

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relaxin-3 receptor 1 (Homo sapiens (Human)) | BDBM50605987 (CHEMBL5185575) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00508 BindingDB Entry DOI: 10.7270/Q2S186MP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 1 (Homo sapiens (Human)) | BDBM50581019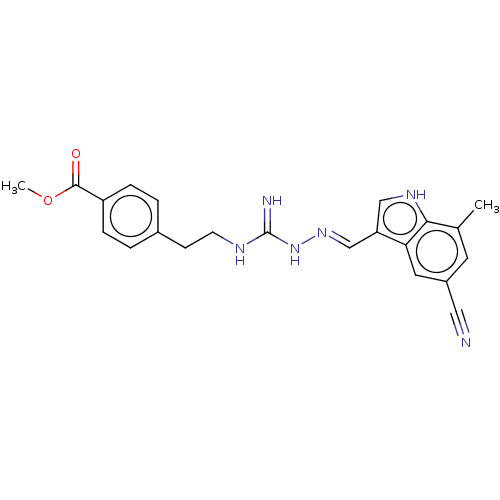 (CHEMBL5089949) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]R3/I5 from human RXFP3 expressed in human CHO-K1 cells incubated for 1 hr by microplate scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01081 BindingDB Entry DOI: 10.7270/Q2ZK5MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 1 (Homo sapiens (Human)) | BDBM50581009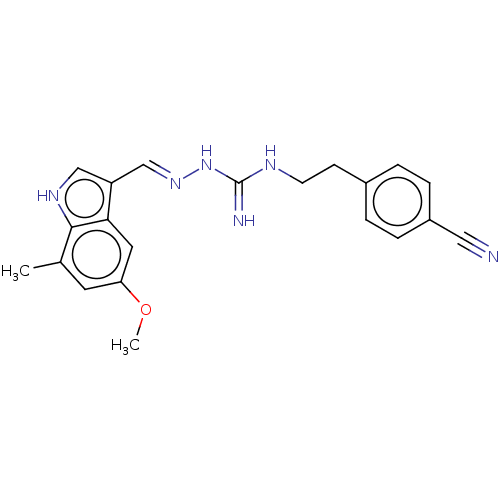 (CHEMBL5094670) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]R3/I5 from human RXFP3 expressed in human CHO-K1 cells incubated for 1 hr by microplate scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01081 BindingDB Entry DOI: 10.7270/Q2ZK5MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 1 (Homo sapiens (Human)) | BDBM50526409 (CHEMBL4522365) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 274 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]R3/I5 from human RXFP3 expressed in human CHO-K1 cells incubated for 1 hr by microplate scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01081 BindingDB Entry DOI: 10.7270/Q2ZK5MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin-3 receptor 1 (Homo sapiens (Human)) | BDBM50526408 (CHEMBL4586446) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]R3/I5 from human RXFP3 expressed in human CHO-K1 cells incubated for 1 hr by microplate scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01081 BindingDB Entry DOI: 10.7270/Q2ZK5MJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50606009 (CHEMBL5207128) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00508 BindingDB Entry DOI: 10.7270/Q2S186MP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM357253 ((5-Amino-1-(2-cyclopropyl-1H-benzo[d]imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The following assay was used to determine the inhibition rate of the preferred compounds of the present invention to the kinase activity of the recom... | US Patent US10214515 (2019) BindingDB Entry DOI: 10.7270/Q22J6F46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKA (unknown origin) by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128409 BindingDB Entry DOI: 10.7270/Q2CZ3C28 