 Found 1315 hits with Last Name = 'guan' and Initial = 'h'
Found 1315 hits with Last Name = 'guan' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50005120
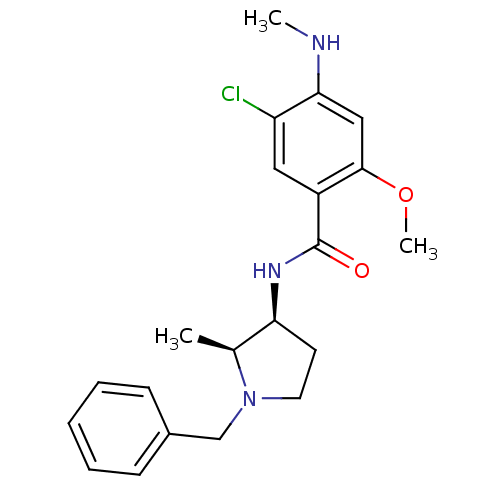
(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50005120

(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor. |
J Med Chem 38: 708-14 (1995)
BindingDB Entry DOI: 10.7270/Q2PG1SCG |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 from canine striatum |
J Med Chem 35: 67-72 (1992)
BindingDB Entry DOI: 10.7270/Q2P84CHC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM81793
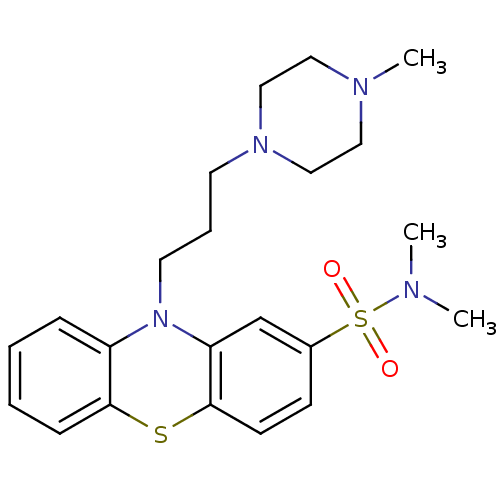
(CAS_316-81-4 | NSC_92178 | Thioproperazine)Show SMILES CN(C)S(=O)(=O)c1ccc2Sc3ccccc3N(CCCN3CCN(C)CC3)c2c1 Show InChI InChI=1S/C22H30N4O2S2/c1-23(2)30(27,28)18-9-10-22-20(17-18)26(19-7-4-5-8-21(19)29-22)12-6-11-25-15-13-24(3)14-16-25/h4-5,7-10,17H,6,11-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50010301
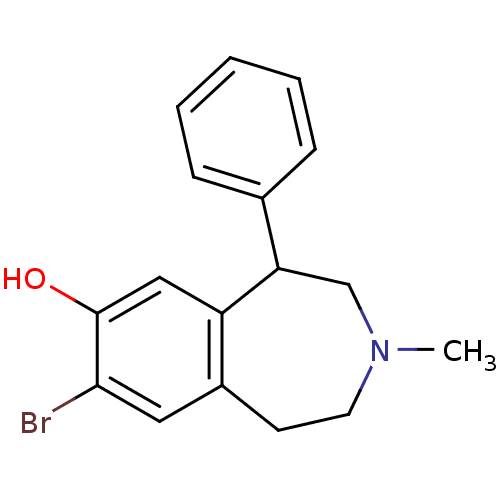
(8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM78433
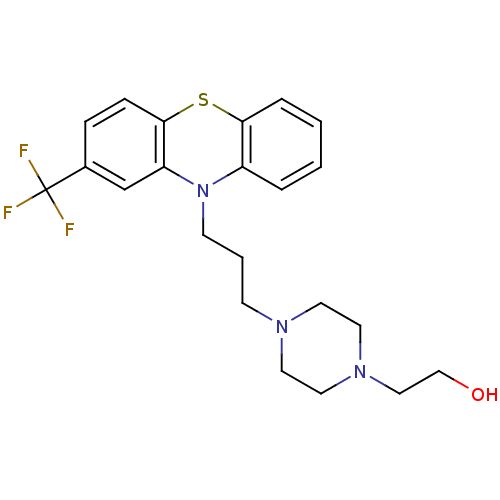
(2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...)Show SMILES OCCN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C22H26F3N3OS/c23-22(24,25)17-6-7-21-19(16-17)28(18-4-1-2-5-20(18)30-21)9-3-8-26-10-12-27(13-11-26)14-15-29/h1-2,4-7,16,29H,3,8-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Synapse 25: 137-46 (1997)
Article DOI: 10.1002/(SICI)1098-2396(199702)25:2
BindingDB Entry DOI: 10.7270/Q2WH2NH6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM81872
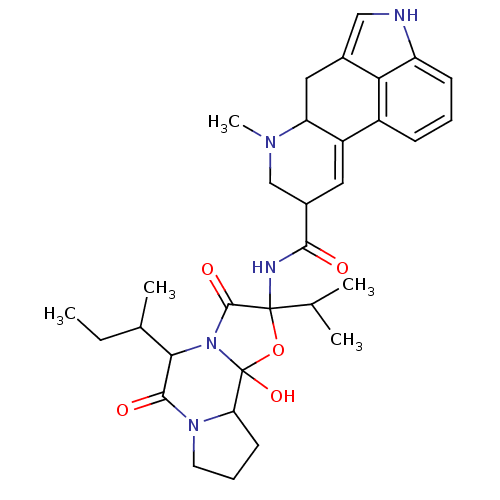
(CAS_161309 | Ergocryptine, beta | NSC_161309)Show SMILES CCC(C)C1N2C(=O)C(NC(=O)C3CN(C)C4Cc5c[nH]c6cccc(C4=C3)c56)(OC2(O)C2CCCN2C1=O)C(C)C |c:27| Show InChI InChI=1S/C32H41N5O5/c1-6-18(4)27-29(39)36-12-8-11-25(36)32(41)37(27)30(40)31(42-32,17(2)3)34-28(38)20-13-22-21-9-7-10-23-26(21)19(15-33-23)14-24(22)35(5)16-20/h7,9-10,13,15,17-18,20,24-25,27,33,41H,6,8,11-12,14,16H2,1-5H3,(H,34,38) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50010301

