 Found 170 hits with Last Name = 'gutierrez' and Initial = 'ja'
Found 170 hits with Last Name = 'gutierrez' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase
(Escherichia coli (strain K12)) | BDBM36435
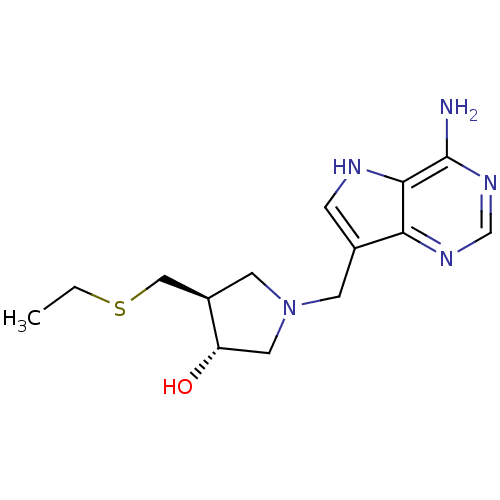
((3R,4S)-1-[(9-Deaza-adenin-9-yl)methyl]-4-ethylthi...)Show SMILES CCSC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O Show InChI InChI=1S/C14H21N5OS/c1-2-21-7-10-5-19(6-11(10)20)4-9-3-16-13-12(9)17-8-18-14(13)15/h3,8,10-11,16,20H,2,4-7H2,1H3,(H2,15,17,18)/t10-,11+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albert Einstein College of Medicine
| Assay Description
Purified MTAN activity in MTAN enzyme inhibition assay |
Nat Chem Biol 5: 251-7 (2009)
Article DOI: 10.1038/nchembio.153
BindingDB Entry DOI: 10.7270/Q28C9TM9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase
(Escherichia coli (strain K12)) | BDBM22113
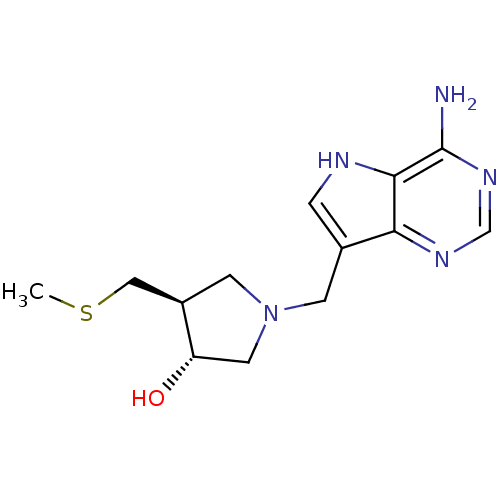
((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...)Show SMILES CSC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C13H19N5OS/c1-20-6-9-4-18(5-10(9)19)3-8-2-15-12-11(8)16-7-17-13(12)14/h2,7,9-10,15,19H,3-6H2,1H3,(H2,14,16,17)/t9-,10+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0730 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albert Einstein College of Medicine
| Assay Description
Purified MTAN activity in MTAN enzyme inhibition assay |
Nat Chem Biol 5: 251-7 (2009)
Article DOI: 10.1038/nchembio.153
BindingDB Entry DOI: 10.7270/Q28C9TM9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM22113

((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...)Show SMILES CSC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C13H19N5OS/c1-20-6-9-4-18(5-10(9)19)3-8-2-15-12-11(8)16-7-17-13(12)14/h2,7,9-10,15,19H,3-6H2,1H3,(H2,14,16,17)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase
(Escherichia coli (strain K12)) | BDBM36436
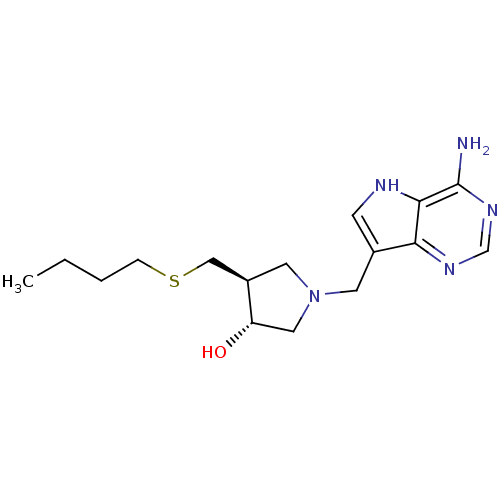
((3R,4S)-1-[(9-Deaza-adenin-9-yl)methyl]-4-ethylthi...)Show SMILES CCCCSC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O Show InChI InChI=1S/C16H25N5OS/c1-2-3-4-23-9-12-7-21(8-13(12)22)6-11-5-18-15-14(11)19-10-20-16(15)17/h5,10,12-13,18,22H,2-4,6-9H2,1H3,(H2,17,19,20)/t12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.208 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albert Einstein College of Medicine
| Assay Description
Purified MTAN activity in MTAN enzyme inhibition assay |
Nat Chem Biol 5: 251-7 (2009)
Article DOI: 10.1038/nchembio.153
BindingDB Entry DOI: 10.7270/Q28C9TM9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326399

((+/-)-trans-4-Butyl-1-[(9-deazaadenin-9-yl)methyl]...)Show SMILES CCCC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C15H23N5O/c1-2-3-4-10-6-20(8-12(10)21)7-11-5-17-14-13(11)18-9-19-15(14)16/h5,9-10,12,17,21H,2-4,6-8H2,1H3,(H2,16,18,19)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326399

