

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50030612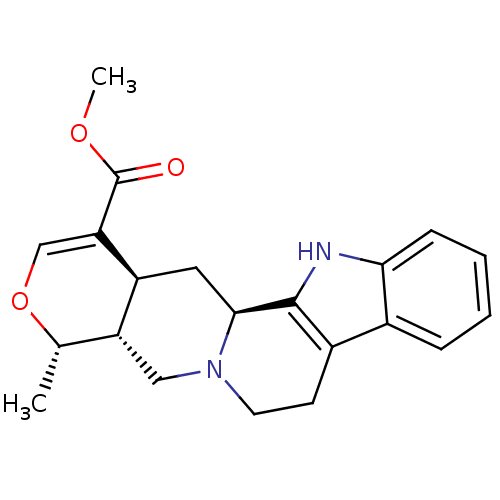 ((7aR,8S,11aS,12aS)-8-Methyl-5,6,7a,8,11a,12,12a,13...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory constant for cytochrome P450 2D6 | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50030612 ((7aR,8S,11aS,12aS)-8-Methyl-5,6,7a,8,11a,12,12a,13...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50030612 ((7aR,8S,11aS,12aS)-8-Methyl-5,6,7a,8,11a,12,12a,13...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Apparent inhibitory constant (Ki) for Bufuralol 1'-hydroxylation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50030612 ((7aR,8S,11aS,12aS)-8-Methyl-5,6,7a,8,11a,12,12a,13...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047020 (1-Isopropyl-4,4-diphenyl-piperidine (prodipine) | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory constant for cytochrome P450 2D6 | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50030612 ((7aR,8S,11aS,12aS)-8-Methyl-5,6,7a,8,11a,12,12a,13...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of partially purified cytochrome P450 2D6 1'-hydroxybufuralol formation | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50030612 ((7aR,8S,11aS,12aS)-8-Methyl-5,6,7a,8,11a,12,12a,13...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50030612 ((7aR,8S,11aS,12aS)-8-Methyl-5,6,7a,8,11a,12,12a,13...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50407151 (CHEMBL2079609) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50407152 (CHEMBL2079555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50017681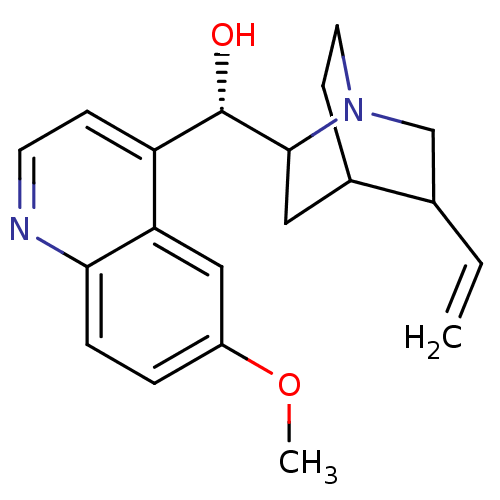 ((1S,2R,4S,5R)-2-[(S)-Hydroxy-(6-methoxy-quinolin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50017681 ((1S,2R,4S,5R)-2-[(S)-Hydroxy-(6-methoxy-quinolin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of partially purified cytochrome P450 2D6 1'-hydroxybufuralol formation | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50017681 ((1S,2R,4S,5R)-2-[(S)-Hydroxy-(6-methoxy-quinolin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of partially purified cytochrome P450 2D6 1'-hydroxybufuralol formation | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50407157 (DIHYDROQUINIDINE | GNF-Pf-5606) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50017681 ((1S,2R,4S,5R)-2-[(S)-Hydroxy-(6-methoxy-quinolin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50027058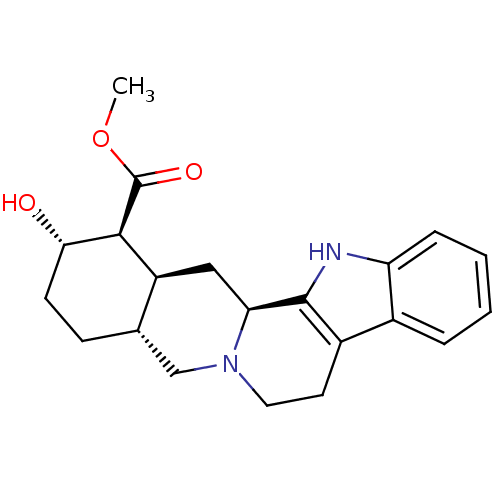 ((1S,2S,4aR,13bS,14aS)-2-Hydroxy-1,2,3,4,4a,5,7,8,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50422013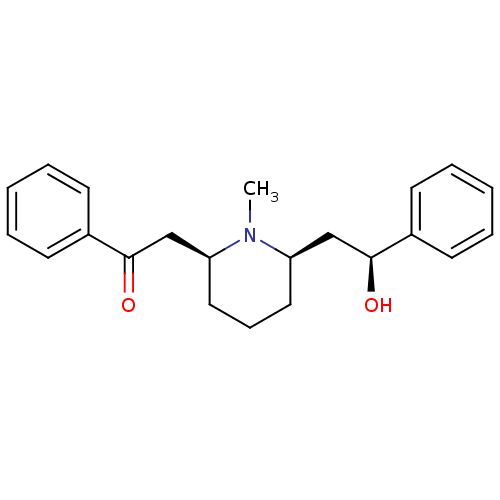 (LOBELINE | Lobeline Hydrochloride) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory constant for cytochrome P450 2D6 | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047016 (1-(4-Fluoro-phenyl)-4-[4-hydroxy-4-(3-trifluoromet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory constant for cytochrome P450 2D6 | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of partially purified cytochrome P450 2D6 1'-hydroxybufuralol formation | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50407158 (CHEMBL2079613) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047017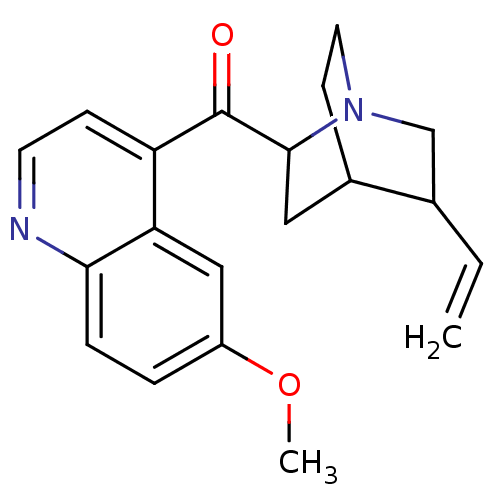 