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443226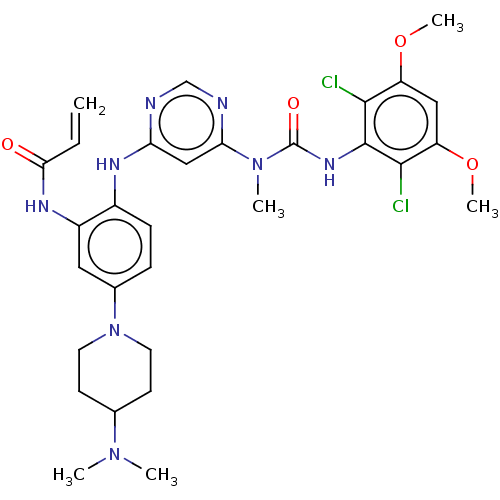 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The following method was used to determine the inhibition degree of the kinase activity of recombinant human FGFR protein by the compounds of the pre... | US Patent US11001572 (2021) BindingDB Entry DOI: 10.7270/Q2Z322RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443226 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The experimental procedure is briefly described as follows: the test compound was first dissolved in DMSO to prepare a stock solution, and then gradi... | US Patent US10654836 (2020) BindingDB Entry DOI: 10.7270/Q25M68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443223 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The experimental procedure is briefly described as follows: the test compound was first dissolved in DMSO to prepare a stock solution, and then gradi... | US Patent US10654836 (2020) BindingDB Entry DOI: 10.7270/Q25M68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443223 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The following method was used to determine the inhibition degree of the kinase activity of recombinant human FGFR protein by the compounds of the pre... | US Patent US11001572 (2021) BindingDB Entry DOI: 10.7270/Q2Z322RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Mus musculus (Mouse)) | BDBM109275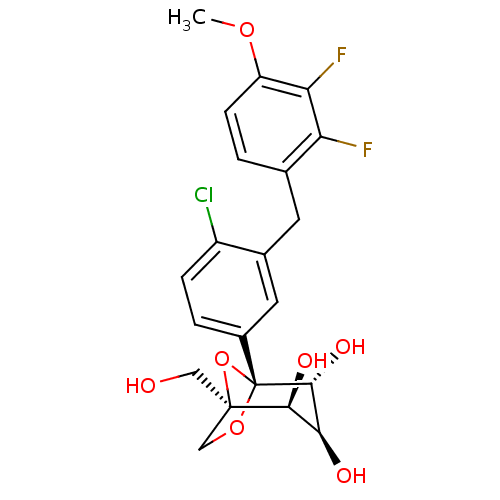 (US8609622, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.42 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd.; Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description Inhibitory activity of the compounds using SGLT1 and SGLT2. | US Patent US8609622 (2013) BindingDB Entry DOI: 10.7270/Q2BC3X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Mus musculus (Mouse)) | BDBM109278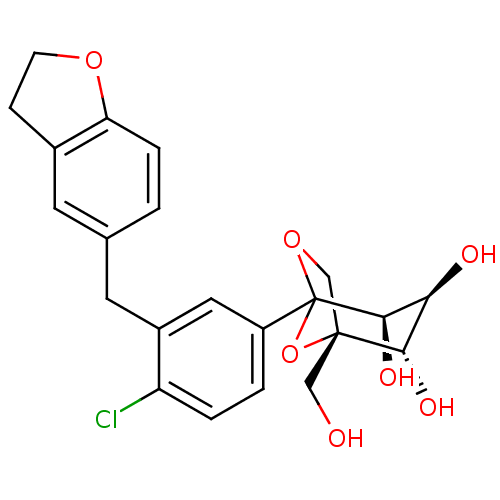 (US8609622, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd.; Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description Inhibitory activity of the compounds using SGLT1 and SGLT2. | US Patent US8609622 (2013) BindingDB Entry DOI: 10.7270/Q2BC3X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443222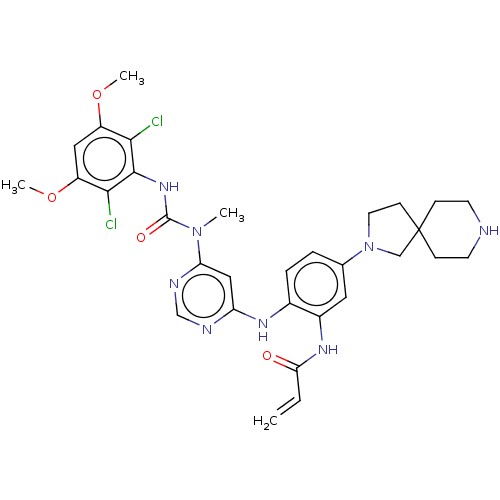 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The following method was used to determine the inhibition degree of the kinase activity of recombinant human FGFR protein by the compounds of the pre... | US Patent US11001572 (2021) BindingDB Entry DOI: 