((+/-)-trans-4-Butyl-1-[(9-deazaadenin-9-yl)methyl]...)Show SMILES CCCC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C15H23N5O/c1-2-3-4-10-6-20(8-12(10)21)7-11-5-17-14-13(11)18-9-19-15(14)16/h5,9-10,12,17,21H,2-4,6-8H2,1H3,(H2,16,18,19)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390240
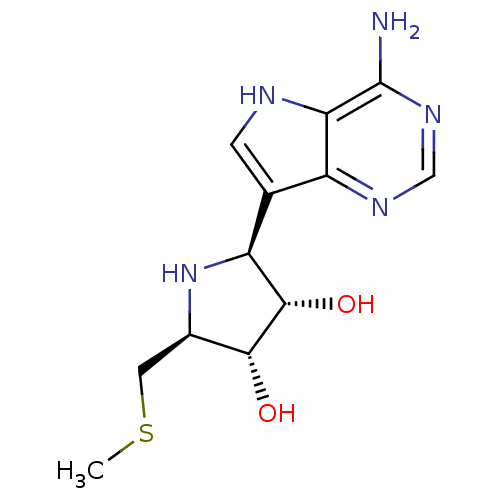
(CHEMBL1195586)Show SMILES CSC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c(N)ncnc12 |r| Show InChI InChI=1S/C12H17N5O2S/c1-20-3-6-10(18)11(19)8(17-6)5-2-14-9-7(5)15-4-16-12(9)13/h2,4,6,8,10-11,14,17-19H,3H2,1H3,(H2,13,15,16)/t6-,8+,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326400
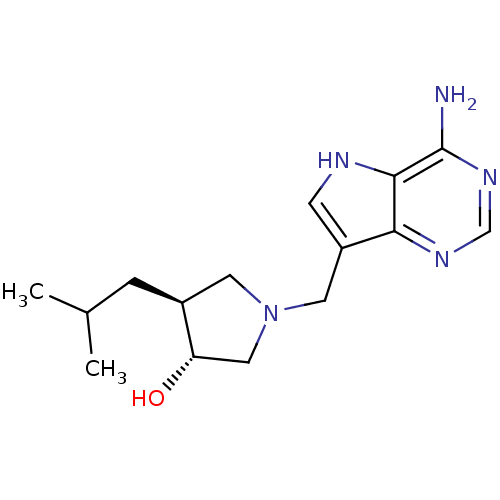
((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES CC(C)C[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C15H23N5O/c1-9(2)3-10-5-20(7-12(10)21)6-11-4-17-14-13(11)18-8-19-15(14)16/h4,8-10,12,17,21H,3,5-7H2,1-2H3,(H2,16,18,19)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326402

((+/-)-trans-4-Cyclopropyl-1-[(9-deazaadenin-9-yl)m...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)C3CC3)c[nH]c12 |r| Show InChI InChI=1S/C14H19N5O/c15-14-13-12(17-7-18-14)9(3-16-13)4-19-5-10(8-1-2-8)11(20)6-19/h3,7-8,10-11,16,20H,1-2,4-6H2,(H2,15,17,18)/t10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326403
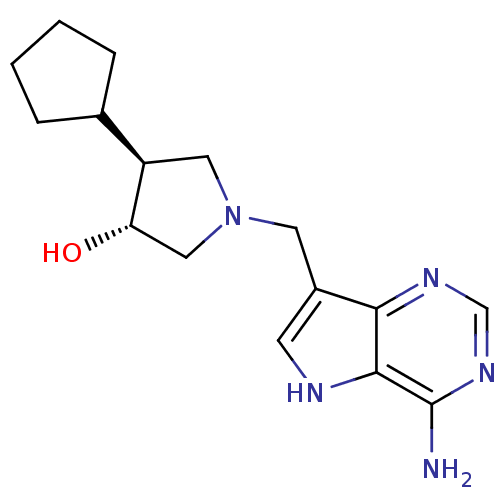
((+/-)-trans-4-Cyclopentyl-1-[(9-deazaadenin-9-yl)m...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)C3CCCC3)c[nH]c12 |r| Show InChI InChI=1S/C16H23N5O/c17-16-15-14(19-9-20-16)11(5-18-15)6-21-7-12(13(22)8-21)10-3-1-2-4-10/h5,9-10,12-13,18,22H,1-4,6-8H2,(H2,17,19,20)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326407
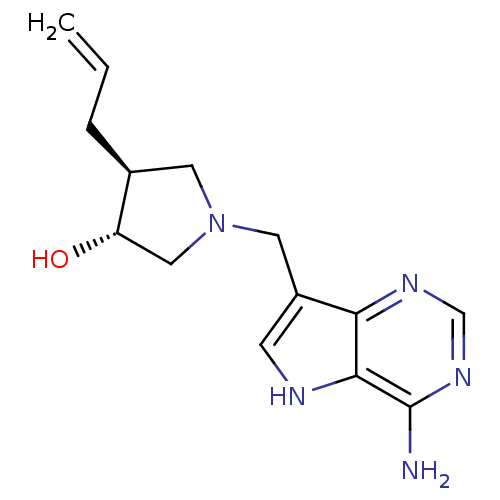
((+/-)-trans-4-Allyl-1-[(9-deazaadenin-9-yl)methyl]...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CC=C)C3)c[nH]c12 |r| Show InChI InChI=1S/C14H19N5O/c1-2-3-9-5-19(7-11(9)20)6-10-4-16-13-12(10)17-8-18-14(13)15/h2,4,8-9,11,16,20H,1,3,5-7H2,(H2,15,17,18)/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326406
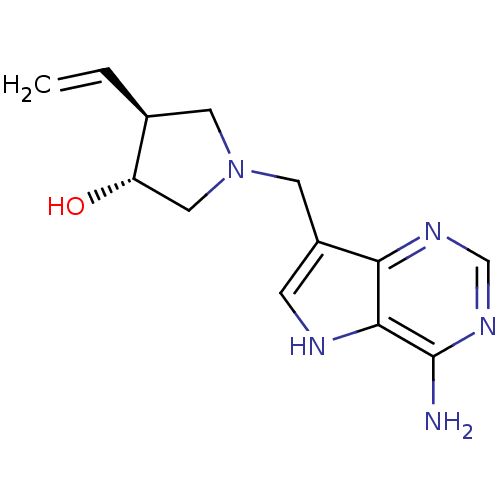
((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)C=C)c[nH]c12 |r| Show InChI InChI=1S/C13H17N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h2-3,7-8,10,15,19H,1,4-6H2,(H2,14,16,17)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326398

((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyl...)Show SMILES CC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C13H19N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h3,7-8,10,15,19H,2,4-6H2,1H3,(H2,14,16,17)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326398

