

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242333 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260295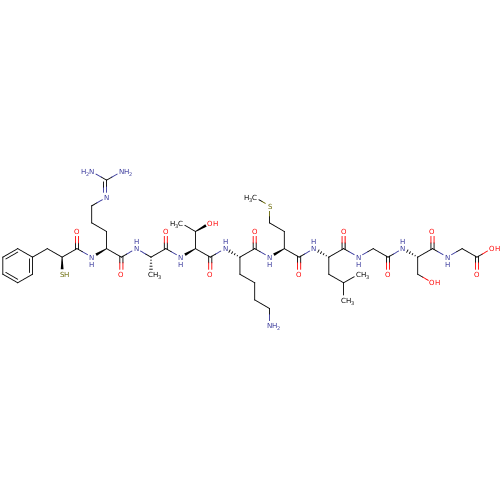 (2-((S)-2-(2-((S)-2-((S)-2-((S)-6-amino-2-((2S,3R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384950 (CHEMBL2037386) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260294 ((S)-2-((S)-6-amino-2-((2S,3R)-2-((S)-2-((S)-5-guan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384951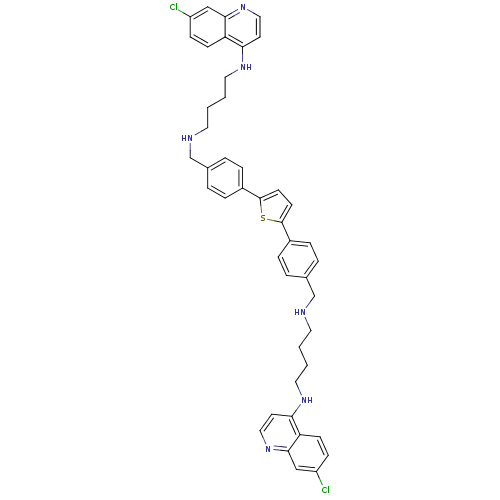 (CHEMBL2037387) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 535 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384440 (CHEMBL2035505 | CHEMBL2037389) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384440 (CHEMBL2035505 | CHEMBL2037389) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50302032 (2-(4-(4-carbamimidoylphenoxy)phenyl)-N'-(3-(7-chlo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50302031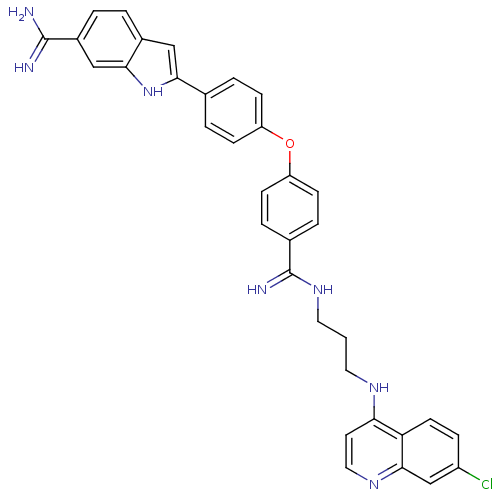 (2-(4-(4-(N'-(3-(7-chloroquinolin-4-ylamino)propyl)...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242339 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242337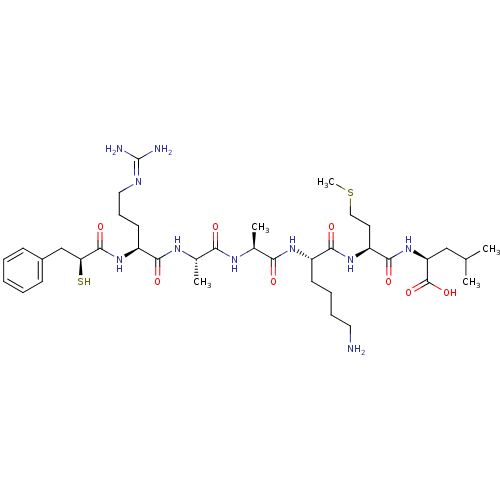 ((S)-2-{(S)-2-[(S)-6-Amino-2-((S)-2-{(S)-2-[(S)-5-g...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384949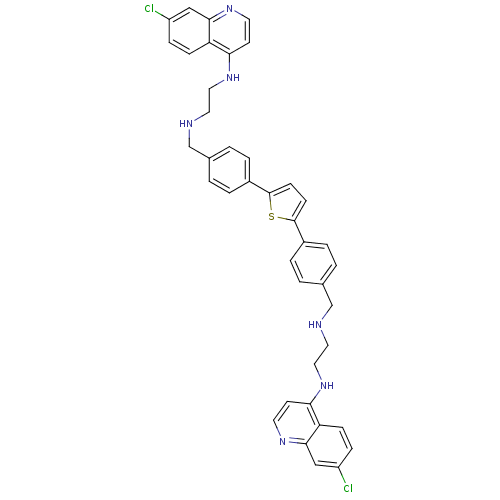 (CHEMBL2037288) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 882 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384952 (CHEMBL2037388) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 889 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384439 (CHEMBL2035506) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260296 (CHEMBL501525 | CRATKML) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242336 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384441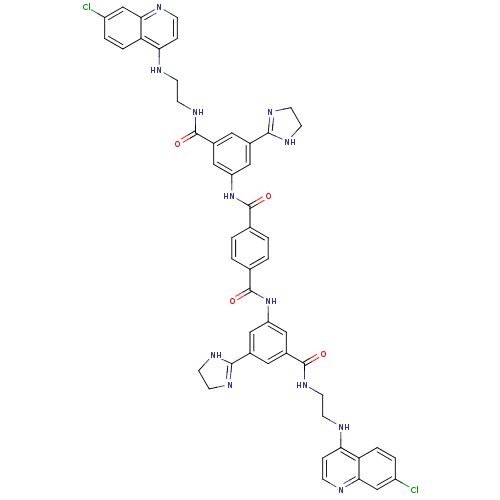 (CHEMBL2035503) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50240902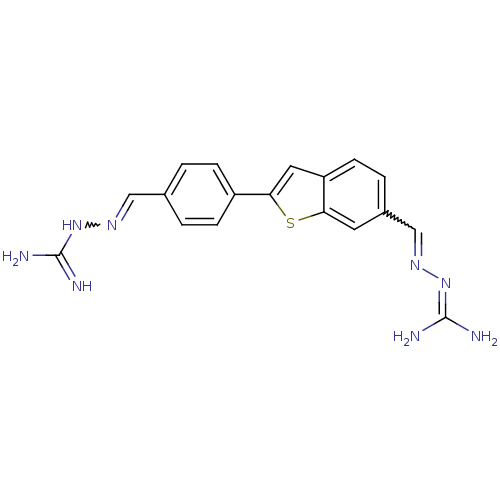 ((2E)-2-{4-[6-((E)-{[(E)-amino(imino)methyl]hydrazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242338 ((S)-2-{(S)-2-[(S)-2-((2S,3R)-2-{(S)-2-[(S)-5-Guani...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260293 ((S)-6-amino-2-((2S,3R)-2-((S)-2-((S)-5-guanidino-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50240901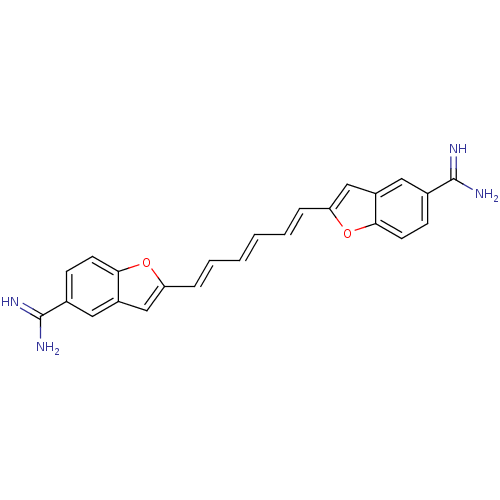 (2-((1E,3E,5E)-6-{5-[(E)-amino(imino)methyl]-1-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242334 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50343187 (CHEMBL1773155 | N,N'-Bis(3-aminopropyl)-3,9-dimeth...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uM | J Med Chem 54: 1157-69 (2011) Article DOI: 10.1021/jm100938u BindingDB Entry DOI: 10.7270/Q26T0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384438 (CHEMBL2035504) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384438 (CHEMBL2035504) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50343188 (CHEMBL1773156 | N,N'-Bis(2-aminoethyl)-3,9-dimethy...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uM | J Med Chem 54: 1157-69 (2011) Article DOI: 10.1021/jm100938u BindingDB Entry DOI: 10.7270/Q26T0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260300 (6-(4,5-dihydro-1H-imidazol-2-yl)-2-(2-(2-(4,5-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50100895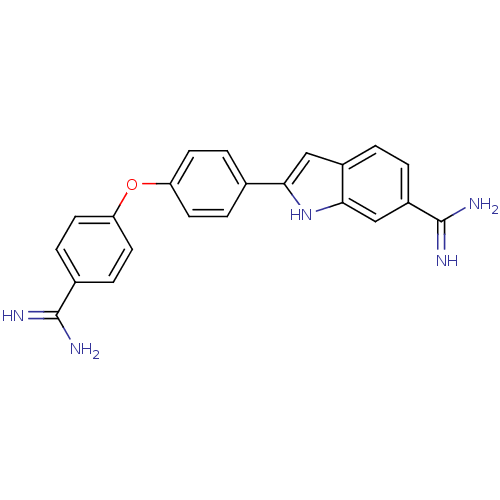 (2-(4-(4-carbamimidoylphenoxy)phenyl)-1H-indole-6-c...