(8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM81777
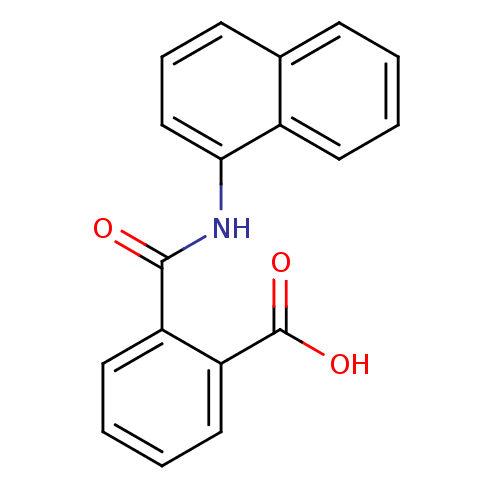
(CAS_132-66-1 | NPA | NPA,(+) | NPA,(-) | NSC_8594)Show InChI InChI=1S/C18H13NO3/c20-17(14-9-3-4-10-15(14)18(21)22)19-16-11-5-7-12-6-1-2-8-13(12)16/h1-11H,(H,19,20)(H,21,22) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM78433

(2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...)Show SMILES OCCN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C22H26F3N3OS/c23-22(24,25)17-6-7-21-19(16-17)28(18-4-1-2-5-20(18)30-21)9-3-8-26-10-12-27(13-11-26)14-15-29/h1-2,4-7,16,29H,3,8-15H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50028600
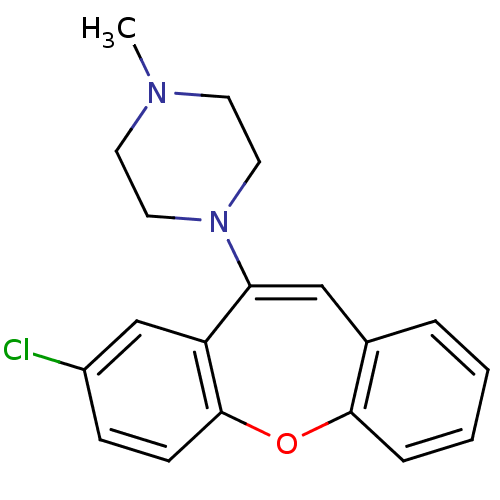
(1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...)Show SMILES CN1CCN(CC1)C1=Cc2ccccc2Oc2ccc(Cl)cc12 |t:8| Show InChI InChI=1S/C19H19ClN2O/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,12-13H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor |
J Med Chem 37: 2686-96 (1994)
BindingDB Entry DOI: 10.7270/Q28051NX |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50028600