((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyl...)Show SMILES CC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C13H19N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h3,7-8,10,15,19H,2,4-6H2,1H3,(H2,14,16,17)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390242
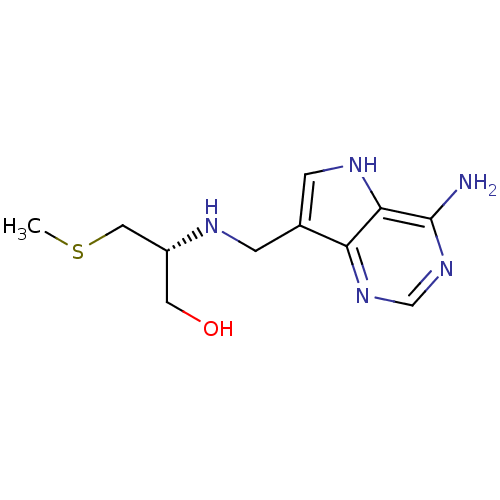
(CHEMBL2070308)Show InChI InChI=1S/C11H17N5OS/c1-18-5-8(4-17)13-2-7-3-14-10-9(7)15-6-16-11(10)12/h3,6,8,13-14,17H,2,4-5H2,1H3,(H2,12,15,16)/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319585

(US10174007, Example 4 | US10787438, Example 4 | US...)Show SMILES C[C@H]1CCN1c1nc(cc(n1)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C16H19F3N4O2/c1-8-2-3-23(8)15-20-12(16(17,18)19)5-13(21-15)22-6-10-9(4-14(24)25)11(10)7-22/h5,8-11H,2-4,6-7H2,1H3,(H,24,25)/t8-,9-,10-,11+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed noncompetitive inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390245
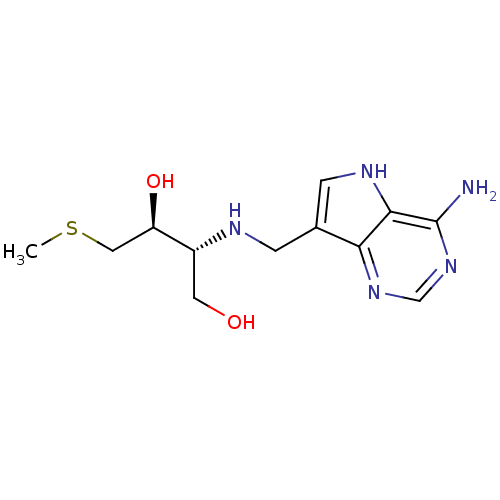
(CHEMBL2070311)Show SMILES CSC[C@@H](O)[C@@H](CO)NCc1c[nH]c2c(N)ncnc12 |r| Show InChI InChI=1S/C12H19N5O2S/c1-20-5-9(19)8(4-18)14-2-7-3-15-11-10(7)16-6-17-12(11)13/h3,6,8-9,14-15,18-19H,2,4-5H2,1H3,(H2,13,16,17)/t8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326405
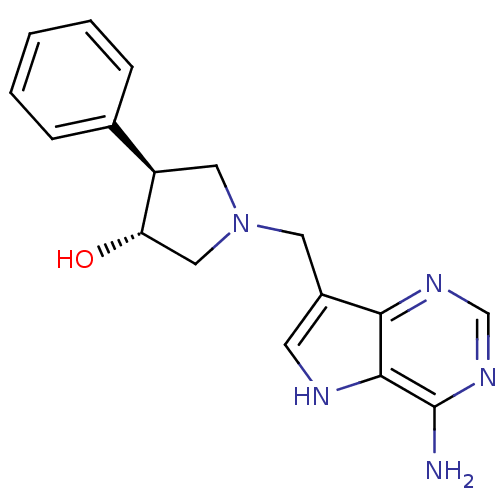
((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)c3ccccc3)c[nH]c12 |r| Show InChI InChI=1S/C17H19N5O/c18-17-16-15(20-10-21-17)12(6-19-16)7-22-8-13(14(23)9-22)11-4-2-1-3-5-11/h1-6,10,13-14,19,23H,7-9H2,(H2,18,20,21)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390244
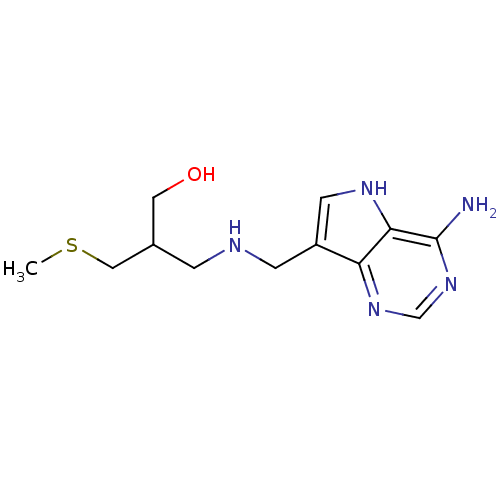
(CHEMBL2070310)Show InChI InChI=1S/C12H19N5OS/c1-19-6-8(5-18)2-14-3-9-4-15-11-10(9)16-7-17-12(11)13/h4,7-8,14-15,18H,2-3,5-6H2,1H3,(H2,13,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390241
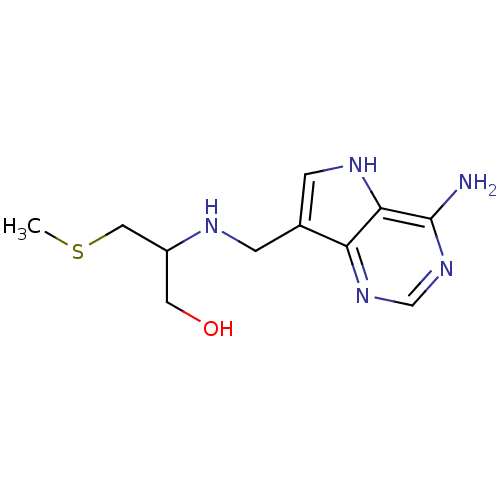
(CHEMBL2070307)Show InChI InChI=1S/C11H17N5OS/c1-18-5-8(4-17)13-2-7-3-14-10-9(7)15-6-16-11(10)12/h3,6,8,13-14,17H,2,4-5H2,1H3,(H2,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390255
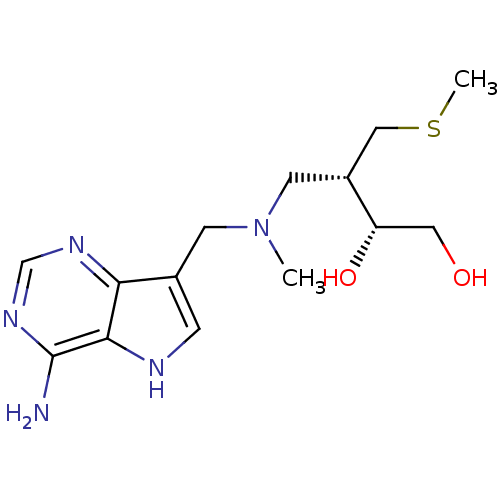
(CHEMBL2070405)Show SMILES CSC[C@@H](CN(C)Cc1c[nH]c2c(N)ncnc12)[C@@H](O)CO |r| Show InChI InChI=1S/C14H23N5O2S/c1-19(5-10(7-22-2)11(21)6-20)4-9-3-16-13-12(9)17-8-18-14(13)15/h3,8,10-11,16,20-21H,4-7H2,1-2H3,(H2,15,17,18)/t10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326404
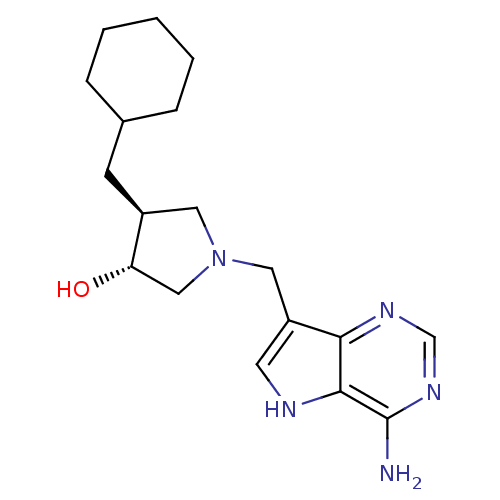
((+/-)-trans-4-(Cyclohexylmethyl)-1-[(9-deaza-adeni...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CC4CCCCC4)C3)c[nH]c12 |r| Show InChI InChI=1S/C18H27N5O/c19-18-17-16(21-11-22-18)14(7-20-17)9-23-8-13(15(24)10-23)6-12-4-2-1-3-5-12/h7,11-13,15,20,24H,1-6,8-10H2,(H2,19,21,22)/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326401

