26)25-15)24-16(18(23)28)10-1-2-10/h3-4,7-8,10,13,16-17,24H,1-2,5-6,9H2,(H2,23,28)/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor mu 1 in chinese Hamster Ovary (CHO) cells membranes was determined using [3H]-DAMGO radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50106857
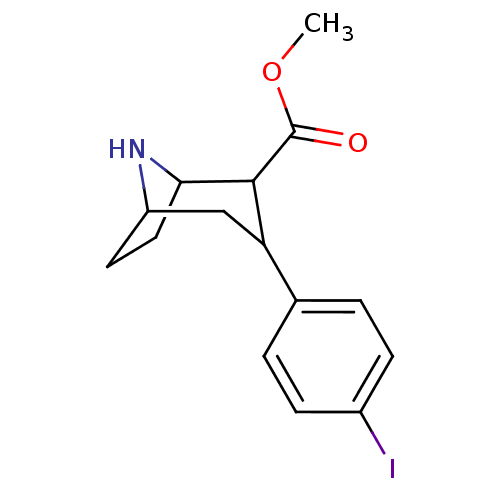
(3-(4-Iodo-phenyl)-8-aza-bicyclo[3.2.1]octane-2-car...)Show SMILES COC(=O)C1C2CCC(CC1c1ccc(I)cc1)N2 |THB:2:4:18:6.7| Show InChI InChI=1S/C15H18INO2/c1-19-15(18)14-12(8-11-6-7-13(14)17-11)9-2-4-10(16)5-3-9/h2-5,11-14,17H,6-8H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcohol and Drug Abuse Research Center
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- paroxetine from Serotonin transporter in rat cerebral cortical homogenate |
Bioorg Med Chem Lett 11: 3049-53 (2001)
BindingDB Entry DOI: 10.7270/Q23N22PN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602305
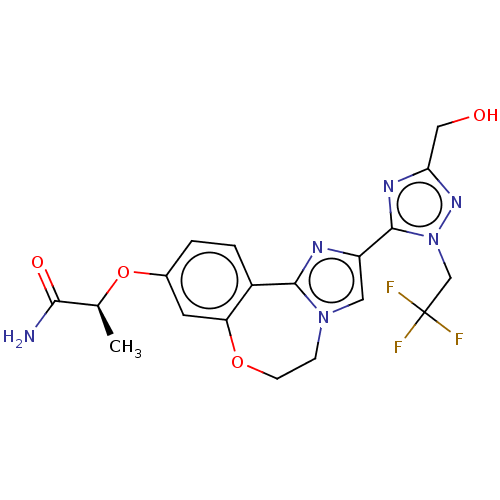
(CHEMBL5209048)Show SMILES C[C@H](Oc1ccc2-c3nc(cn3CCOc2c1)-c1nc(CO)nn1CC(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50135808
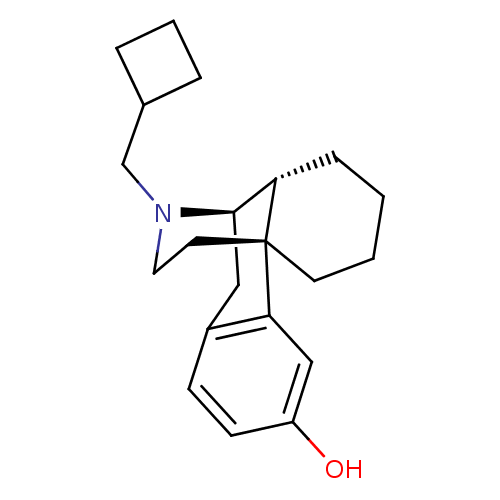
((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 |r,TLB:16:15:4.21.5:7| Show InChI InChI=1S/C21H29NO/c23-17-8-7-16-12-20-18-6-1-2-9-21(18,19(16)13-17)10-11-22(20)14-15-4-3-5-15/h7-8,13,15,18,20,23H,1-6,9-12,14H2/t18-,20+,21+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135796
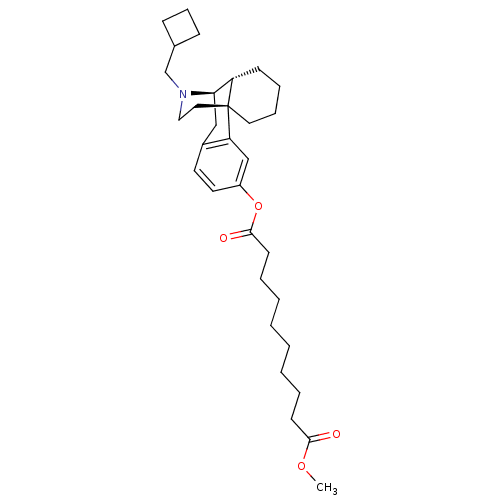