(1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...)Show SMILES CN1CCN(CC1)C1=Cc2ccccc2Oc2ccc(Cl)cc12 |t:8| Show InChI InChI=1S/C19H19ClN2O/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,12-13H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D4 in COS7 cells |
J Med Chem 38: 708-14 (1995)
BindingDB Entry DOI: 10.7270/Q2PG1SCG |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50368313
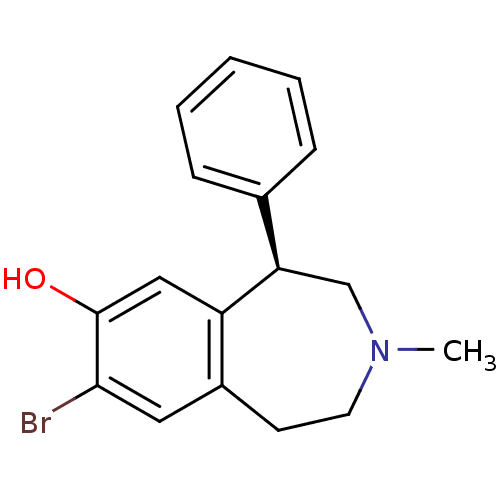
(CHEMBL1744079)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 from canine striatum |
J Med Chem 35: 67-72 (1992)
BindingDB Entry DOI: 10.7270/Q2P84CHC |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007568
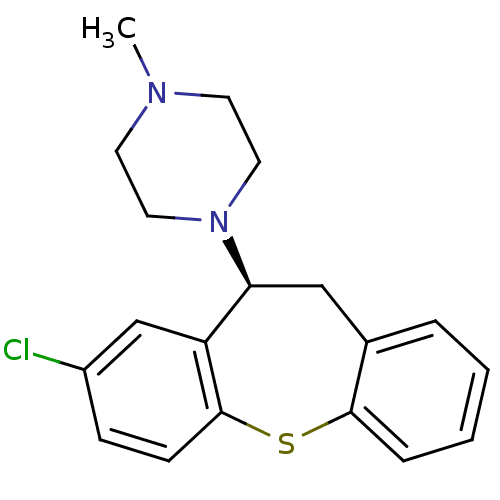
(1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3/t17-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50054062
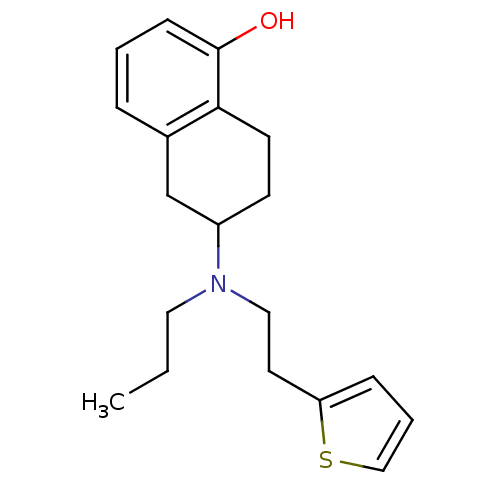
(6-[Propyl-(2-thiophen-2-yl-ethyl)-amino]-5,6,7,8-t...)Show InChI InChI=1S/C19H25NOS/c1-2-11-20(12-10-17-6-4-13-22-17)16-8-9-18-15(14-16)5-3-7-19(18)21/h3-7,13,16,21H,2,8-12,14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433602
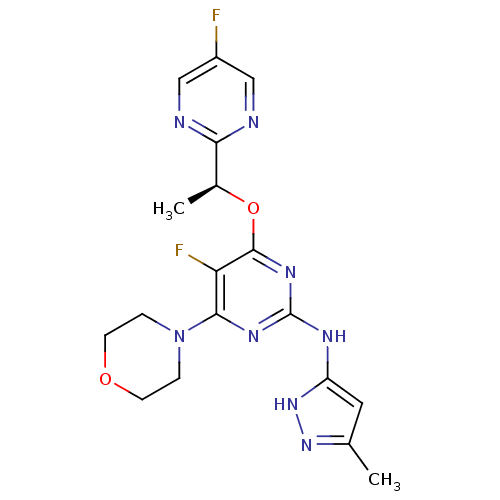
(CHEMBL2381980)Show SMILES C[C@H](Oc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cn1 |r| Show InChI InChI=1S/C18H20F2N8O2/c1-10-7-13(27-26-10)23-18-24-16(28-3-5-29-6-4-28)14(20)17(25-18)30-11(2)15-21-8-12(19)9-22-15/h7-9,11H,3-6H2,1-2H3,(H2,23,24,25,26,27)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50007702