((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES CCC(CC)C[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C17H27N5O/c1-3-11(4-2)5-12-7-22(9-14(12)23)8-13-6-19-16-15(13)20-10-21-17(16)18/h6,10-12,14,19,23H,3-5,7-9H2,1-2H3,(H2,18,20,21)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326408
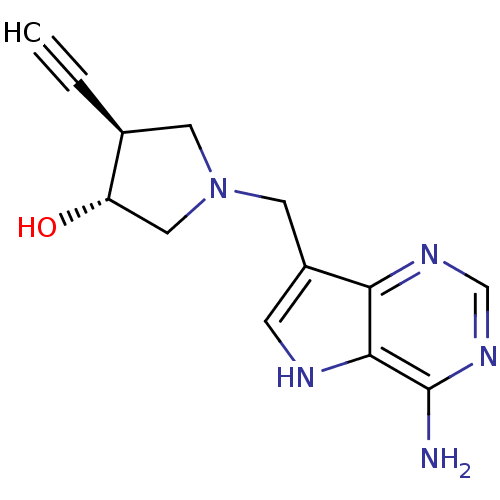
((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyn...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)C#C)c[nH]c12 |r| Show InChI InChI=1S/C13H15N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h1,3,7-8,10,15,19H,4-6H2,(H2,14,16,17)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326411

((+/-)-Benzyl cis-3-(Benzoyloxy)-4-ethylpyrrolidine...)Show SMILES CC[C@H]1CN(Cc2c[nH]c3c(N)ncnc23)C[C@H]1O |r| Show InChI InChI=1S/C13H19N5O/c1-2-8-4-18(6-10(8)19)5-9-3-15-12-11(9)16-7-17-13(12)14/h3,7-8,10,15,19H,2,4-6H2,1H3,(H2,14,16,17)/t8-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390251
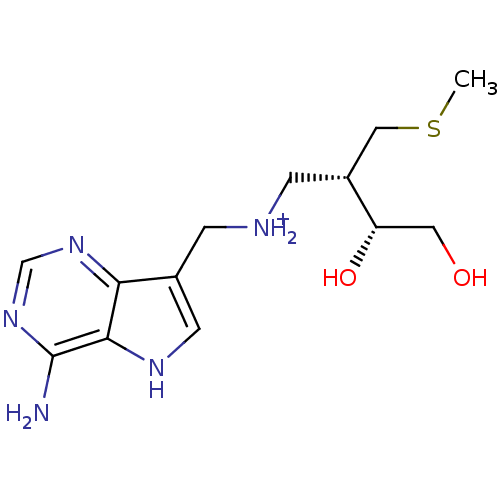
(CHEMBL2070404)Show SMILES CSC[C@@H](C[NH2+]Cc1c[nH]c2c(N)ncnc12)[C@@H](O)CO |r| Show InChI InChI=1S/C13H21N5O2S/c1-21-6-9(10(20)5-19)3-15-2-8-4-16-12-11(8)17-7-18-13(12)14/h4,7,9-10,15-16,19-20H,2-3,5-6H2,1H3,(H2,14,17,18)/p+1/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390249
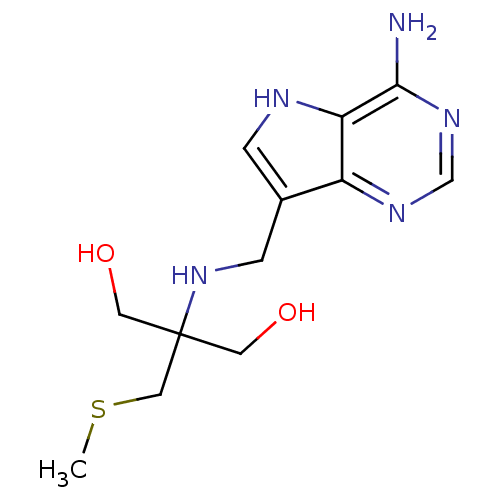
(CHEMBL2070402)Show InChI InChI=1S/C12H19N5O2S/c1-20-6-12(4-18,5-19)17-3-8-2-14-10-9(8)15-7-16-11(10)13/h2,7,14,17-19H,3-6H2,1H3,(H2,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390248
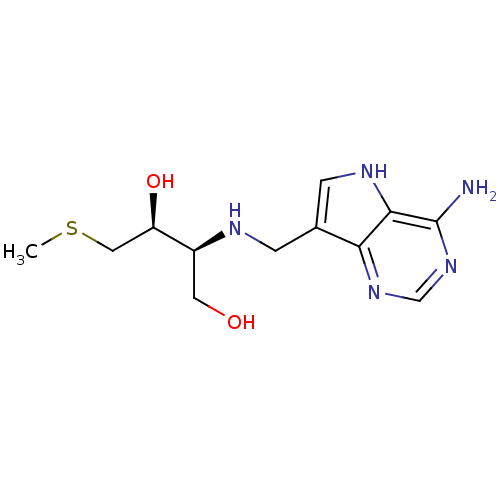
(CHEMBL2070401)Show SMILES CSC[C@@H](O)[C@H](CO)NCc1c[nH]c2c(N)ncnc12 |r| Show InChI InChI=1S/C12H19N5O2S/c1-20-5-9(19)8(4-18)14-2-7-3-15-11-10(7)16-6-17-12(11)13/h3,6,8-9,14-15,18-19H,2,4-5H2,1H3,(H2,13,16,17)/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390243
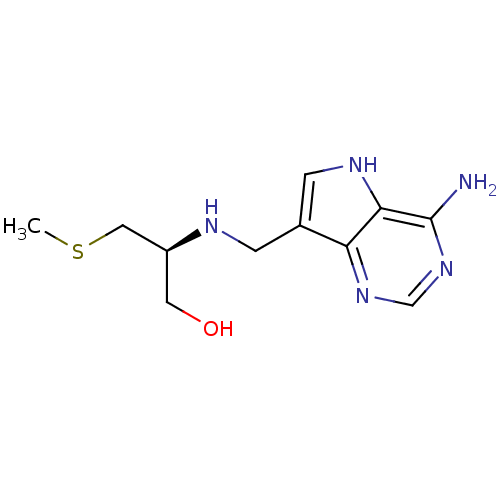
(CHEMBL2070309)Show InChI InChI=1S/C11H17N5OS/c1-18-5-8(4-17)13-2-7-3-14-10-9(7)15-6-16-11(10)12/h3,6,8,13-14,17H,2,4-5H2,1H3,(H2,12,15,16)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326409