(1-[17-cyclobutylmethyl-(1R,9R,10R)-17-azatetracycl...)Show SMILES COC(=O)CCCCCCCCC(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 Show InChI InChI=1S/C32H47NO4/c1-36-30(34)14-6-4-2-3-5-7-15-31(35)37-26-17-16-25-21-29-27-13-8-9-18-32(27,28(25)22-26)19-20-33(29)23-24-11-10-12-24/h16-17,22,24,27,29H,2-15,18-21,23H2,1H3/t27-,29+,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135797
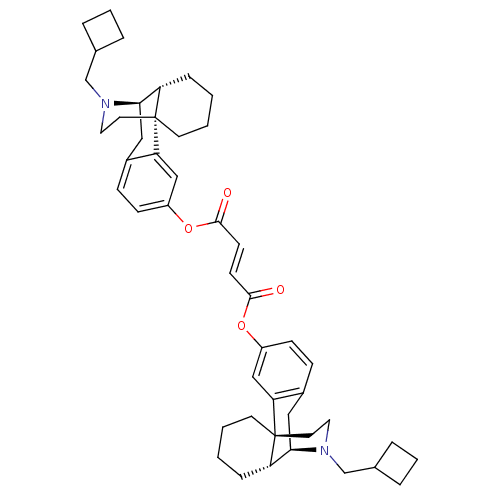
(CHEMBL146756 | di[17-cyclobutylmethyl-(1R,9R,10R)-...)Show SMILES O=C(Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1)\C=C\C(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 Show InChI InChI=1S/C46H58N2O4/c49-43(51-35-15-13-33-25-41-37-11-1-3-19-45(37,39(33)27-35)21-23-47(41)29-31-7-5-8-31)17-18-44(50)52-36-16-14-34-26-42-38-12-2-4-20-46(38,40(34)28-36)22-24-48(42)30-32-9-6-10-32/h13-18,27-28,31-32,37-38,41-42H,1-12,19-26,29-30H2/b18-17+/t37-,38-,41+,42+,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135798
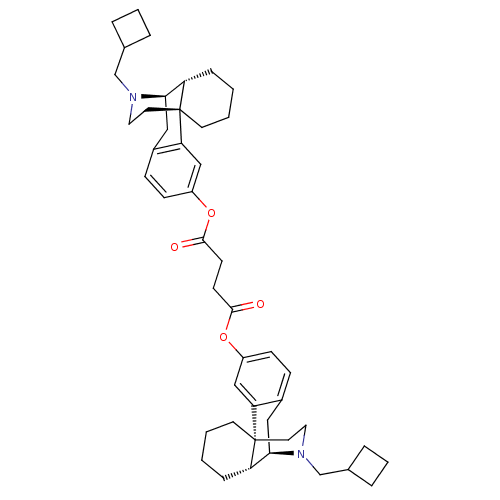
(CHEMBL433697 | di[17-cyclobutylmethyl-(10R)-17-aza...)Show SMILES O=C(CCC(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 Show InChI InChI=1S/C46H60N2O4/c49-43(51-35-15-13-33-25-41-37-11-1-3-19-45(37,39(33)27-35)21-23-47(41)29-31-7-5-8-31)17-18-44(50)52-36-16-14-34-26-42-38-12-2-4-20-46(38,40(34)28-36)22-24-48(42)30-32-9-6-10-32/h13-16,27-28,31-32,37-38,41-42H,1-12,17-26,29-30H2/t37-,38-,41+,42+,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135808

((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 |r,TLB:16:15:4.21.5:7| Show InChI InChI=1S/C21H29NO/c23-17-8-7-16-12-20-18-6-1-2-9-21(18,19(16)13-17)10-11-22(20)14-15-4-3-5-15/h7-8,13,15,18,20,23H,1-6,9-12,14H2/t18-,20+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50105498