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384948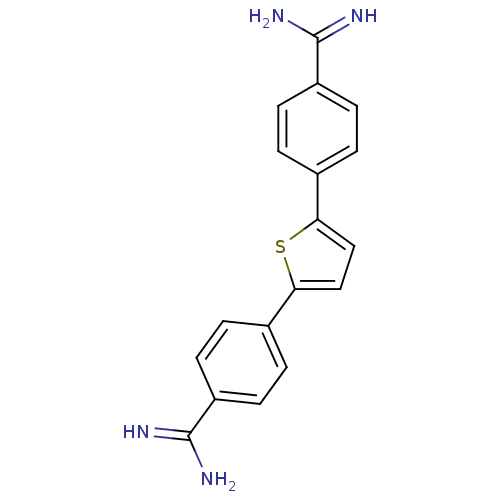 (CHEMBL2037287) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260292 ((2S,3R)-2-{(S)-2-[(S)-5-Guanidino-2-((S)-2-mercapt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242311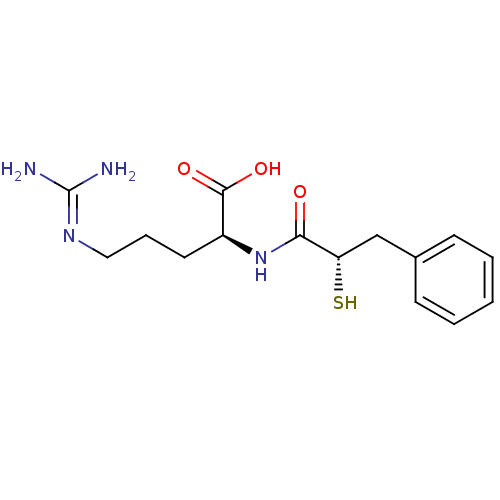 ((S)-5-Guanidino-2-((S)-2-mercapto-3-phenyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260291 ((S)-2-[(S)-5-Guanidino-2-((S)-2-mercapto-3-phenyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242335 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM84533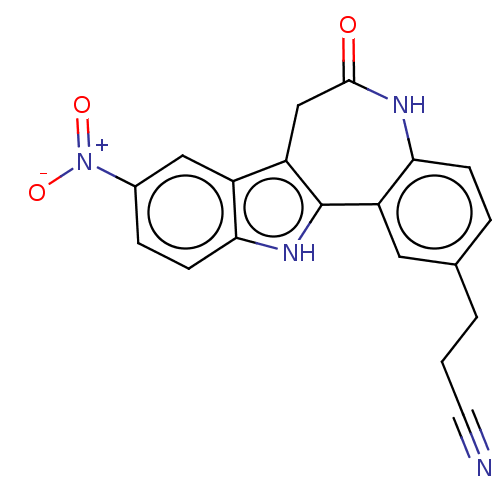 (Alsterpaullone derivative, 7 | BDBM50375663) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Technische Universität Braunschweig | Assay Description Kinase activity assay using GSK-3beta, CDK1/cyclin B and CDK5/p25. | Chembiochem 6: 541-9 (2005) Article DOI: 10.1002/cbic.200400099 BindingDB Entry DOI: 10.7270/Q2FX780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM84533 (Alsterpaullone derivative, 7 | BDBM50375663) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Technische Universität Braunschweig | Assay Description Kinase activity assay using GSK-3beta, CDK1/cyclin B and CDK5/p25. | Chembiochem 6: 541-9 (2005) Article DOI: 10.1002/cbic.200400099 BindingDB Entry DOI: 10.7270/Q2FX780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM84530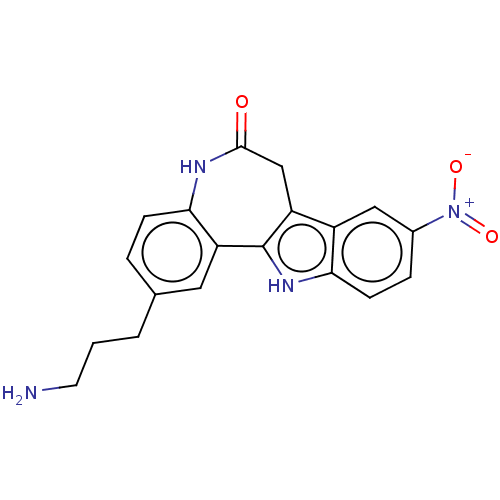 (Alsterpaullone derivative, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Technische Universität Braunschweig | Assay Description Kinase activity assay using GSK-3beta, CDK1/cyclin B and CDK5/p25. | Chembiochem 6: 541-9 (2005) Article DOI: 10.1002/cbic.200400099 BindingDB Entry DOI: 10.7270/Q2FX780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Rattus norvegicus (rat)) | BDBM7262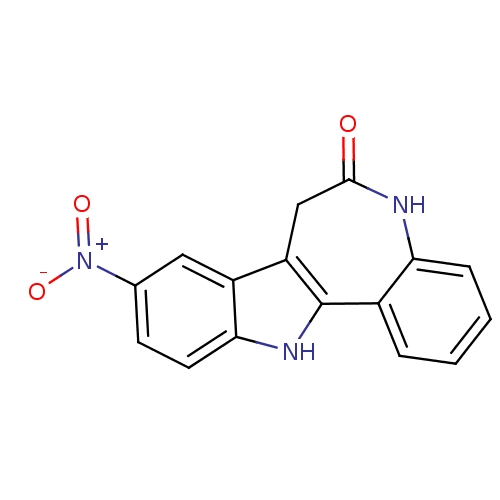 (14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 30 |