((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@H](C3)n3ccnn3)c[nH]c12 |r| Show InChI InChI=1S/C13H16N8O/c14-13-12-11(16-7-17-13)8(3-15-12)4-20-5-9(10(22)6-20)21-2-1-18-19-21/h1-3,7,9-10,15,22H,4-6H2,(H2,14,16,17)/t9-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50326410

((+/-)-trans-4-[3-(Benzylthio)propyl]-1-[(9-deazaad...)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CCCSCc4ccccc4)C3)c[nH]c12 |r| Show InChI InChI=1S/C21H27N5OS/c22-21-20-19(24-14-25-21)17(9-23-20)11-26-10-16(18(27)12-26)7-4-8-28-13-15-5-2-1-3-6-15/h1-3,5-6,9,14,16,18,23,27H,4,7-8,10-13H2,(H2,22,24,25)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MTAP |
J Med Chem 53: 6730-46 (2010)
Article DOI: 10.1021/jm100898v
BindingDB Entry DOI: 10.7270/Q22R3RWQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390247
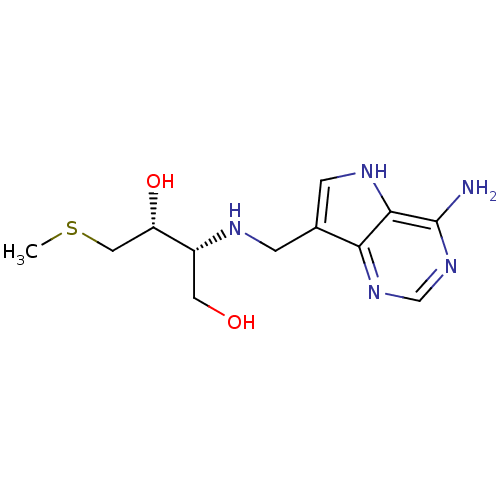
(CHEMBL2070400)Show SMILES CSC[C@H](O)[C@@H](CO)NCc1c[nH]c2c(N)ncnc12 |r| Show InChI InChI=1S/C12H19N5O2S/c1-20-5-9(19)8(4-18)14-2-7-3-15-11-10(7)16-6-17-12(11)13/h3,6,8-9,14-15,18-19H,2,4-5H2,1H3,(H2,13,16,17)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390246
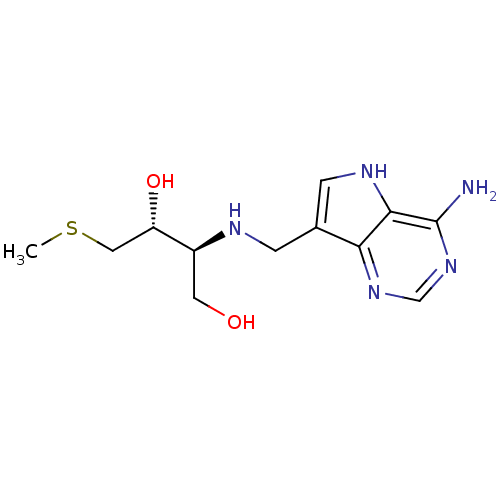
(CHEMBL2070312)Show SMILES CSC[C@H](O)[C@H](CO)NCc1c[nH]c2c(N)ncnc12 |r| Show InChI InChI=1S/C12H19N5O2S/c1-20-5-9(19)8(4-18)14-2-7-3-15-11-10(7)16-6-17-12(11)13/h3,6,8-9,14-15,18-19H,2,4-5H2,1H3,(H2,13,16,17)/t8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390252

