 Found 3421 hits with Last Name = 'hall' and Initial = 'm'
Found 3421 hits with Last Name = 'hall' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM391716

(N-[(2R)-3-(7-methyl-1H- | US10300056, Example 1 | ...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)C(=O)N[C@@H](CC2CCNCC2)C(=O)N2CCN(CC2)c2ccncc2)cc2cn[nH]c12 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... |
J Med Chem 63: 7906-7920 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01003
BindingDB Entry DOI: 10.7270/Q2NK3JKR |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50539955

(CHEMBL4638938)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)C(=O)N[C@@H](CCCCN)C(=O)N2CCN(CC2)c2ccncc2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C38H48N12O4/c1-25-21-26(22-27-24-42-46-33(25)27)23-31(44-37(53)49-15-9-29(10-16-49)50-32-6-4-12-41-34(32)45-38(50)54)35(51)43-30(5-2-3-11-39)36(52)48-19-17-47(18-20-48)28-7-13-40-14-8-28/h4,6-8,12-14,21-22,24,29-31H,2-3,5,9-11,15-20,23,39H2,1H3,(H,42,46)(H,43,51)(H,44,53)(H,41,45,54)/t30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... |
J Med Chem 63: 7906-7920 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01003
BindingDB Entry DOI: 10.7270/Q2NK3JKR |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM391726
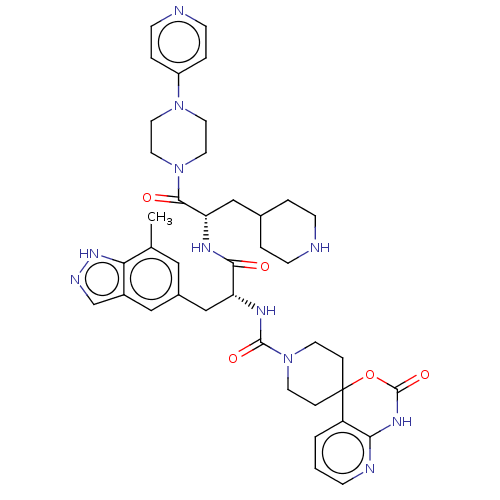
(N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC3(CC2)OC(=O)Nc2ncccc32)C(=O)N[C@@H](CC2CCNCC2)C(=O)N2CCN(CC2)c2ccncc2)cc2cn[nH]c12 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... |
J Med Chem 63: 7906-7920 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01003
BindingDB Entry DOI: 10.7270/Q2NK3JKR |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50184069
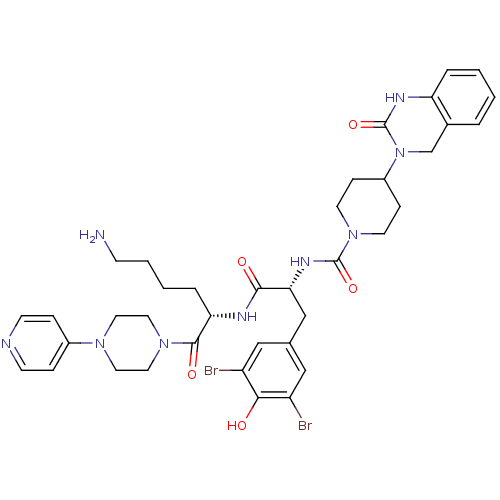
(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... |
J Med Chem 63: 7906-7920 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01003
BindingDB Entry DOI: 10.7270/Q2NK3JKR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM391723

(3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 8...)Show SMILES Oc1c(Br)cc(C[C@@H](NC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)C(=O)N[C@@H](CC2CCNCC2)C(=O)N2CCN(CC2)c2ccncc2)cc1Br |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... |
J Med Chem 63: 7906-7920 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01003
BindingDB Entry DOI: 10.7270/Q2NK3JKR |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM391725

(N-[(2R)-3-(7-methyl-1H- | US10300056, Example 10 |...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N[C@@H](CC2CCNCC2)C(=O)N2CCN(CC2)c2ccncc2)cc2cn[nH]c12 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... |
J Med Chem 63: 7906-7920 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01003
BindingDB Entry DOI: 10.7270/Q2NK3JKR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558721