CNRS | Assay Description Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/[gamma-32P] ATP. 32P... | Eur J Biochem 267: 5983-94 (2000) Article DOI: 10.1046/j.1432-1327.2000.01673.x BindingDB Entry DOI: 10.7270/Q2CR5RJS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM7262 (14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Technische Universität Braunschweig | Assay Description Kinase activity assay using GSK-3beta, CDK1/cyclin B and CDK5/p25. | Chembiochem 6: 541-9 (2005) Article DOI: 10.1002/cbic.200400099 BindingDB Entry DOI: 10.7270/Q2FX780X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM84529 (Alsterpaullone derivative, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Technische Universität Braunschweig | Assay Description Kinase activity assay using GSK-3beta, CDK1/cyclin B and CDK5/p25. | Chembiochem 6: 541-9 (2005) Article DOI: 10.1002/cbic.200400099 BindingDB Entry DOI: 10.7270/Q2FX780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM84531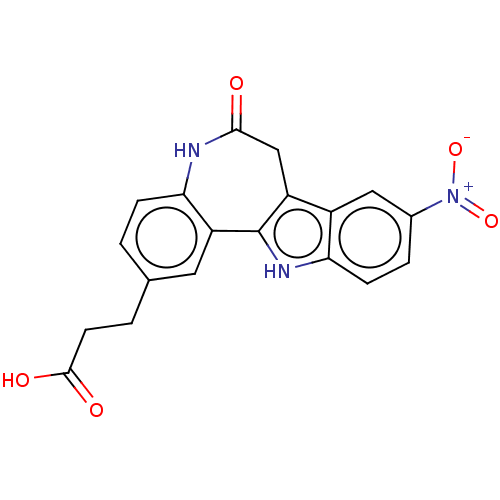 (Alsterpaullone derivative, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Technische Universität Braunschweig | Assay Description Kinase activity assay using GSK-3beta, CDK1/cyclin B and CDK5/p25. | Chembiochem 6: 541-9 (2005) Article DOI: 10.1002/cbic.200400099 BindingDB Entry DOI: 10.7270/Q2FX780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Rattus norvegicus (rat)) | BDBM7263 (9-cyanopaullone | 9-oxo-8,18-diazatetracyclo[9.7.0...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 30 |
CNRS | Assay Description Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/[gamma-32P] ATP. 32P... | Eur J Biochem 267: 5983-94 (2000) Article DOI: 10.1046/j.1432-1327.2000.01673.x BindingDB Entry DOI: 10.7270/Q2CR5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Rattus norvegicus (rat)) | BDBM7264 (2,3-dimethoxy-9-nitropaullone | 4,5-dimethoxy-14-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 30 |
