

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CXCR4-mediated chemotaxis in SDF1-stimulated human U937 cells treated 15 mins before SDF1 challenge measured after 2 hrs by luminescenc... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated 30 mins before agonist challenge... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated before agonist challenge measure... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 45 mg/ml human ... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of alpha-acid glyc... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 HXB2 gp120-mediated viral infusion into HEK293 cells after 24 hrs by luciferase reporter gene assay in presence of 1 mg/ml alpha-a... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Mus musculus) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR4 expressed in human U2OS cells assessed as inhibition of SDF1-induced increase in intracellular calcium level by FL... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.41 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced increase in intracellular calcium level treated 1... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated before agonist challenge measure... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50316633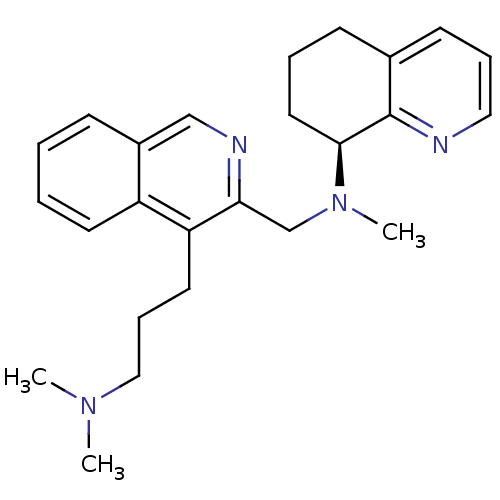 ((S)-N-((4-(3-(dimethylamino)propyl)isoquinolin-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to human CXCR4 expressed in HEK293 cells | Bioorg Med Chem Lett 20: 3026-30 (2010) Article DOI: 10.1016/j.bmcl.2010.03.118 BindingDB Entry DOI: 10.7270/Q2J67H2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Rattus norvegicus (Rat)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rat CXCR4 expressed in human U2OS cells assessed as inhibition of SDF1-induced increase in intracellular calcium level by FLIP... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50314758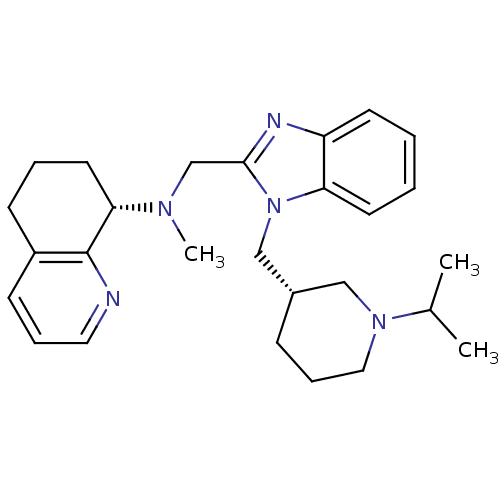 ((S)-N-((1-(((S)-1-isopropylpiperidin-3-yl)methyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of human CXCR4 | Bioorg Med Chem Lett 20: 2125-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.053 BindingDB Entry DOI: 10.7270/Q2ZW1M20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in human U2OS cells assessed as inhibition of SDF1-induced increase in intracellular calcium level by FL... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Macaca mulatta) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at monkey CXCR4 expressed in human U2OS cells assessed as inhibition of SDF1-induced increase in intracellular calcium level by F... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50316634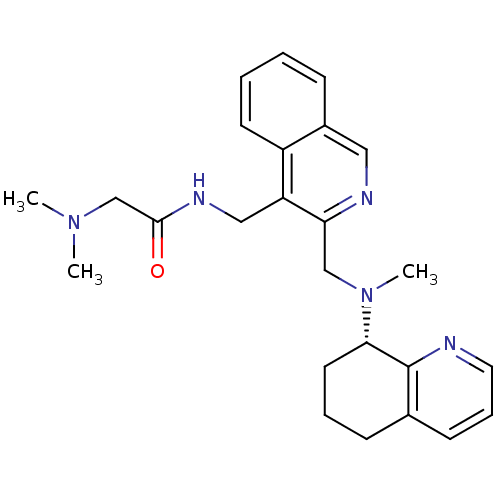 ((S)-2-(dimethylamino)-N-((3-((methyl(5,6,7,8-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to human CXCR4 expressed in HEK293 cells | Bioorg Med Chem Lett 20: 3026-30 (2010) Article DOI: 10.1016/j.bmcl.2010.03.118 BindingDB Entry DOI: 10.7270/Q2J67H2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 26.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated 30 mins before agonist challenge... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315287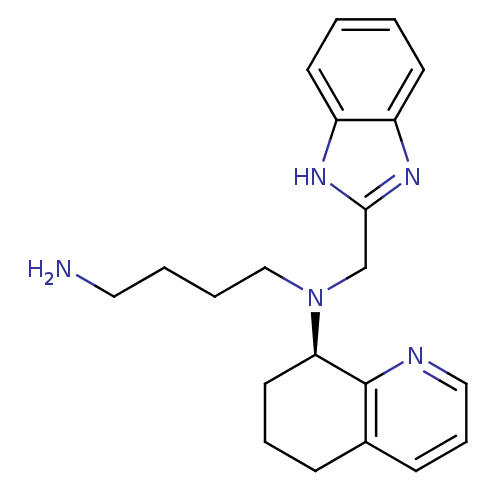 ((R)-N1-((1H-benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to human CXCR4 expressed in HEK293 cells | Bioorg Med Chem Lett 20: 3026-30 (2010) Article DOI: 10.1016/j.bmcl.2010.03.118 BindingDB Entry DOI: 10.7270/Q2J67H2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50314756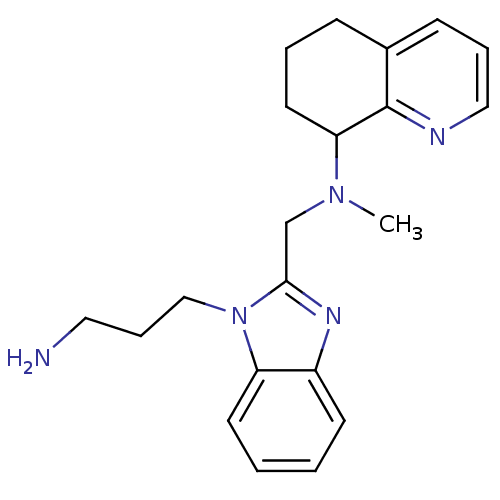 (CHEMBL577268 | N-((1-(3-aminopropyl)-1H-benzo[d]im...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of human CXCR4 | Bioorg Med Chem Lett 20: 2125-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.053 BindingDB Entry DOI: 10.7270/Q2ZW1M20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50314757 (CHEMBL1091591 | N-methyl-N-((1-(1-methylpiperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of human CXCR4 | Bioorg Med Chem Lett 20: 2125-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.053 BindingDB Entry DOI: 10.7270/Q2ZW1M20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50269061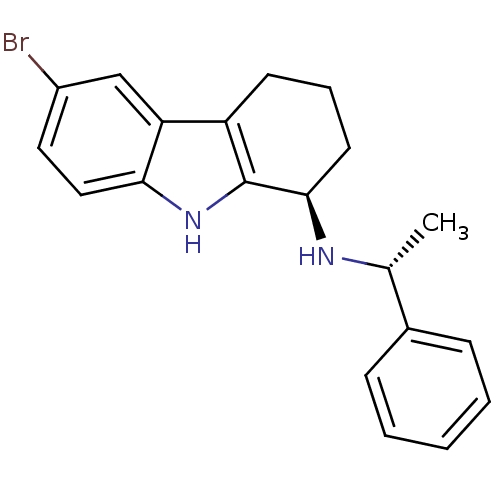 ((1R)-6-Bromo-N-[(1R)-1-phenylethyl]-2,3,4,9-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of ACE | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50269061 ((1R)-6-Bromo-N-[(1R)-1-phenylethyl]-2,3,4,9-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50295256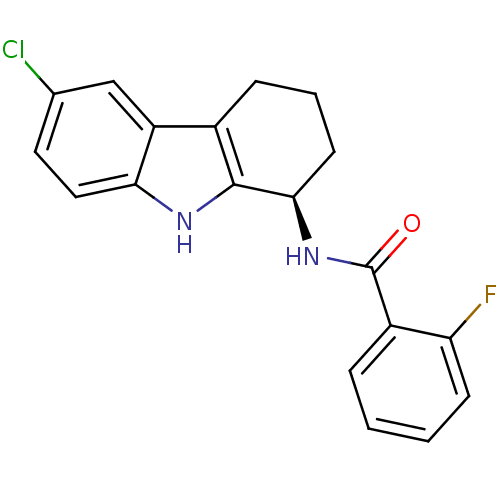 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50295257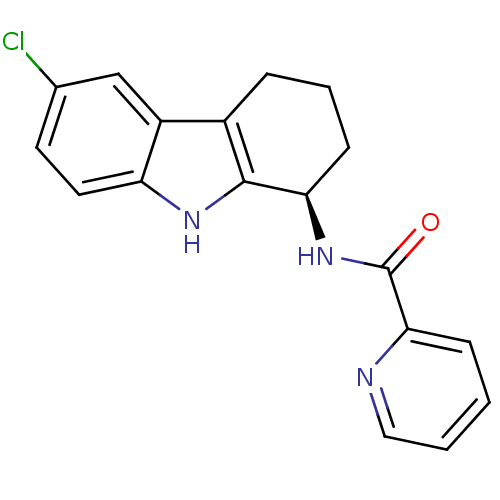 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50295256 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50295257 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50295255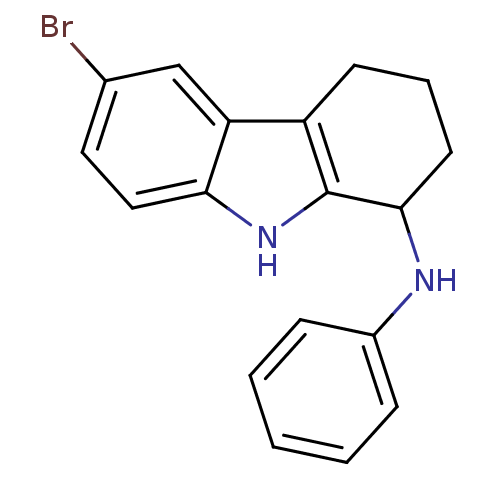 (6-bromo-N-phenyl-2,3,4,9-tetrahydro-1H-carbazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50295255 (6-bromo-N-phenyl-2,3,4,9-tetrahydro-1H-carbazol-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||