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370411 (CINCHORINE | GNF-PF-3189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047006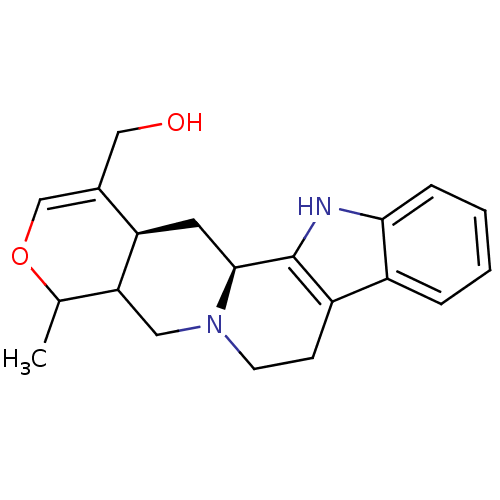 ((8-Methyl-5,6,7a,8,11a,12,12a,13-octahydro-7H-9-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50407156 (TETRAHYDROALSTONINE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047003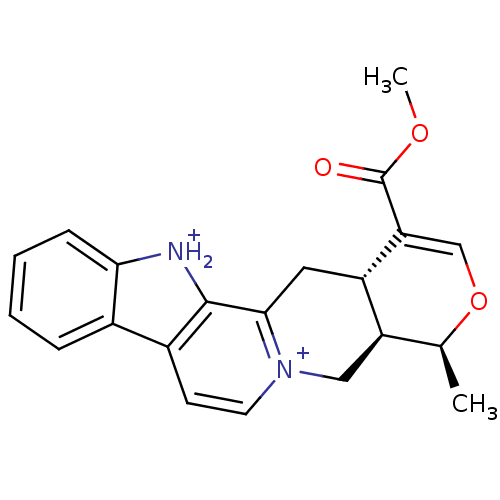 (CHEMBL14755 | Serpentine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370411 (CINCHORINE | GNF-PF-3189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of partially purified cytochrome P450 2D6 1'-hydroxybufuralol formation | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047001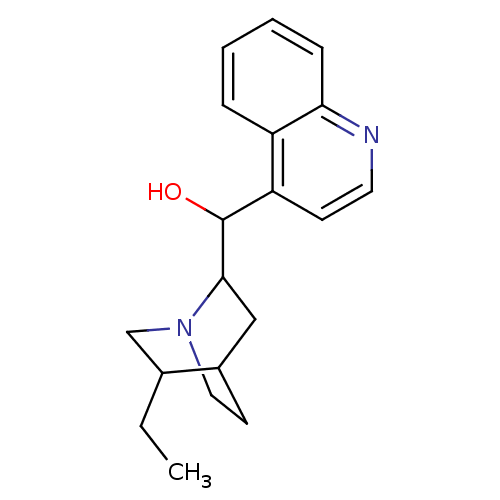 ((5-Ethyl-1-aza-bicyclo[2.2.2]oct-2-yl)-quinolin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047001 ((5-Ethyl-1-aza-bicyclo[2.2.2]oct-2-yl)-quinolin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047018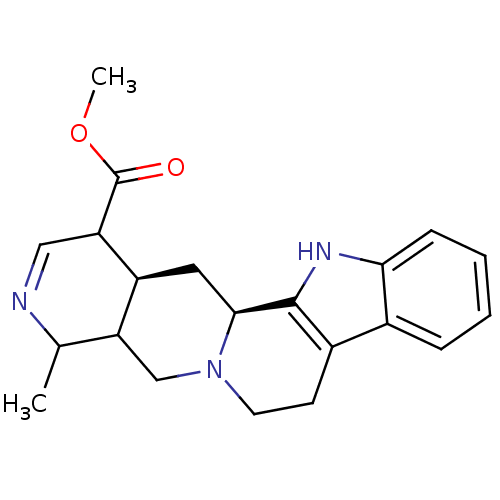 (8-Methyl-5,6,7,7a,8,9,11a,12,12a,13-decahydro-6a,9...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047010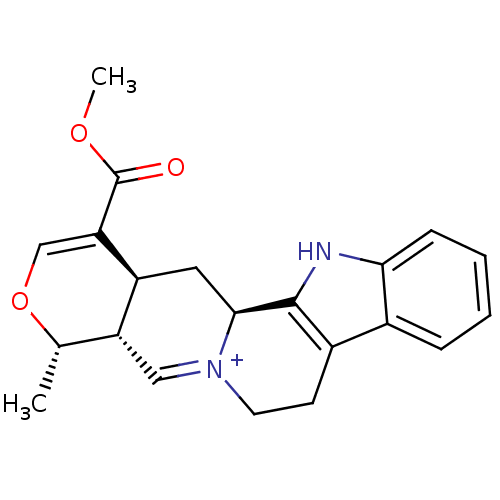 (11-Methoxycarbonyl-8-methyl-5,6,7a,8,11a,12,12a,13...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047001 ((5-Ethyl-1-aza-bicyclo[2.2.2]oct-2-yl)-quinolin-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370411 (CINCHORINE | GNF-PF-3189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370411 (CINCHORINE | GNF-PF-3189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Apparent inhibitory constant (Ki) for Bufuralol 1'-hydroxylation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50407155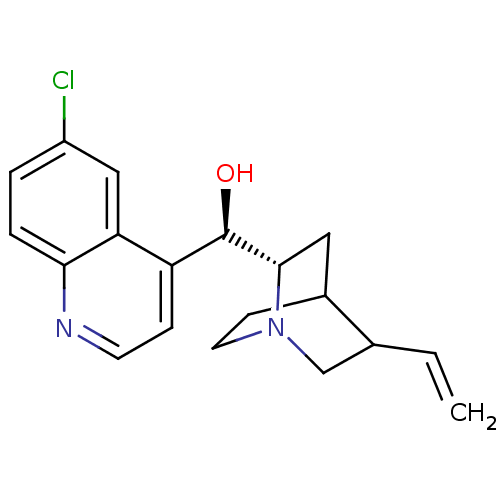 (CHEMBL2079556) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370411 (CINCHORINE | GNF-PF-3189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370411 (CINCHORINE | GNF-PF-3189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50407154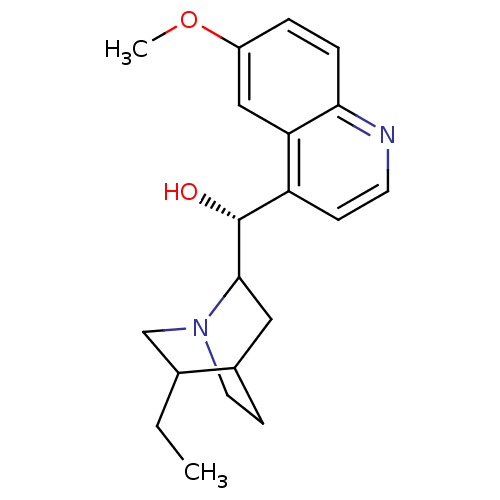 (CHEMBL2079611) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50367247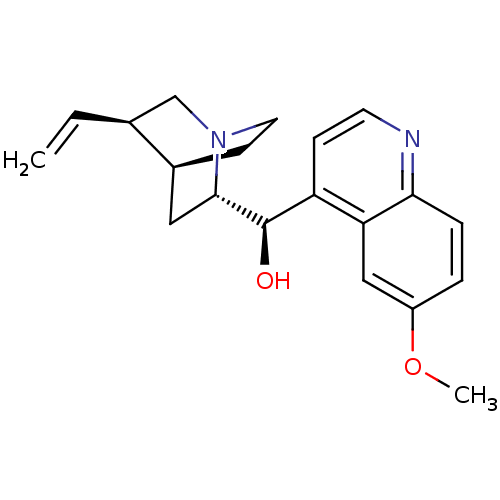 (QUININE | Quinamm | Quinsan | cid_3034034) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370411 (CINCHORINE | GNF-PF-3189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50047011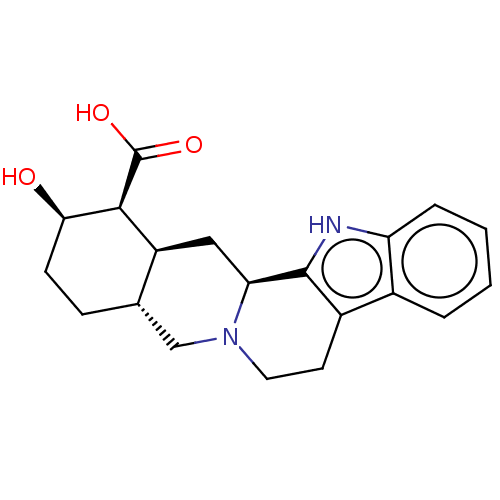 (2-Hydroxy-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory constant for cytochrome P450 2D6 | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370411 (CINCHORINE | GNF-PF-3189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibitory effect on Bufuralol 1'-hydroxylation by human liver microsomes (Ki = apparent inhibition constant) | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50407153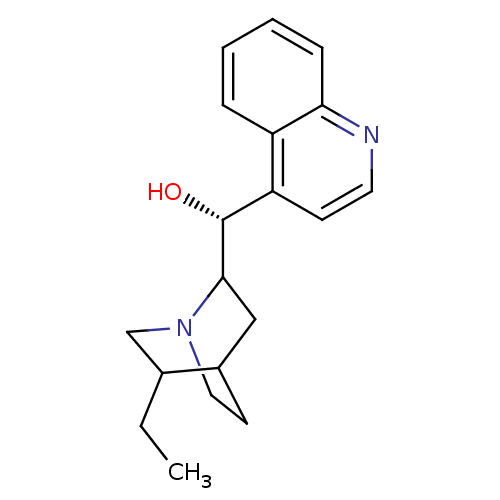 (CHEMBL2079610) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50407153 (CHEMBL2079610) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50370411 (CINCHORINE | GNF-PF-3189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Toxikologie Curated by ChEMBL | Assay Description Inhibition of 1'-hydroxybufuralol formation by human liver microsomes | J Med Chem 36: 1136-45 (1993) BindingDB Entry DOI: 10.7270/Q2GM87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 157 total ) | Next | Last >> |