(CHEMBL2070406)Show SMILES CSC[C@H](O)[C@@H](CO)CN(C)Cc1c[nH]c2c(N)ncnc12 |r| Show InChI InChI=1S/C14H23N5O2S/c1-19(5-10(6-20)11(21)7-22-2)4-9-3-16-13-12(9)17-8-18-14(13)15/h3,8,10-11,16,20-21H,4-7H2,1-2H3,(H2,15,17,18)/t10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390254
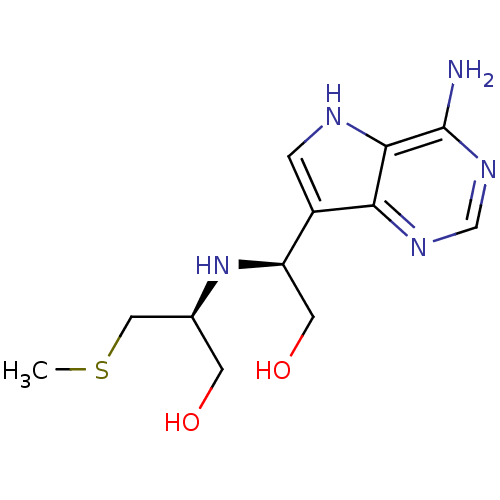
(CHEMBL2070408)Show SMILES CSC[C@H](CO)N[C@H](CO)c1c[nH]c2c(N)ncnc12 |r| Show InChI InChI=1S/C12H19N5O2S/c1-20-5-7(3-18)17-9(4-19)8-2-14-11-10(8)15-6-16-12(11)13/h2,6-7,9,14,17-19H,3-5H2,1H3,(H2,13,15,16)/t7-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390253
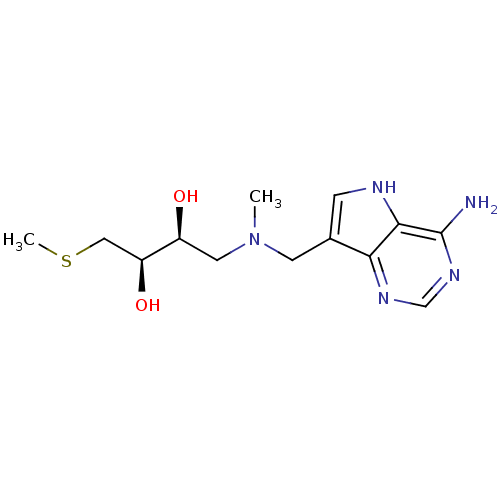
(CHEMBL2070407)Show SMILES CSC[C@H](O)[C@@H](O)CN(C)Cc1c[nH]c2c(N)ncnc12 |r| Show InChI InChI=1S/C13H21N5O2S/c1-18(5-9(19)10(20)6-21-2)4-8-3-15-12-11(8)16-7-17-13(12)14/h3,7,9-10,15,19-20H,4-6H2,1-2H3,(H2,14,16,17)/t9-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 368 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50390250
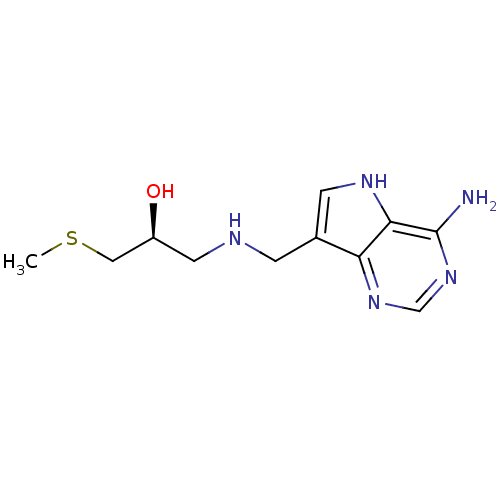
(CHEMBL2070403)Show InChI InChI=1S/C11H17N5OS/c1-18-5-8(17)4-13-2-7-3-14-10-9(7)15-6-16-11(10)12/h3,6,8,13-14,17H,2,4-5H2,1H3,(H2,12,15,16)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 602 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis |
Bioorg Med Chem 20: 5181-7 (2012)
Article DOI: 10.1016/j.bmc.2012.07.006
BindingDB Entry DOI: 10.7270/Q2XG9S6F |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319582
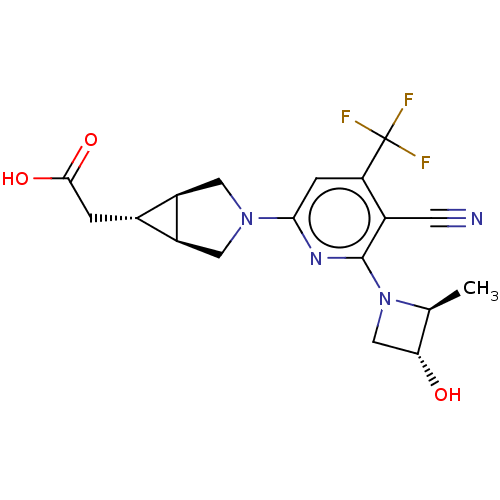
(US10174007, Example 1 | US10787438, Example 1 | US...)Show SMILES C[C@H]1[C@H](O)CN1c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C18H19F3N4O3/c1-8-14(26)7-25(8)17-10(4-22)13(18(19,20)21)3-15(23-17)24-5-11-9(2-16(27)28)12(11)6-24/h3,8-9,11-12,14,26H,2,5-7H2,1H3,(H,27,28)/t8-,9-,11-,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM195572
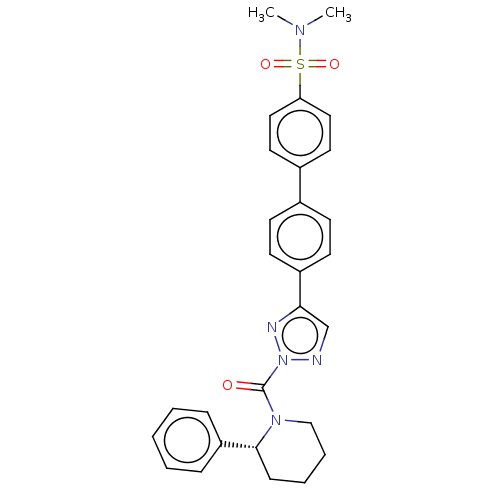
((R)-N,N-dimethyl-4'-(2-(2-phenylpiperidine-1-c...)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)-c1cnn(n1)C(=O)N1CCCC[C@@H]1c1ccccc1 Show InChI InChI=1S/C28H29N5O3S/c1-31(2)37(35,36)25-17-15-22(16-18-25)21-11-13-23(14-12-21)26-20-29-33(30-26)28(34)32-19-7-6-10-27(32)24-8-4-3-5-9-24/h3-5,8-9,11-18,20,27H,6-7,10,19H2,1-2H3/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM195570