10.7270/Q2Z322RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443222 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The experimental procedure is briefly described as follows: the test compound was first dissolved in DMSO to prepare a stock solution, and then gradi... | US Patent US10654836 (2020) BindingDB Entry DOI: 10.7270/Q25M68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443225 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The following method was used to determine the inhibition degree of the kinase activity of recombinant human FGFR protein by the compounds of the pre... | US Patent US11001572 (2021) BindingDB Entry DOI: 10.7270/Q2Z322RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443225 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The experimental procedure is briefly described as follows: the test compound was first dissolved in DMSO to prepare a stock solution, and then gradi... | US Patent US10654836 (2020) BindingDB Entry DOI: 10.7270/Q25M68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443224 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The experimental procedure is briefly described as follows: the test compound was first dissolved in DMSO to prepare a stock solution, and then gradi... | US Patent US10654836 (2020) BindingDB Entry DOI: 10.7270/Q25M68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443224 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The following method was used to determine the inhibition degree of the kinase activity of recombinant human FGFR protein by the compounds of the pre... | US Patent US11001572 (2021) BindingDB Entry DOI: 10.7270/Q2Z322RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443227 (N-(5-(4-Cyclopropylpiperazin-1-yl)-2-((6-(3-(2,6-d...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The experimental procedure is briefly described as follows: the test compound was first dissolved in DMSO to prepare a stock solution, and then gradi... | US Patent US10654836 (2020) BindingDB Entry DOI: 10.7270/Q25M68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443227 (N-(5-(4-Cyclopropylpiperazin-1-yl)-2-((6-(3-(2,6-d...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The following method was used to determine the inhibition degree of the kinase activity of recombinant human FGFR protein by the compounds of the pre... | US Patent US11001572 (2021) BindingDB Entry DOI: 10.7270/Q2Z322RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM357251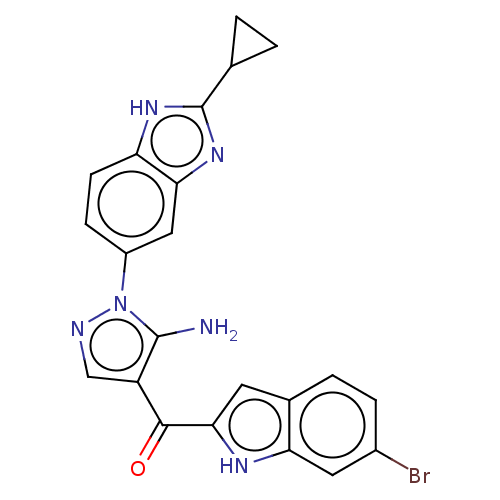 ((5-Amino-1-(2-cyclopropyl-1H-benzo[d]imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The following assay was used to determine the inhibition rate of the preferred compounds of the present invention to the kinase activity of the recom... | US Patent US10214515 (2019) BindingDB Entry DOI: 10.7270/Q22J6F46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM520084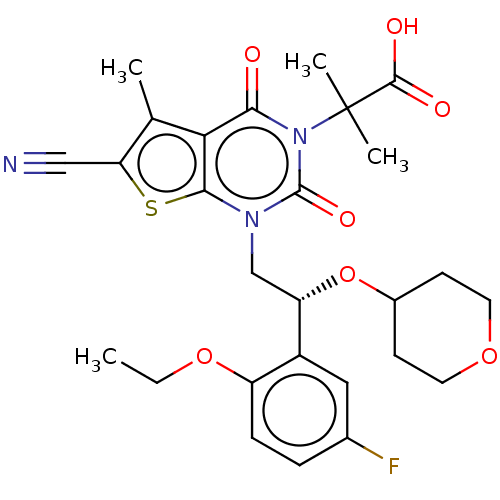 ((R)-2-(6-cyano-1-(2-(2-ethoxy-5-fluoro-phenyl)-2-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The degree of inhibition of the enzymatic activity of recombinant human ACC1, ACC2 proteins under in-vitro conditions by the preferred compounds of t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N3014R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM357252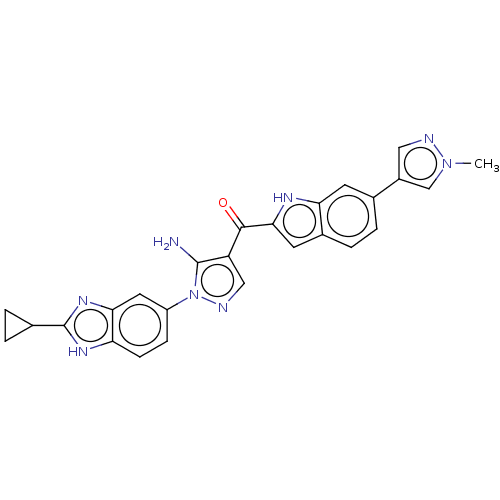 ((5-Amino-1-(2-cyclopropyl-1H-benzo[d]imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The following assay was used to determine the inhibition rate of the preferred compounds of the present invention to the kinase activity of the recom... | US Patent US10214515 (2019) BindingDB Entry DOI: 10.7270/Q22J6F46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKB (unknown origin) by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128409 BindingDB Entry DOI: 10.7270/Q2CZ3C28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Mus musculus (Mouse)) | BDBM109277 (US8609622, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd.; Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description Inhibitory activity of the compounds using SGLT1 and SGLT2. | US Patent US8609622 (2013) BindingDB Entry DOI: 10.7270/Q2BC3X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Mus musculus (Mouse)) | BDBM109275 (US8609622, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.65 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd.; Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description Inhibitory activity of the compounds using SGLT1 and SGLT2. | US Patent US8609622 (2013) BindingDB Entry DOI: 10.7270/Q2BC3X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443228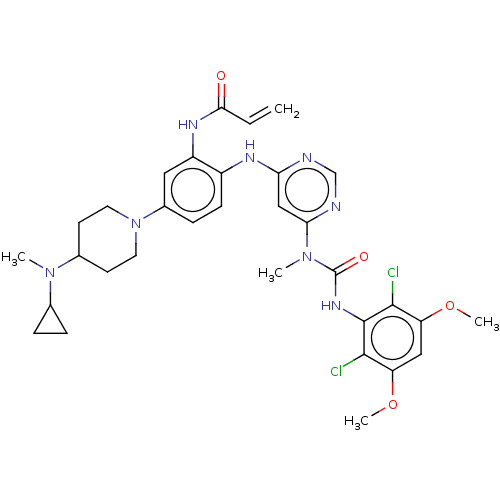 (N-(5-(4-(Cyclopropyl(methyl)amino)piperidin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The experimental procedure is briefly described as follows: the test compound was first dissolved in DMSO to prepare a stock solution, and then gradi... | US Patent US10654836 (2020) BindingDB Entry DOI: 10.7270/Q25M68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443228 (N-(5-(4-(Cyclopropyl(methyl)amino)piperidin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The following method was used to determine the inhibition degree of the kinase activity of recombinant human FGFR protein by the compounds of the pre... | US Patent US11001572 (2021) BindingDB Entry DOI: 10.7270/Q2Z322RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM520082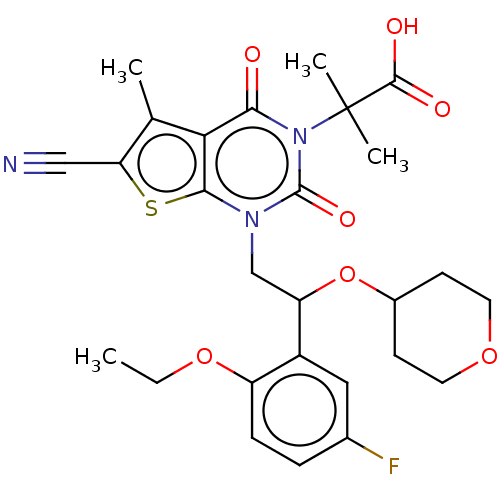 (2-(6-Cyano-1-(2-(2-ethoxy-5-fluorophenyl)-2-((tetr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The degree of inhibition of the enzymatic activity of recombinant human ACC1, ACC2 proteins under in-vitro conditions by the preferred compounds of t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N3014R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Mus musculus (Mouse)) | BDBM109279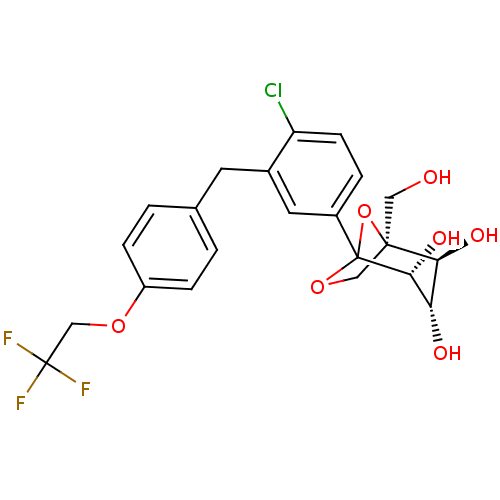 (US8609622, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd.; Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description Inhibitory activity of the compounds using SGLT1 and SGLT2. | US Patent US8609622 (2013) BindingDB Entry DOI: 10.7270/Q2BC3X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443221 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The following method was used to determine the inhibition degree of the kinase activity of recombinant human FGFR protein by the compounds of the pre... | US Patent US11001572 (2021) BindingDB Entry DOI: 10.7270/Q2Z322RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443221 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The experimental procedure is briefly described as follows: the test compound was first dissolved in DMSO to prepare a stock solution, and then gradi... | US Patent US10654836 (2020) BindingDB Entry DOI: 10.7270/Q25M68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM357253 ((5-Amino-1-(2-cyclopropyl-1H-benzo[d]imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The following assay was used to determine the inhibition rate of the preferred compounds of the present invention to the kinase activity of the recom... | US Patent US10214515 (2019) BindingDB Entry DOI: 10.7270/Q22J6F46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM136597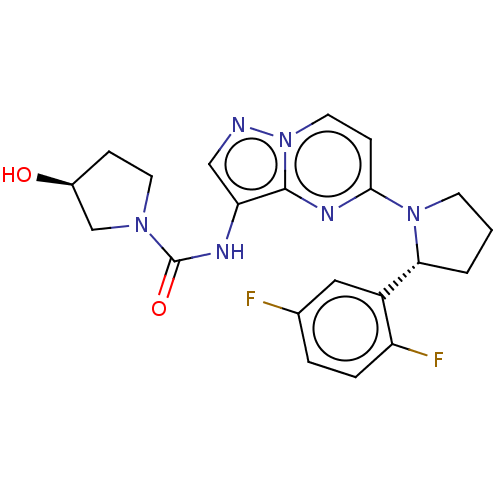 (US10005783, 14 | US10047097, 14 | US10774085, Exam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type TRKC (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128409 BindingDB Entry DOI: 10.7270/Q2CZ3C28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM136597 (US10005783, 