CNRS | Assay Description Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/[gamma-32P] ATP. 32P... | Eur J Biochem 267: 5983-94 (2000) Article DOI: 10.1046/j.1432-1327.2000.01673.x BindingDB Entry DOI: 10.7270/Q2CR5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Rattus norvegicus (rat)) | BDBM7322 (14-bromo-5-hydroxy-8,18-diazatetracyclo[9.7.0.0^{2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 30 |
Universitat Hamburg | Assay Description Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32... | J Med Chem 47: 22-36 (2004) Article DOI: 10.1021/jm0308904 BindingDB Entry DOI: 10.7270/Q28050TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Rattus norvegicus (rat)) | BDBM7265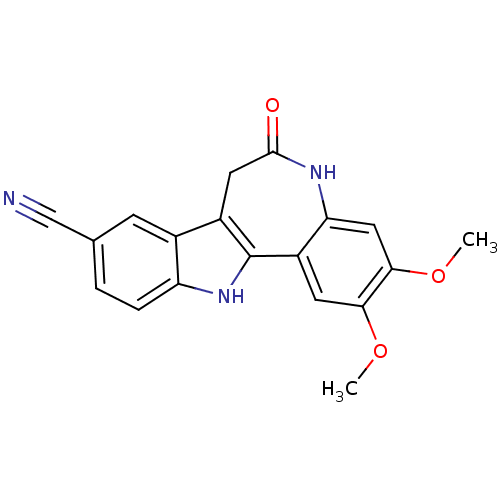 (4,5-dimethoxy-9-oxo-8,18-diazatetracyclo[9.7.0.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 30 |
CNRS | Assay Description Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/[gamma-32P] ATP. 32P... | Eur J Biochem 267: 5983-94 (2000) Article DOI: 10.1046/j.1432-1327.2000.01673.x BindingDB Entry DOI: 10.7270/Q2CR5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 [99-307] (Homo sapiens (Human)) | BDBM7264 (2,3-dimethoxy-9-nitropaullone | 4,5-dimethoxy-14-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | 30 |
CNRS | Assay Description Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/[gamma-32P] ATP. 32P... | Eur J Biochem 267: 5983-94 (2000) Article DOI: 10.1046/j.1432-1327.2000.01673.x BindingDB Entry DOI: 10.7270/Q2CR5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Rattus norvegicus (rat)) | BDBM7330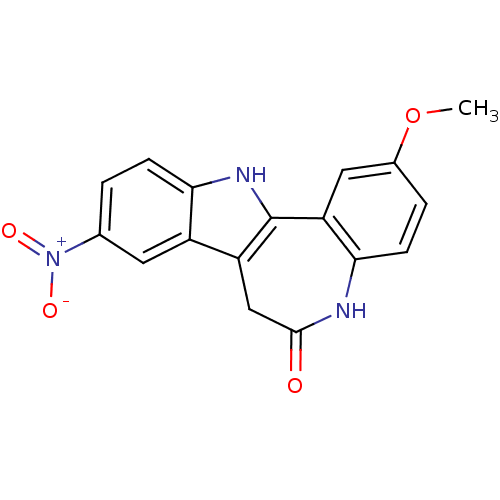 (2-Methoxy-9-nitro-7,12-dihydro-indolo[3,2-d][1]ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | 30 |
