

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408704 (CHEMBL282229) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474384 (CHEMBL2113364) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474398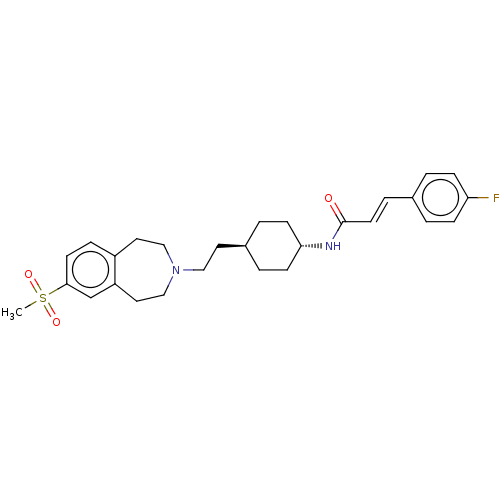 (CHEMBL2368629) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM85166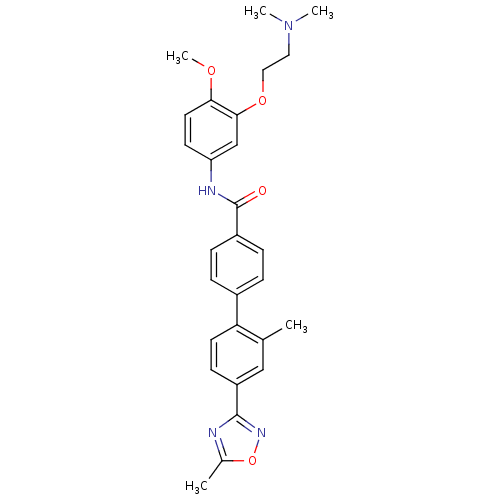 (CAS_3292447 | NSC_3292447 | SB 216641) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474396 (CHEMBL2113356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50098551 ((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Med Chem 43: 342-5 (2000) Article DOI: 10.1021/jm991151j BindingDB Entry DOI: 10.7270/Q28S4NGP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474403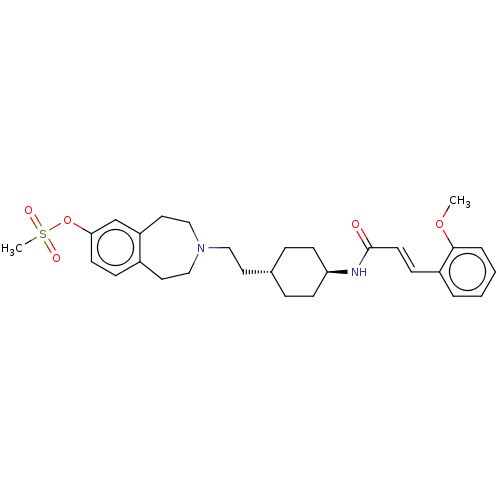 (CHEMBL2368633) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474399 (CHEMBL2368625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474389 (CHEMBL3084597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474406 (CHEMBL3084598) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474412 (CHEMBL2368622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474395 (CHEMBL2368626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408701 (CHEMBL21724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474393 (CHEMBL2368623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474410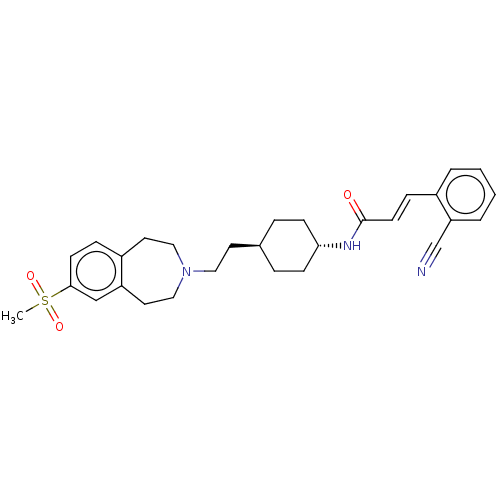 (CHEMBL2368620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474401 (CHEMBL2368618) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474404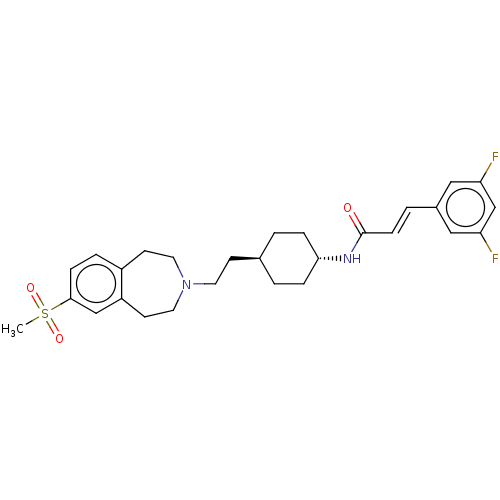 (CHEMBL2368627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474413 (CHEMBL3084626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474407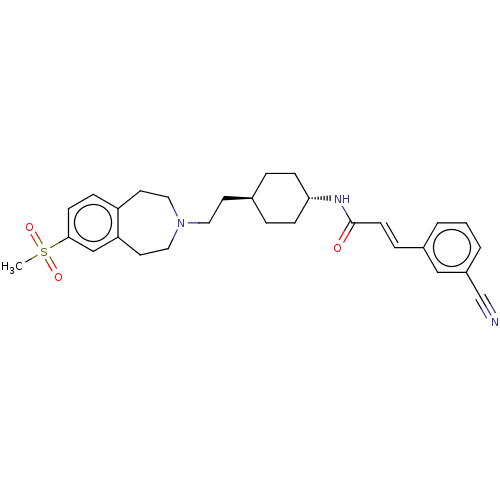 (CHEMBL2368624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474392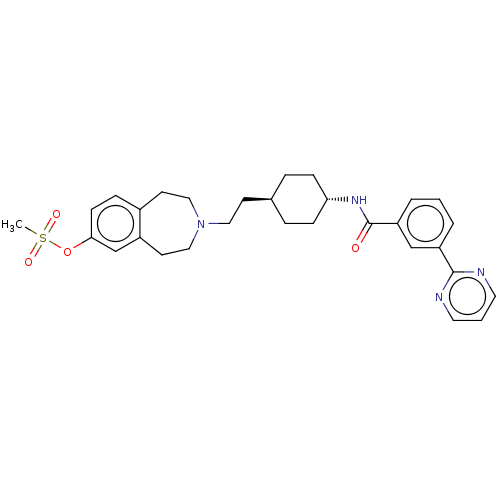 (CHEMBL3084601) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474414 (CHEMBL2368617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474388 (CHEMBL3084625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM85610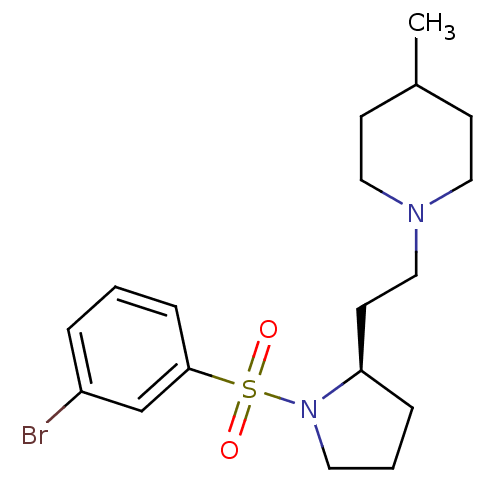 ((R)-1-(2-(1-(3-bromo-benzenesulfonyl)-pyrrolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Med Chem 43: 342-5 (2000) Article DOI: 10.1021/jm991151j BindingDB Entry DOI: 10.7270/Q28S4NGP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474400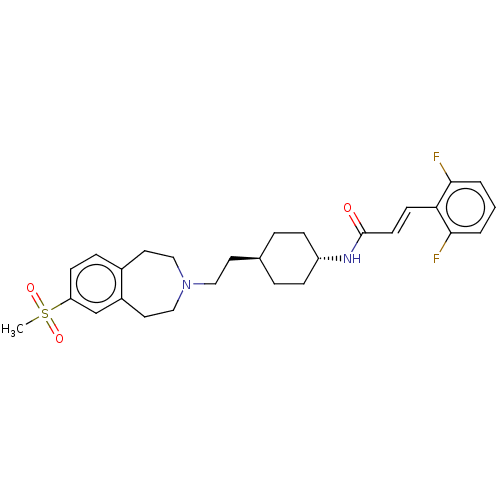 (CHEMBL2368619) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474408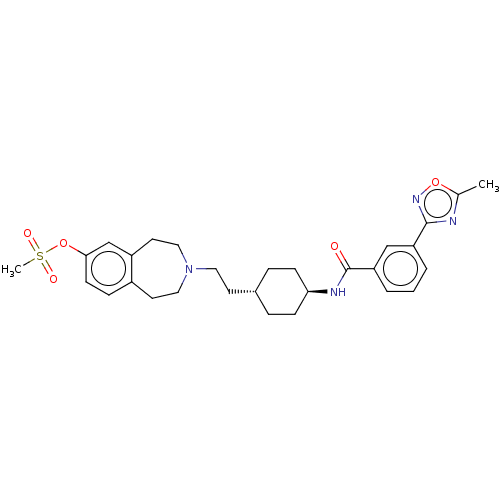 (CHEMBL3084613) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474390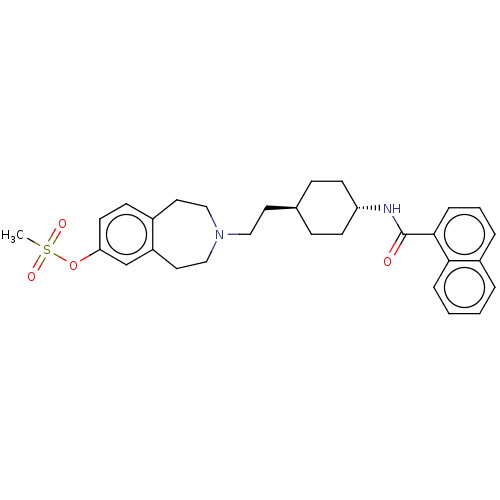 (CHEMBL3084624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408705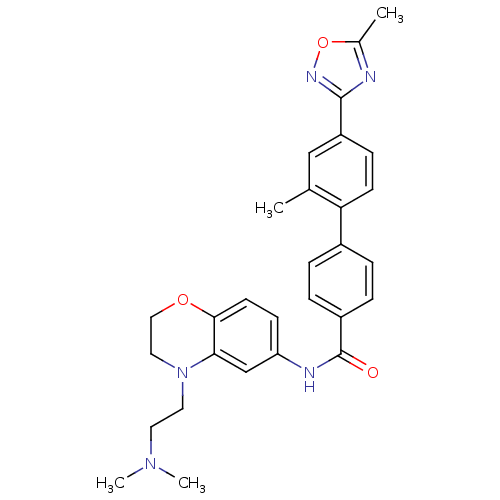 (CHEMBL20791) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408700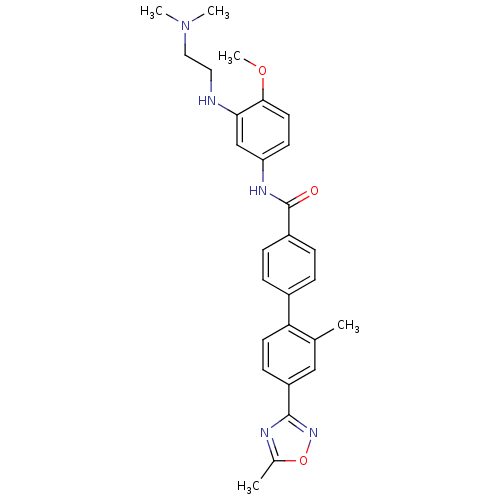 (CHEMBL282693) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408702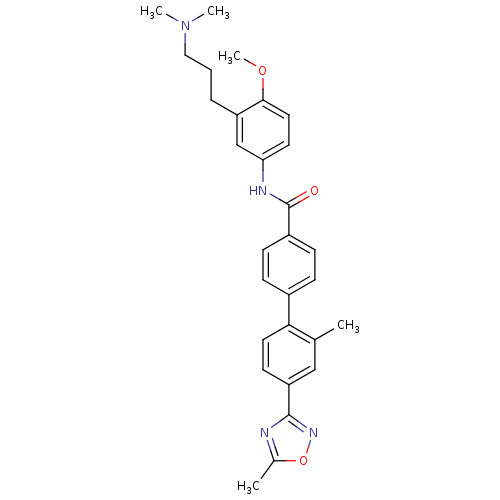 (CHEMBL21790) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM78940 (METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | Br J Pharmacol 124: 1300-6 (1998) Article DOI: 10.1038/sj.bjp.0701946 BindingDB Entry DOI: 