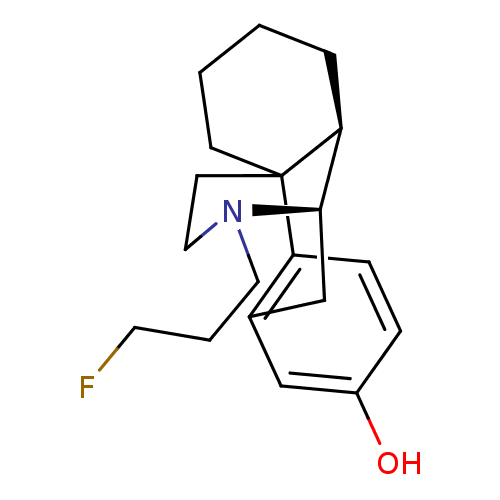
(17-(3-fluoropropyl)-(1R,9R,10R)-17-azatetracyclo[7...)Show SMILES Oc1ccc2c(C[C@@H]3[C@@H]4CCCCC24CCN3CCCF)c1 |THB:17:16:8:4.5.6| Show InChI InChI=1S/C19H26FNO/c20-9-3-10-21-11-8-19-7-2-1-4-17(19)18(21)13-14-12-15(22)5-6-16(14)19/h5-6,12,17-18,22H,1-4,7-11,13H2/t17-,18+,19?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor kappa 1 in guinea pig brain membranes using [3H]U-69593 as radioligand |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806
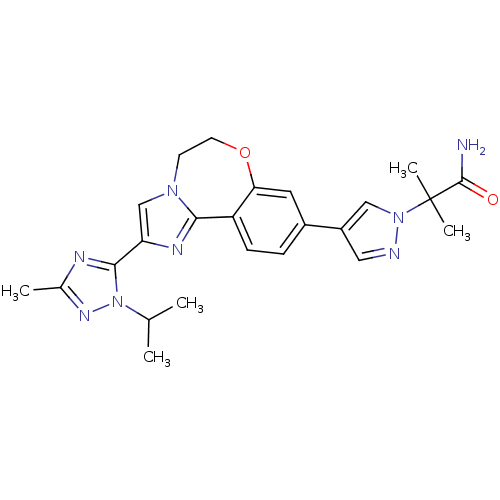
(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50135806

(CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...)Show SMILES O=C(CCCCCCCCC(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 Show InChI InChI=1S/C52H72N2O4/c55-49(57-41-23-21-39-31-47-43-17-7-9-25-51(43,45(39)33-41)27-29-53(47)35-37-13-11-14-37)19-5-3-1-2-4-6-20-50(56)58-42-24-22-40-32-48-44-18-8-10-26-52(44,46(40)34-42)28-30-54(48)36-38-15-12-16-38/h21-24,33-34,37-38,43-44,47-48H,1-20,25-32,35-36H2/t43-,44-,47+,48+,51+,52+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor mu 1 in chinese Hamster Ovary (CHO) cells membranes was determined using [3H]-DAMGO radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50105477