14 | US10047097, 14 | US10774085, Exam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type TRKA (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128409 BindingDB Entry DOI: 10.7270/Q2CZ3C28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM136597 (US10005783, 14 | US10047097, 14 | US10774085, Exam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type TRKB (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128409 BindingDB Entry DOI: 10.7270/Q2CZ3C28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM357250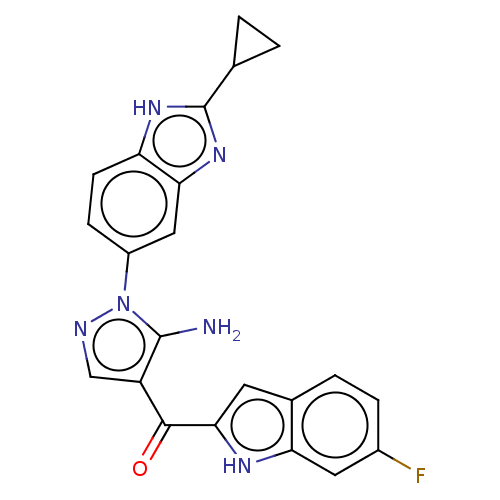 ((5-Amino-1-(2-cyclopropyl-1H-benzo[d]imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The following assay was used to determine the inhibition rate of the preferred compounds of the present invention to the kinase activity of the recom... | US Patent US10214515 (2019) BindingDB Entry DOI: 10.7270/Q22J6F46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKC (unknown origin) by radiometric assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128409 BindingDB Entry DOI: 10.7270/Q2CZ3C28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443220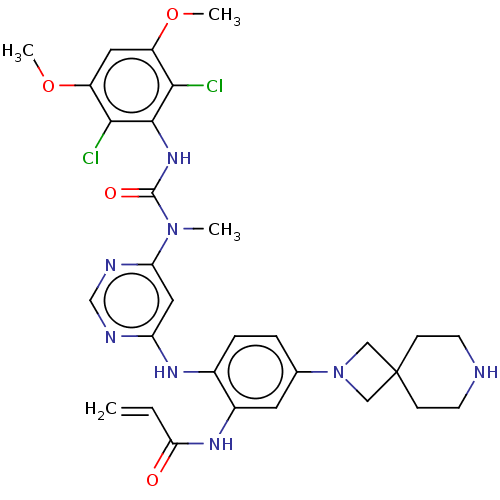 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The following method was used to determine the inhibition degree of the kinase activity of recombinant human FGFR protein by the compounds of the pre... | US Patent US11001572 (2021) BindingDB Entry DOI: 10.7270/Q2Z322RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM357254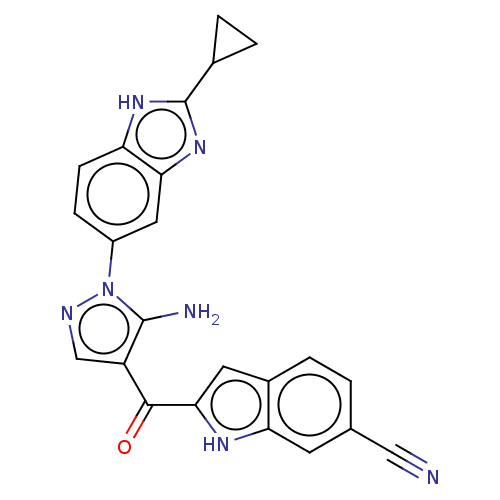 (2-(5-Amino-1-(2-cyclopropyl-1H-benzo[d]imidazol-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The following assay was used to determine the inhibition rate of the preferred compounds of the present invention to the kinase activity of the recom... | US Patent US10214515 (2019) BindingDB Entry DOI: 10.7270/Q22J6F46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM443220 (N-(2-((6-(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The experimental procedure is briefly described as follows: the test compound was first dissolved in DMSO to prepare a stock solution, and then gradi... | US Patent US10654836 (2020) BindingDB Entry DOI: 10.7270/Q25M68RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM357251 ((5-Amino-1-(2-cyclopropyl-1H-benzo[d]imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The following assay was used to determine the inhibition rate of the preferred compounds of the present invention to the kinase activity of the recom... | US