(CHEMBL4784791)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM391718
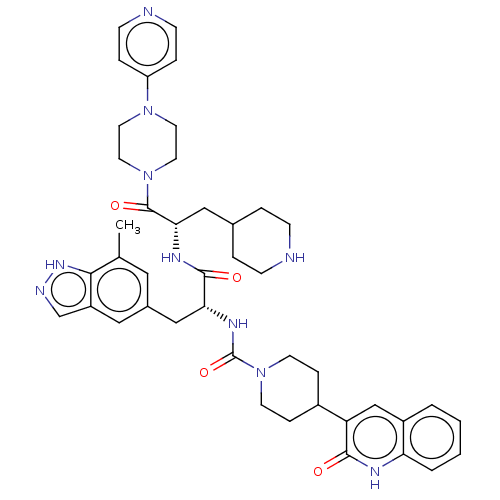
(N-[(2R)-3-(7-methyl-1H- | US10300056, Example 3 | ...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)c2cc3ccccc3[nH]c2=O)C(=O)N[C@@H](CC2CCNCC2)C(=O)N2CCN(CC2)c2ccncc2)cc2cn[nH]c12 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... |
J Med Chem 63: 7906-7920 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01003
BindingDB Entry DOI: 10.7270/Q2NK3JKR |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM391724

(3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 9...)Show SMILES Oc1c(Br)cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N[C@@H](CC2CCNCC2)C(=O)N2CCN(CC2)c2ccncc2)cc1Br |r| Show InChI InChI=1S/C40H49Br2N9O5/c41-31-21-27(22-32(42)36(31)52)24-34(47-39(55)50-15-9-30(10-16-50)51-25-28-3-1-2-4-33(28)46-40(51)56)37(53)45-35(23-26-5-11-43-12-6-26)38(54)49-19-17-48(18-20-49)29-7-13-44-14-8-29/h1-4,7-8,13-14,21-22,26,30,34-35,43,52H,5-6,9-12,15-20,23-25H2,(H,45,53)(H,46,56)(H,47,55)/t34-,35+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... |
J Med Chem 63: 7906-7920 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01003
BindingDB Entry DOI: 10.7270/Q2NK3JKR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM214798
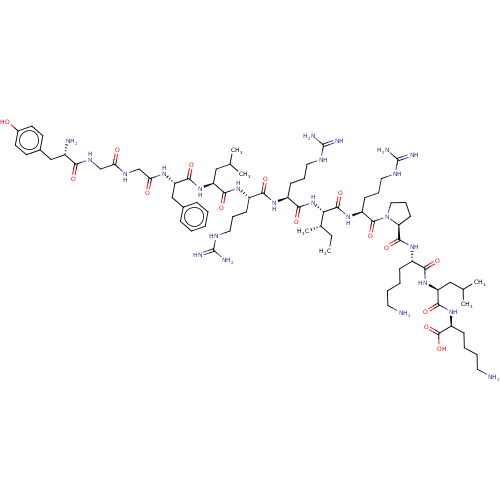
(Dynorphin A (1-17) | YGGFLRRIRPKLK)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50539954