(6-Chloro-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO2/c1-19-8-7-12-13(9-15(20)17(21)16(12)18)14(10-19)11-5-3-2-4-6-11/h2-6,9,14,20-21H,7-8,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at dopamine receptor D1 |
J Med Chem 34: 3366-71 (1992)
BindingDB Entry DOI: 10.7270/Q26H4J17 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004793

(3-Allyl-8-bromo-5-phenyl-2,3,4,5-tetrahydro-1H-ben...)Show InChI InChI=1S/C19H20BrNO/c1-2-9-21-10-8-15-11-18(20)19(22)12-16(15)17(13-21)14-6-4-3-5-7-14/h2-7,11-12,17,22H,1,8-10,13H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 from canine striatum |
J Med Chem 35: 67-72 (1992)
BindingDB Entry DOI: 10.7270/Q2P84CHC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50028602

(1-(2-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...)Show SMILES CN1CCN(CC1)C1=Cc2cc(Cl)ccc2Oc2ccccc12 |t:8| Show InChI InChI=1S/C19H19ClN2O/c1-21-8-10-22(11-9-21)17-13-14-12-15(20)6-7-18(14)23-19-5-3-2-4-16(17)19/h2-7,12-13H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor |
J Med Chem 37: 2686-96 (1994)
BindingDB Entry DOI: 10.7270/Q28051NX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50028601
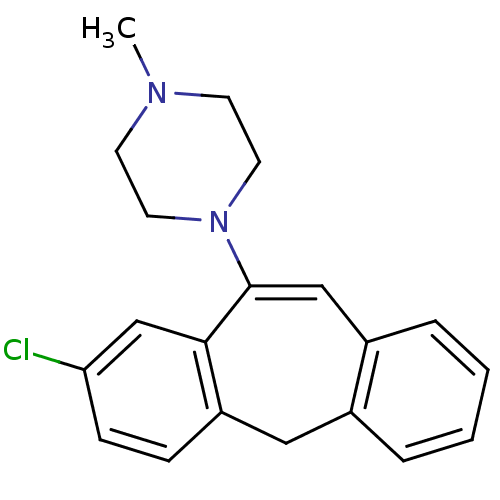
(1-(8-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...)Show SMILES CN1CCN(CC1)C1=Cc2ccccc2Cc2ccc(Cl)cc12 |t:8| Show InChI InChI=1S/C20H21ClN2/c1-22-8-10-23(11-9-22)20-13-16-5-3-2-4-15(16)12-17-6-7-18(21)14-19(17)20/h2-7,13-14H,8-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-2(long) receptor |
J Med Chem 37: 2686-96 (1994)
BindingDB Entry DOI: 10.7270/Q28051NX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50028601