(17-(2-methoxyethyl)-(1R,9R,10R)-17-azatetracyclo[7...)Show SMILES COCCN1CCC23CCCC[C@H]2[C@H]1Cc1cc(O)ccc31 |TLB:3:4:12:21.15.14| Show InChI InChI=1S/C19H27NO2/c1-22-11-10-20-9-8-19-7-3-2-4-17(19)18(20)13-14-12-15(21)5-6-16(14)19/h5-6,12,17-18,21H,2-4,7-11,13H2,1H3/t17-,18+,19?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards guinea pig Opioid receptor kappa 1 using radioligand [3H]U-69593 |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602328
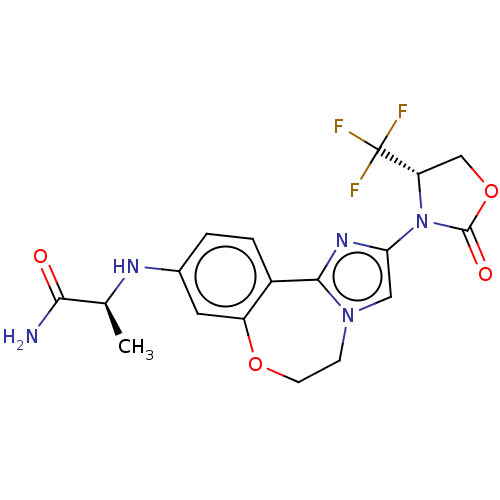
(CHEMBL5205438)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602326
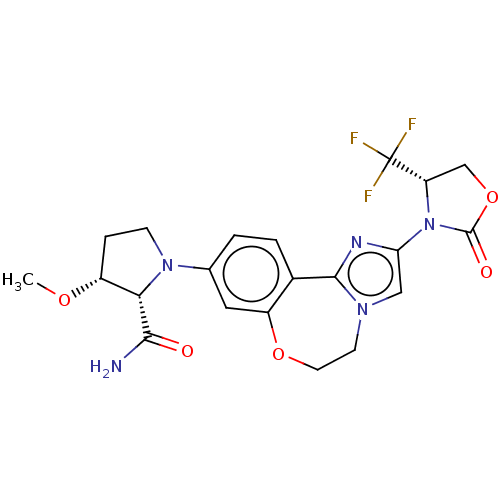
(CHEMBL5181348)Show SMILES CO[C@@H]1CCN([C@@H]1C(N)=O)c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602331
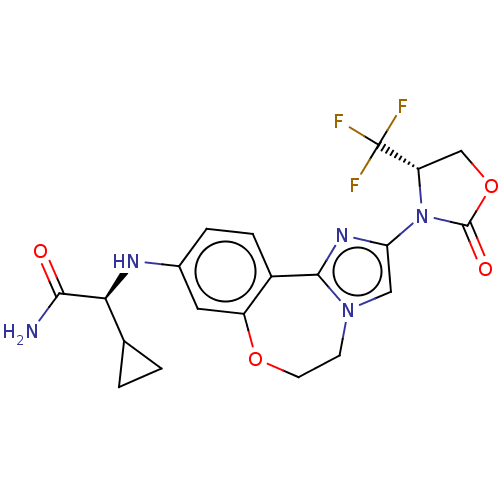
(CHEMBL5182371)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135801

(CHEMBL413512 | di[17-cyclopropylmethyl-(10R)-17-az...)Show SMILES O=C(CCC(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C44H56N2O4/c47-41(49-33-13-11-31-23-39-35-5-1-3-17-43(35,37(31)25-33)19-21-45(39)27-29-7-8-29)15-16-42(48)50-34-14-12-32-24-40-36-6-2-4-18-44(36,38(32)26-34)20-22-46(40)28-30-9-10-30/h11-14,25-26,29-30,35-36,39-40H,1-10,15-24,27-28H2/t35-,36-,39+,40+,43+,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50163035
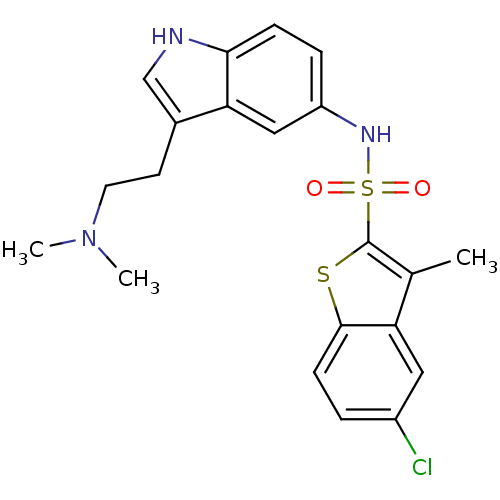
(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic aci...)Show SMILES CN(C)CCc1c[nH]c2ccc(NS(=O)(=O)c3sc4ccc(Cl)cc4c3C)cc12 Show InChI InChI=1S/C21H22ClN3O2S2/c1-13-17-10-15(22)4-7-20(17)28-21(13)29(26,27)24-16-5-6-19-18(11-16)14(12-23-19)8-9-25(2)3/h4-7,10-12,23-24H,8-9H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve S.A.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LSD binding to human 5-hydroxytryptamine 6 receptor expressed in HEK293 cells |