Patent US10214515 (2019) BindingDB Entry DOI: 10.7270/Q22J6F46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM520084 ((R)-2-(6-cyano-1-(2-(2-ethoxy-5-fluoro-phenyl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The degree of inhibition of the enzymatic activity of recombinant human ACC1, ACC2 proteins under in-vitro conditions by the preferred compounds of t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N3014R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM357246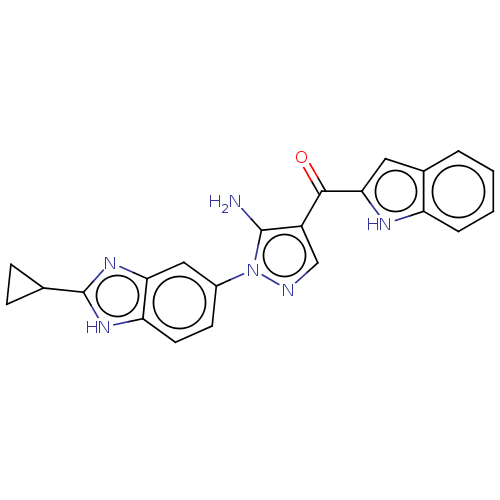 ((5-amino-1-(2-cyclopropyl-1H-benzo[d]imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The following assay was used to determine the inhibition rate of the preferred compounds of the present invention to the kinase activity of the recom... | US Patent US10214515 (2019) BindingDB Entry DOI: 10.7270/Q22J6F46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Mus musculus (Mouse)) | BDBM109277 (US8609622, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd.; Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description Inhibitory activity of the compounds using SGLT1 and SGLT2. | US Patent US8609622 (2013) BindingDB Entry DOI: 10.7270/Q2BC3X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM520086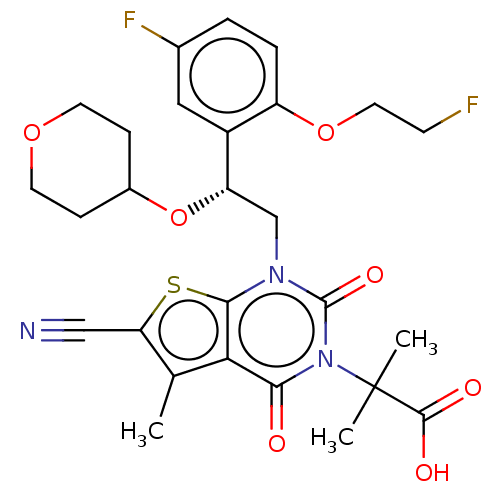 ((R)-2-(6-Cyano-1-(2-(5-fluoro-2-(2-fluoroethoxy)ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The degree of inhibition of the enzymatic activity of recombinant human ACC1, ACC2 proteins under in-vitro conditions by the preferred compounds of t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N3014R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM357253 ((5-Amino-1-(2-cyclopropyl-1H-benzo[d]imidazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Hisun Pharmaceutical Co., Ltd. US Patent | Assay Description The following assay was used to determine the inhibition rate of the preferred compounds of the present invention to the kinase activity of the recom... | US Patent US10214515 (2019) BindingDB Entry DOI: 10.7270/Q22J6F46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Mus musculus (Mouse)) | BDBM109277 (US8609622, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.92 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd.; Jiangsu Hengrui Medicine Co., Ltd. US Patent | Assay Description Inhibitory activity of the compounds using SGLT1 and SGLT2. | US Patent US8609622 (2013) BindingDB Entry DOI: 10.7270/Q2BC3X6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 384 total ) | Next | Last >> |