Universitat Hamburg | Assay Description Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32... | J Med Chem 47: 22-36 (2004) Article DOI: 10.1021/jm0308904 BindingDB Entry DOI: 10.7270/Q28050TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Rattus norvegicus (rat)) | BDBM7266 (14-bromo-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 30 |
CNRS | Assay Description Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/[gamma-32P] ATP. 32P... | Eur J Biochem 267: 5983-94 (2000) Article DOI: 10.1046/j.1432-1327.2000.01673.x BindingDB Entry DOI: 10.7270/Q2CR5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 (Homo sapiens (Human)) | BDBM84530 (Alsterpaullone derivative, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Technische Universität Braunschweig | Assay Description Kinase activity assay using GSK-3beta, CDK1/cyclin B and CDK5/p25. | Chembiochem 6: 541-9 (2005) Article DOI: 10.1002/cbic.200400099 BindingDB Entry DOI: 10.7270/Q2FX780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B (Marthasterias glacialis (starfish)) | BDBM7264 (2,3-dimethoxy-9-nitropaullone | 4,5-dimethoxy-14-n...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.2 | 30 |
CNRS | Assay Description Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/[gamma-32P] ATP. 32P... | Eur J Biochem 267: 5983-94 (2000) Article DOI: 10.1046/j.1432-1327.2000.01673.x BindingDB Entry DOI: 10.7270/Q2CR5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM7263 (9-cyanopaullone | 9-oxo-8,18-diazatetracyclo[9.7.0...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Hamburg Curated by ChEMBL | Assay Description In vitro inhibitory activity against cyclin-dependent kinase 1-cyclin B (Cyclin-Dependent Kinase) harvested from starfish oocytes. | J Med Chem 42: 2909-19 (1999) Article DOI: 10.1021/jm9900570 BindingDB Entry DOI: 10.7270/Q21C1W2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 377 total ) | Next | Last >> |