(1-(8-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...)Show SMILES CN1CCN(CC1)C1=Cc2ccccc2Cc2ccc(Cl)cc12 |t:8| Show InChI InChI=1S/C20H21ClN2/c1-22-8-10-23(11-9-22)20-13-16-5-3-2-4-15(16)12-17-6-7-18(21)14-19(17)20/h2-7,13-14H,8-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor |
J Med Chem 37: 2686-96 (1994)
BindingDB Entry DOI: 10.7270/Q28051NX |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004923
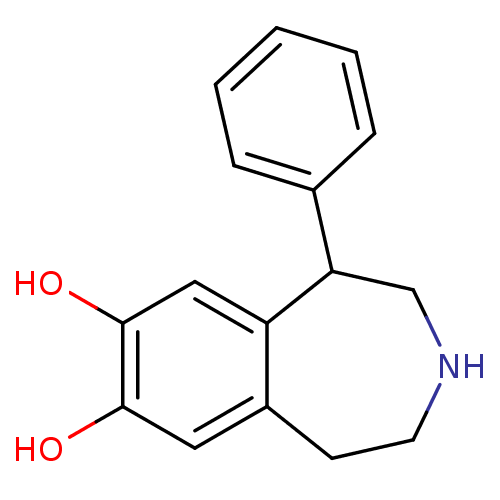
((+/-)-SKF-38393 | 1-Phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at Dopamine receptor D1 |
J Med Chem 34: 3366-71 (1992)
BindingDB Entry DOI: 10.7270/Q26H4J17 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004923

((+/-)-SKF-38393 | 1-Phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at Dopamine receptor D1 |
J Med Chem 34: 3366-71 (1992)
BindingDB Entry DOI: 10.7270/Q26H4J17 |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50020217
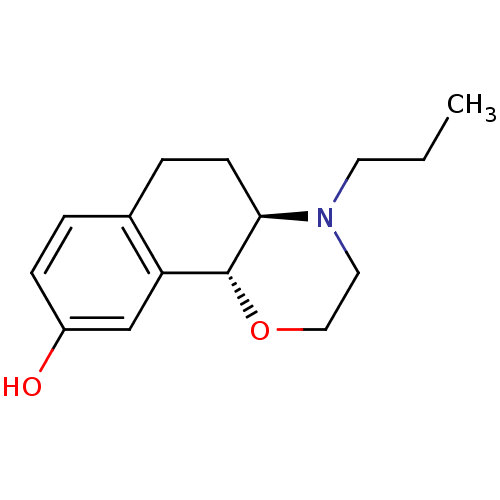
((4aR,10bR)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-na...)Show InChI InChI=1S/C15H21NO2/c1-2-7-16-8-9-18-15-13-10-12(17)5-3-11(13)4-6-14(15)16/h3,5,10,14-15,17H,2,4,6-9H2,1H3/t14-,15-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004792
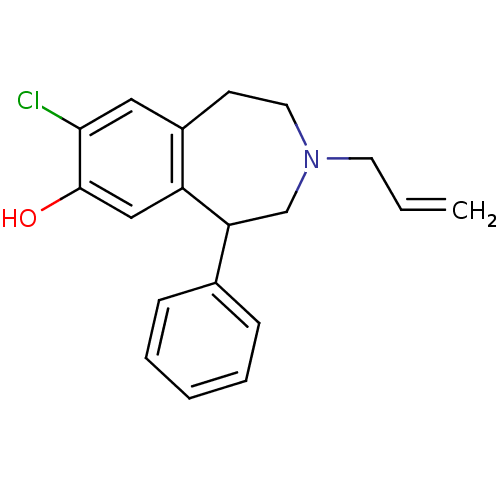
(3-Allyl-8-chloro-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C19H20ClNO/c1-2-9-21-10-8-15-11-18(20)19(22)12-16(15)17(13-21)14-6-4-3-5-7-14/h2-7,11-12,17,22H,1,8-10,13H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-SCH- 23390 binding to Dopamine receptor D1 from canine striatum |
J Med Chem 35: 67-72 (1992)
BindingDB Entry DOI: 10.7270/Q2P84CHC |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50007700