J Med Chem 48: 1781-95 (2005)
Article DOI: 10.1021/jm049615n
BindingDB Entry DOI: 10.7270/Q2ZP45N9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602304
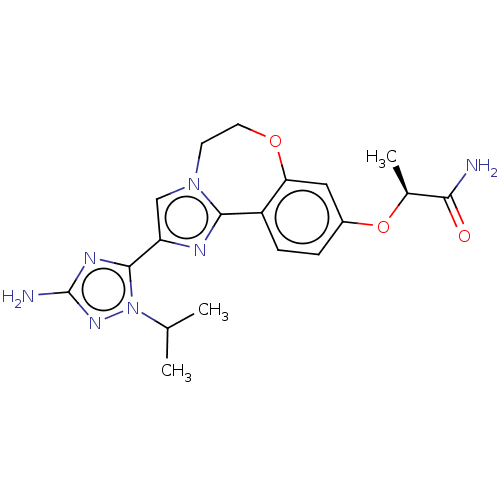
(CHEMBL5182339)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50105477

(17-(2-methoxyethyl)-(1R,9R,10R)-17-azatetracyclo[7...)Show SMILES COCCN1CCC23CCCC[C@H]2[C@H]1Cc1cc(O)ccc31 |TLB:3:4:12:21.15.14| Show InChI InChI=1S/C19H27NO2/c1-22-11-10-20-9-8-19-7-3-2-4-17(19)18(20)13-14-12-15(21)5-6-16(14)19/h5-6,12,17-18,21H,2-4,7-11,13H2,1H3/t17-,18+,19?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50135808

((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 |r,TLB:16:15:4.21.5:7| Show InChI InChI=1S/C21H29NO/c23-17-8-7-16-12-20-18-6-1-2-9-21(18,19(16)13-17)10-11-22(20)14-15-4-3-5-15/h7-8,13,15,18,20,23H,1-6,9-12,14H2/t18-,20+,21+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 in guinea pig brain membranes using [3H]naltrindole as radioligand |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50105496
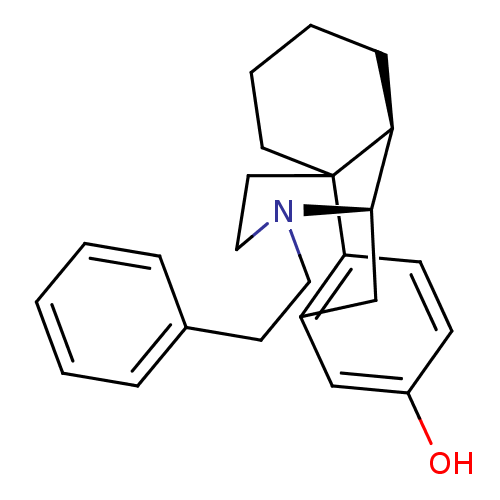
(17-phenethyl-(1R,9R,10R)-17-azatetracyclo[7.5.3.01...)Show SMILES Oc1ccc2c(C[C@@H]3[C@@H]4CCCCC24CCN3CCc2ccccc2)c1 |THB:17:16:8:4.5.6| Show InChI InChI=1S/C24H29NO/c26-20-9-10-21-19(16-20)17-23-22-8-4-5-12-24(21,22)13-15-25(23)14-11-18-6-2-1-3-7-18/h1-3,6-7,9-10,16,22-23,26H,4-5,8,11-15,17H2/t22-,23+,24?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50105474
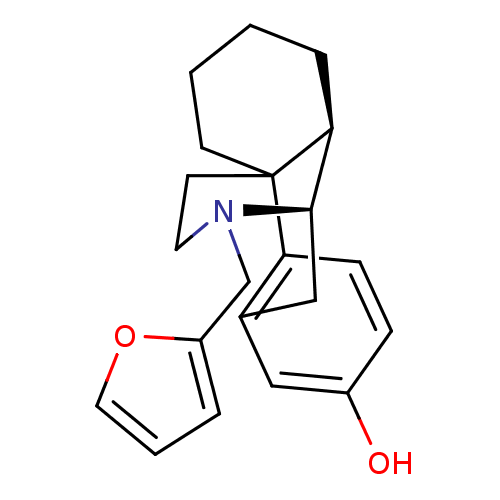