((S)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...)Show SMILES OC(=O)c1ccc(cc1)-c1ccc(cc1)-c1cnn(n1)C(=O)N1CCCC[C@H]1c1ccccc1 Show InChI InChI=1S/C27H24N4O3/c32-26(33)23-15-11-20(12-16-23)19-9-13-21(14-10-19)24-18-28-31(29-24)27(34)30-17-5-4-8-25(30)22-6-2-1-3-7-22/h1-3,6-7,9-16,18,25H,4-5,8,17H2,(H,32,33)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |
Lysophospholipase-like protein 1
(Homo sapiens (Human)) | BDBM195581
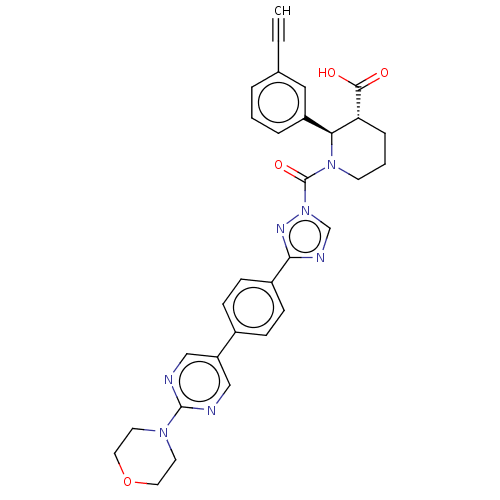
((2R,3R)-2-(3-ethynylphenyl)-1-(3-(4-(2-morpholinop...)Show SMILES OC(=O)[C@@H]1CCCN([C@H]1c1cccc(c1)C#C)C(=O)n1cnc(n1)-c1ccc(cc1)-c1cnc(nc1)N1CCOCC1 Show InChI InChI=1S/C31H29N7O4/c1-2-21-5-3-6-24(17-21)27-26(29(39)40)7-4-12-37(27)31(41)38-20-34-28(35-38)23-10-8-22(9-11-23)25-18-32-30(33-19-25)36-13-15-42-16-14-36/h1,3,5-6,8-11,17-20,26-27H,4,7,12-16H2,(H,39,40)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM195571
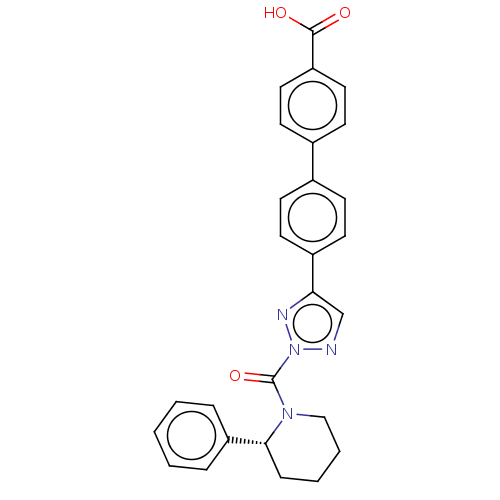
((R)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...)Show SMILES OC(=O)c1ccc(cc1)-c1ccc(cc1)-c1cnn(n1)C(=O)N1CCCC[C@@H]1c1ccccc1 Show InChI InChI=1S/C27H24N4O3/c32-26(33)23-15-11-20(12-16-23)19-9-13-21(14-10-19)24-18-28-31(29-24)27(34)30-17-5-4-8-25(30)22-6-2-1-3-7-22/h1-3,6-7,9-16,18,25H,4-5,8,17H2,(H,32,33)/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM195574
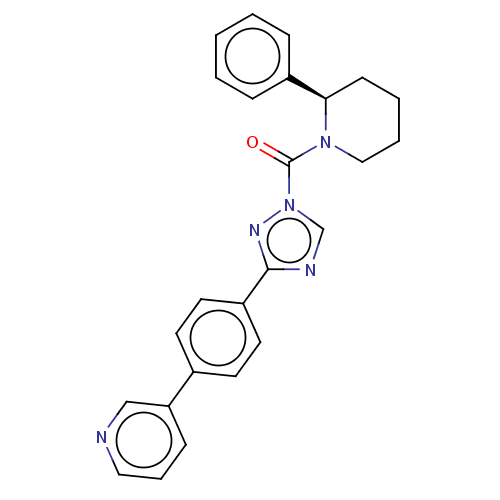
((R)-(2-phenylpiperidin-1-yl)(3-(4-(pyridin-3-yl)ph...)Show SMILES O=C(N1CCCC[C@@H]1c1ccccc1)n1cnc(n1)-c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C25H23N5O/c31-25(29-16-5-4-10-23(29)20-7-2-1-3-8-20)30-18-27-24(28-30)21-13-11-19(12-14-21)22-9-6-15-26-17-22/h1-3,6-9,11-15,17-18,23H,4-5,10,16H2/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM195575
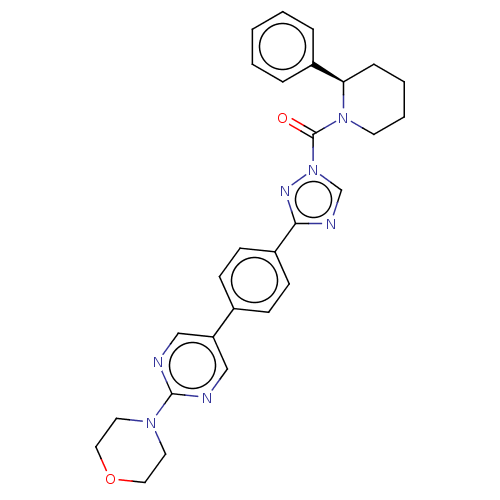
((R)-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H-1...)Show SMILES O=C(N1CCCC[C@@H]1c1ccccc1)n1cnc(n1)-c1ccc(cc1)-c1cnc(nc1)N1CCOCC1 Show InChI InChI=1S/C28H29N7O2/c36-28(34-13-5-4-8-25(34)22-6-2-1-3-7-22)35-20-31-26(32-35)23-11-9-21(10-12-23)24-18-29-27(30-19-24)33-14-16-37-17-15-33/h1-3,6-7,9-12,18-20,25H,4-5,8,13-17H2/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |
Lysophospholipase-like protein 1
(Homo sapiens (Human)) | BDBM195580
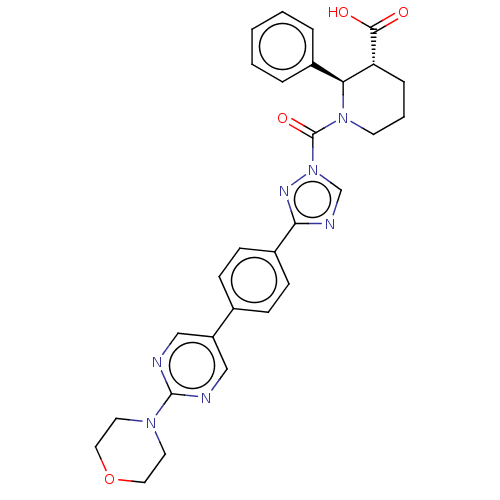
((2R,3R)-1-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl...)Show SMILES OC(=O)[C@@H]1CCCN([C@H]1c1ccccc1)C(=O)n1cnc(n1)-c1ccc(cc1)-c1cnc(nc1)N1CCOCC1 Show InChI InChI=1S/C29H29N7O4/c37-27(38)24-7-4-12-35(25(24)21-5-2-1-3-6-21)29(39)36-19-32-26(33-36)22-10-8-20(9-11-22)23-17-30-28(31-18-23)34-13-15-40-16-14-34/h1-3,5-6,8-11,17-19,24-25H,4,7,12-16H2,(H,37,38)/t24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319582