(6-Bromo-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO2/c1-19-8-7-12-13(9-15(20)17(21)16(12)18)14(10-19)11-5-3-2-4-6-11/h2-6,9,14,20-21H,7-8,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at dopamine receptor D1 |
J Med Chem 34: 3366-71 (1992)
BindingDB Entry DOI: 10.7270/Q26H4J17 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D2L in COS7 cells |
J Med Chem 38: 708-14 (1995)
BindingDB Entry DOI: 10.7270/Q2PG1SCG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433601
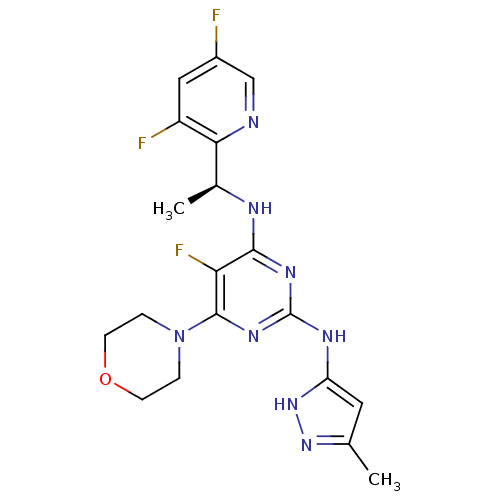
(CHEMBL2381983)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)nc(N2CCOCC2)c1F)c1ncc(F)cc1F |r| Show InChI InChI=1S/C19H21F3N8O/c1-10-7-14(29-28-10)25-19-26-17(15(22)18(27-19)30-3-5-31-6-4-30)24-11(2)16-13(21)8-12(20)9-23-16/h7-9,11H,3-6H2,1-2H3,(H3,24,25,26,27,28,29)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3105-10 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.111
BindingDB Entry DOI: 10.7270/Q23T9JMX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007568

(1-((S)-8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3/t17-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50007567
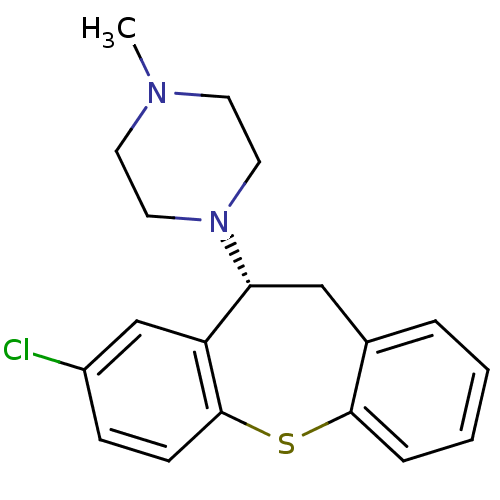
(1-(8-Chloro-10,11-dihydro-dibenzo[b,f]thiepin-10-y...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3/t17-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM81195
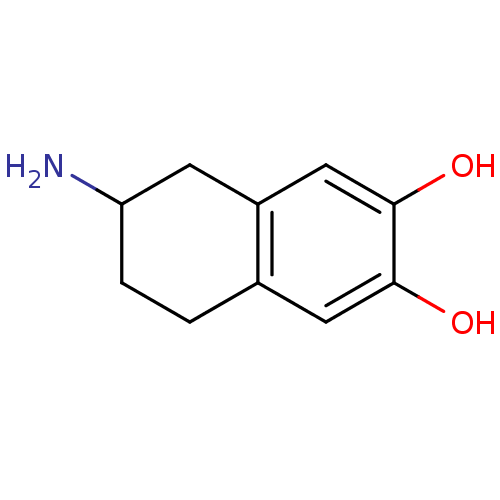
((+/-)-2-Amino-6,7-dihydroxy-1,2,3,4-tetrahydronaph...)Show InChI InChI=1S/C10H13NO2/c11-8-2-1-6-4-9(12)10(13)5-7(6)3-8/h4-5,8,12-13H,1-3,11H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50005118
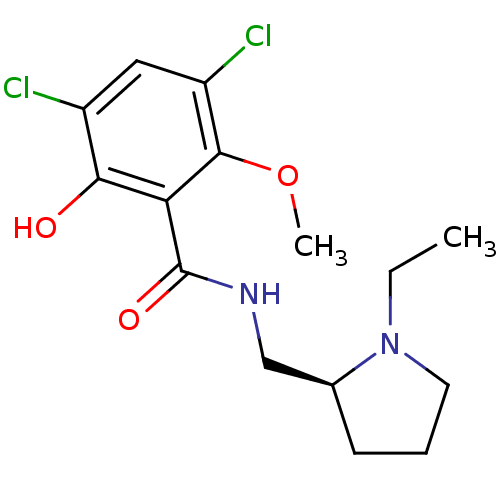
((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...)Show InChI InChI=1S/C15H20Cl2N2O3/c1-3-19-6-4-5-9(19)8-18-15(21)12-13(20)10(16)7-11(17)14(12)22-2/h7,9,20H,3-6,8H2,1-2H3,(H,18,21)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data