(17-(2-furylmethyl)-(1R,9R,10R)-17-azatetracyclo[7....)Show SMILES Oc1ccc2c(C[C@@H]3[C@@H]4CCCCC24CCN3Cc2ccco2)c1 |THB:17:16:8:4.5.6| Show InChI InChI=1S/C21H25NO2/c23-16-6-7-18-15(12-16)13-20-19-5-1-2-8-21(18,19)9-10-22(20)14-17-4-3-11-24-17/h3-4,6-7,11-12,19-20,23H,1-2,5,8-10,13-14H2/t19-,20+,21?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards guinea pig Opioid receptor kappa 1 using radioligand [3H]U-69593 |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50135801

(CHEMBL413512 | di[17-cyclopropylmethyl-(10R)-17-az...)Show SMILES O=C(CCC(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C44H56N2O4/c47-41(49-33-13-11-31-23-39-35-5-1-3-17-43(35,37(31)25-33)19-21-45(39)27-29-7-8-29)15-16-42(48)50-34-14-12-32-24-40-36-6-2-4-18-44(36,38(32)26-34)20-22-46(40)28-30-9-10-30/h11-14,25-26,29-30,35-36,39-40H,1-10,15-24,27-28H2/t35-,36-,39+,40+,43+,44+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor mu 1 in chinese Hamster Ovary (CHO) cells membranes was determined using [3H]-DAMGO radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602321
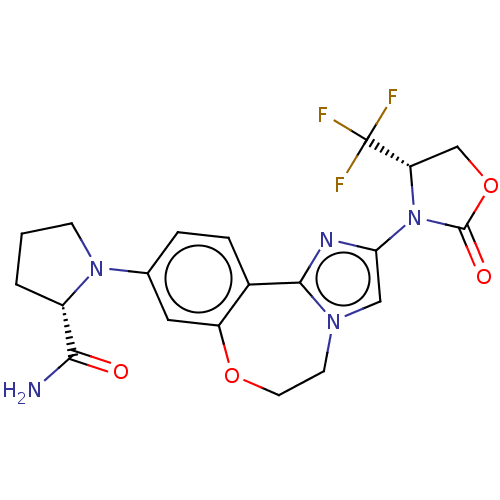
(CHEMBL5202305)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602329
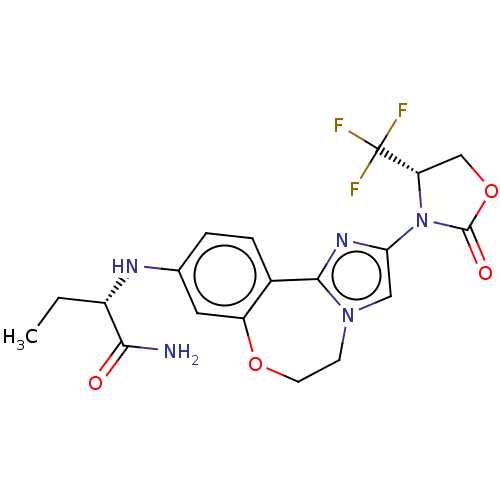
(CHEMBL5189517)Show SMILES CC[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50135798

(CHEMBL433697 | di[17-cyclobutylmethyl-(10R)-17-aza...)Show SMILES O=C(CCC(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 Show InChI