(US10174007, Example 1 | US10787438, Example 1 | US...)Show SMILES C[C@H]1[C@H](O)CN1c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C18H19F3N4O3/c1-8-14(26)7-25(8)17-10(4-22)13(18(19,20)21)3-15(23-17)24-5-11-9(2-16(27)28)12(11)6-24/h3,8-9,11-12,14,26H,2,5-7H2,1H3,(H,27,28)/t8-,9-,11-,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM195573
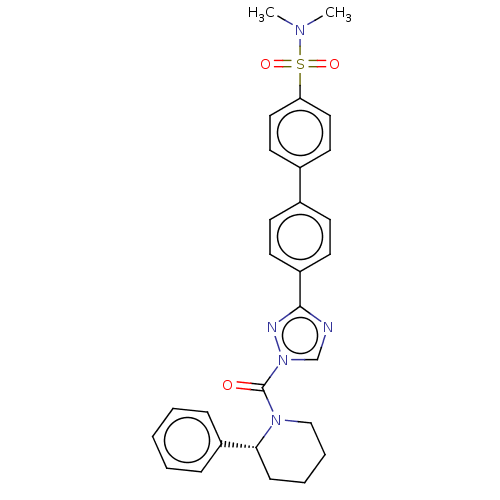
((R)-4'-(1-(2-phenylpiperidine-1-carbonyl)-1H-1...)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)-c1ncn(n1)C(=O)N1CCCC[C@@H]1c1ccccc1 Show InChI InChI=1S/C26H25N5O3S/c27-35(33,34)23-15-13-20(14-16-23)19-9-11-22(12-10-19)25-28-18-31(29-25)26(32)30-17-5-4-8-24(30)21-6-2-1-3-7-21/h1-3,6-7,9-16,18,24H,4-5,8,17H2,(H2,27,33,34)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |
Lysophospholipase-like protein 1
(Homo sapiens (Human)) | BDBM195575

((R)-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H-1...)Show SMILES O=C(N1CCCC[C@@H]1c1ccccc1)n1cnc(n1)-c1ccc(cc1)-c1cnc(nc1)N1CCOCC1 Show InChI InChI=1S/C28H29N7O2/c36-28(34-13-5-4-8-25(34)22-6-2-1-3-7-22)35-20-31-26(32-35)23-11-9-21(10-12-23)24-18-29-27(30-19-24)33-14-16-37-17-15-33/h1-3,6-7,9-12,18-20,25H,4-5,8,13-17H2/t25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319585

(US10174007, Example 4 | US10787438, Example 4 | US...)Show SMILES C[C@H]1CCN1c1nc(cc(n1)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C16H19F3N4O2/c1-8-2-3-23(8)15-20-12(16(17,18)19)5-13(21-15)22-6-10-9(4-14(24)25)11(10)7-22/h5,8-11H,2-4,6-7H2,1H3,(H,24,25)/t8-,9-,10-,11+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
Lysophospholipase-like protein 1
(Homo sapiens (Human)) | BDBM195578
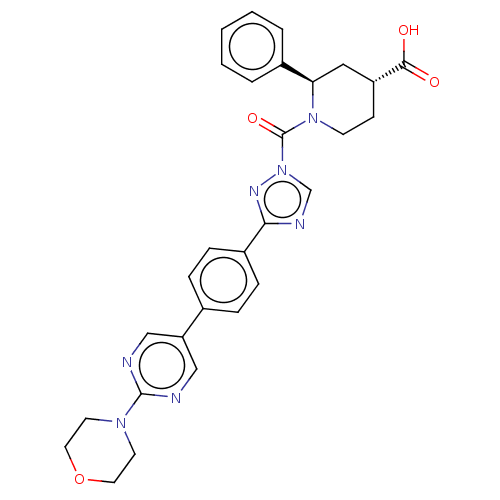
((2R, 4R)-1-(3-(4-(2-morpholinopyrimidin-5-yl)pheny...)Show SMILES OC(=O)[C@@H]1CCN([C@H](C1)c1ccccc1)C(=O)n1cnc(n1)-c1ccc(cc1)-c1cnc(nc1)N1CCOCC1 Show InChI InChI=1S/C29H29N7O4/c37-27(38)23-10-11-35(25(16-23)21-4-2-1-3-5-21)29(39)36-19-32-26(33-36)22-8-6-20(7-9-22)24-17-30-28(31-18-24)34-12-14-40-15-13-34/h1-9,17-19,23,25H,10-16H2,(H,37,38)/t23-,25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
| Assay Description
For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... |
ACS Chem Biol 11: 2529-40 (2016)
Article DOI: 10.1021/acschembio.6b00266
BindingDB Entry DOI: 10.7270/Q24X56K5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data