10.7270/Q269724V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50130279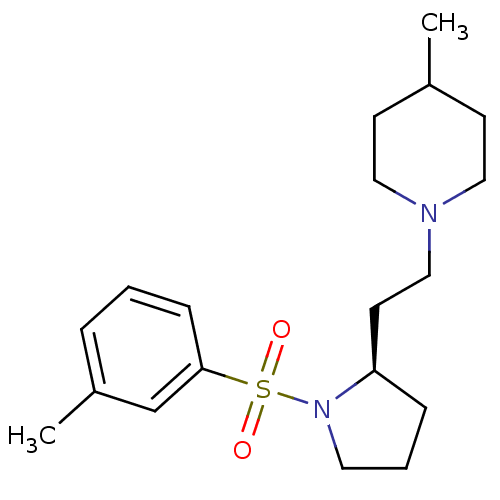 ((R)-4-Methyl-1-(2-(1-toluene-3-sulfonyl)-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Med Chem 43: 342-5 (2000) Article DOI: 10.1021/jm991151j BindingDB Entry DOI: 10.7270/Q28S4NGP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474394 (CHEMBL2113363) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474391 (CHEMBL3084596) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474409 (CHEMBL3084612) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474397 (CHEMBL3084629) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474386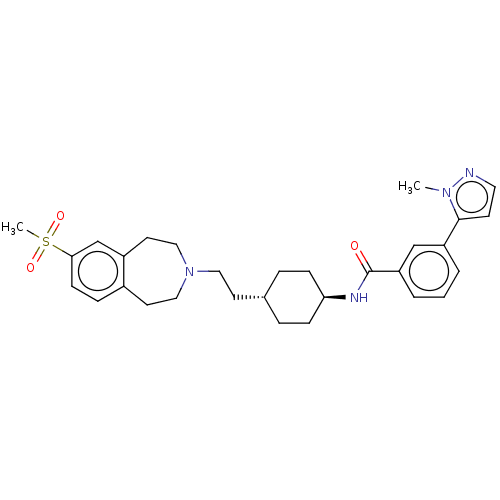 (CHEMBL3084605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM85608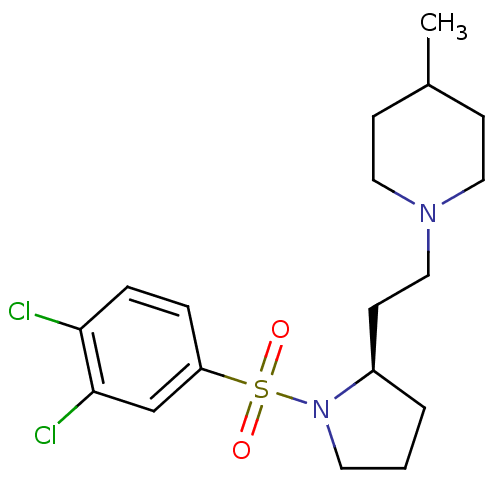 ((R)-1-(2-(1-(3,4-Dichloro-benzene sulfonyl)-pyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Med Chem 43: 342-5 (2000) Article DOI: 10.1021/jm991151j BindingDB Entry DOI: 10.7270/Q28S4NGP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50408705 (CHEMBL20791) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470855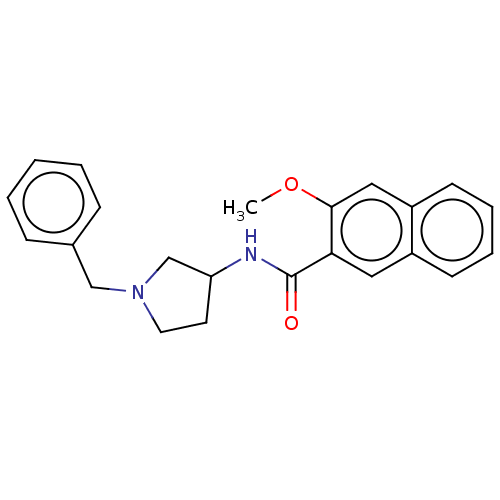 (CHEMBL60708) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474385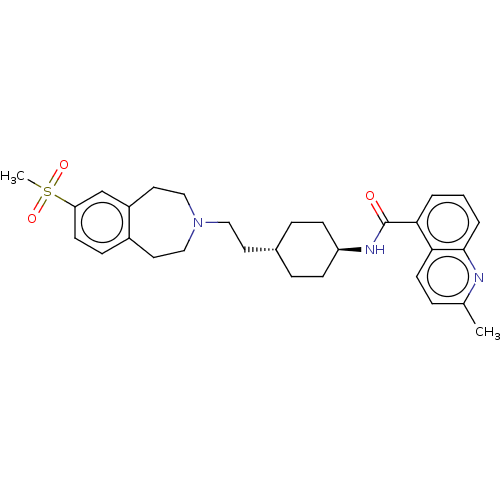 (CHEMBL3084599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474402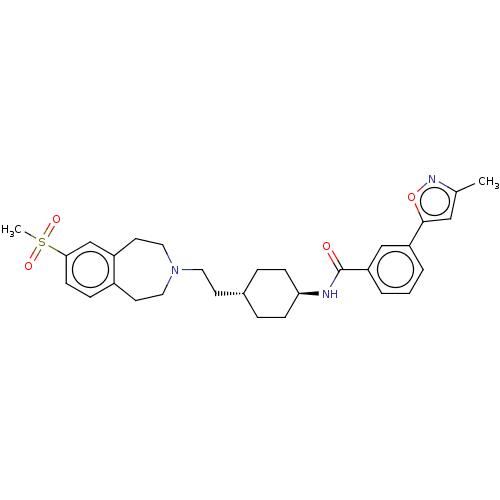 (CHEMBL3084615) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50411370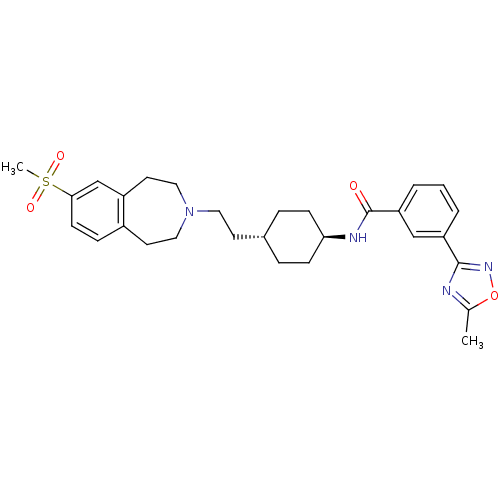 (CHEMBL244083 | SB-414796) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470850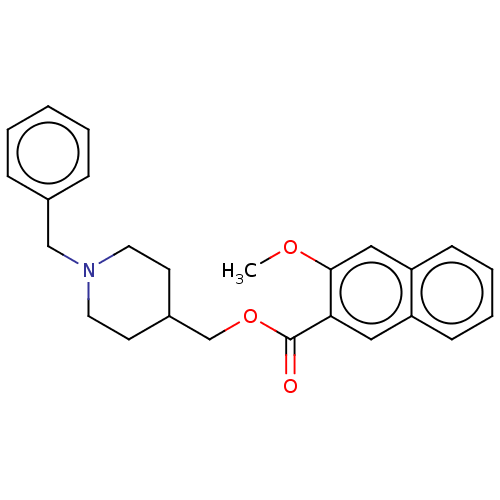 (CHEMBL59326) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50408700 (CHEMBL282693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408704 (CHEMBL282229) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | Br J Pharmacol 124: 1300-6 (1998) Article DOI: 10.1038/sj.bjp.0701946 BindingDB Entry DOI: 10.7270/Q269724V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474387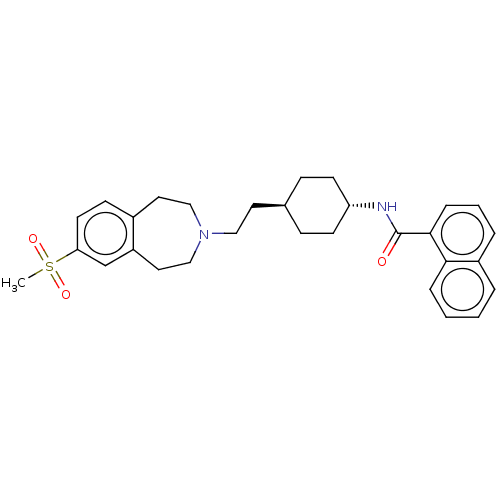 (CHEMBL3084620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474411 (CHEMBL3084602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 321 total ) | Next | Last >> |