InChI=1S/C46H60N2O4/c49-43(51-35-15-13-33-25-41-37-11-1-3-19-45(37,39(33)27-35)21-23-47(41)29-31-7-5-8-31)17-18-44(50)52-36-16-14-34-26-42-38-12-2-4-20-46(38,40(34)28-36)22-24-48(42)30-32-9-6-10-32/h13-16,27-28,31-32,37-38,41-42H,1-12,17-26,29-30H2/t37-,38-,41+,42+,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Opioid receptor mu 1 in chinese Hamster Ovary (CHO) cells membranes was determined using [3H]-DAMGO radioligand |
J Med Chem 46: 5162-70 (2003)
Article DOI: 10.1021/jm030139v
BindingDB Entry DOI: 10.7270/Q2GX4C9C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50105491

(17-allyl-(1R,9R,10R)-17-azatetracyclo[7.5.3.01,10....)Show SMILES Oc1ccc2c(C[C@@H]3[C@@H]4CCCCC24CCN3CC=C)c1 |THB:17:16:8:4.5.6| Show InChI InChI=1S/C19H25NO/c1-2-10-20-11-9-19-8-4-3-5-17(19)18(20)13-14-12-15(21)6-7-16(14)19/h2,6-7,12,17-18,21H,1,3-5,8-11,13H2/t17-,18+,19?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Binding affinity towards guinea pig Opioid receptor kappa 1 using radioligand [3H]U-69593 |
Bioorg Med Chem Lett 11: 2735-40 (2001)
BindingDB Entry DOI: 10.7270/Q25T3M01 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344100
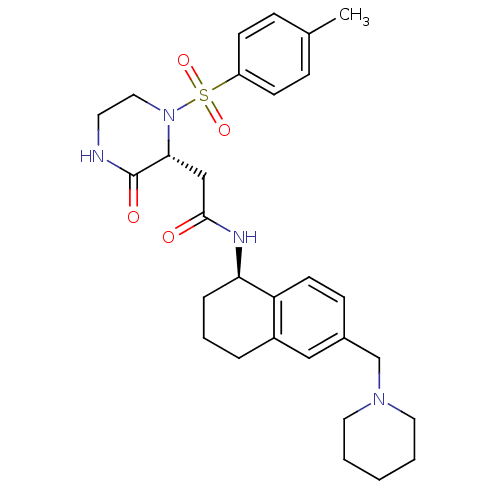
(2-((2R)-1-((4-methylphenyl)sulfonyl)-3-oxo-2-piper...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O4S/c1-21-8-11-24(12-9-21)38(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-23-18-22(10-13-25(23)26)20-32-15-3-2-4-16-32/h8-13,18,26-27H,2-7,14-17,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1061-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.112
BindingDB Entry DOI: 10.7270/Q22J6CB2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data