(CHEMBL4636143)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)C(=O)N[C@@H](Cc2ccncc2)C(=O)N2CCN(CC2)c2ccncc2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C40H44N12O4/c1-26-21-28(22-29-25-44-48-35(26)29)24-32(46-39(55)51-15-8-31(9-16-51)52-34-3-2-10-43-36(34)47-40(52)56)37(53)45-33(23-27-4-11-41-12-5-27)38(54)50-19-17-49(18-20-50)30-6-13-42-14-7-30/h2-7,10-14,21-22,25,31-33H,8-9,15-20,23-24H2,1H3,(H,44,48)(H,45,53)(H,46,55)(H,43,47,56)/t32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... |
J Med Chem 63: 7906-7920 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01003
BindingDB Entry DOI: 10.7270/Q2NK3JKR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50040123
(CHEMBL438223 | HTry-Gly-Gly-Phe-Leu-Arg-Arg-lle-Ar...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C63H103N21O13/c1-5-37(4)51(58(94)80-44(20-13-29-74-63(70)71)59(95)84-30-14-21-48(84)57(93)81-45(60(96)97)17-9-10-26-64)83-54(90)43(19-12-28-73-62(68)69)78-53(89)42(18-11-27-72-61(66)67)79-55(91)46(31-36(2)3)82-56(92)47(33-38-15-7-6-8-16-38)77-50(87)35-75-49(86)34-76-52(88)41(65)32-39-22-24-40(85)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,85H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H,75,86)(H,76,88)(H,77,87)(H,78,89)(H,79,91)(H,80,94)(H,81,93)(H,82,92)(H,83,90)(H,96,97)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030815
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT2 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM85484
(Bombesin)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |wU:82.92,74.81,8.12,4.4,30.31,53.61,16.23,wD:93.101,39.52,62.69,102.104,34.35,(24.84,-22.59,;25.32,-21.13,;24.29,-19.98,;24.77,-18.52,;23.74,-17.37,;24.22,-15.91,;25.72,-15.59,;26.75,-16.74,;26.2,-14.13,;27.71,-13.81,;28.74,-14.96,;30.24,-14.64,;28.26,-16.42,;25.17,-12.98,;25.65,-11.52,;27.16,-11.2,;24.62,-10.37,;25.1,-8.91,;26.61,-8.59,;27.75,-9.62,;29.09,-8.86,;28.77,-7.35,;27.24,-7.19,;23.12,-10.69,;22.09,-9.54,;22.57,-8.08,;20.58,-9.86,;19.55,-8.72,;18.04,-9.03,;17.57,-10.5,;17.02,-7.89,;15.51,-8.2,;14.48,-7.06,;14.96,-5.59,;12.97,-7.38,;12.5,-8.84,;11.95,-6.23,;10.44,-6.55,;9.96,-8.01,;9.41,-5.4,;7.9,-5.72,;7.42,-7.18,;8.32,-8.42,;7.42,-9.66,;5.97,-9.19,;4.63,-9.96,;3.3,-9.19,;3.3,-7.65,;4.63,-6.88,;5.97,-7.65,;9.89,-3.94,;8.86,-2.79,;7.35,-3.11,;9.34,-1.33,;10.85,-1.01,;11.32,.45,;12.83,.77,;13.31,2.24,;13.86,-.37,;8.31,-.18,;8.79,1.28,;10.3,1.6,;7.76,2.43,;6.25,2.11,;5.22,3.26,;3.72,2.94,;5.7,4.72,;8.24,3.89,;9.75,4.21,;10.77,3.06,;10.22,5.67,;11.73,5.99,;12.21,7.46,;11.18,8.6,;13.72,7.77,;14.74,6.63,;16.25,6.94,;17.28,5.8,;16.73,8.41,;14.19,9.24,;13.17,10.38,;11.66,10.07,;13.64,11.85,;15.15,12.17,;15.63,13.63,;17.14,13.95,;17.61,15.41,;19.12,15.73,;19.6,17.19,;20.15,14.58,;12.62,12.99,;13.09,14.46,;14.6,14.78,;12.07,15.6,;10.56,15.29,;9.53,16.43,;8.02,16.11,;7,17.26,;7.55,14.65,;12.54,17.07,;11.52,18.21,;10.01,17.9,;11.99,19.68,;11.09,20.93,;12,22.17,;13.46,21.69,;14.71,22.59,;13.46,20.15,;17.49,-6.42,;19,-6.11,;16.47,-5.28,;22.23,-17.69,;21.75,-19.16,;21.2,-16.55,)| Show InChI InChI=1S/C71H110N24O18S/c1-34(2)24-47(92-62(105)43(14-11-22-79-71(76)77)89-64(107)45(15-18-52(72)96)90-63(106)44-17-20-55(99)85-44)61(104)81-31-56(100)87-51(28-54(74)98)69(112)91-46(16-19-53(73)97)65(108)94-49(26-38-29-80-41-13-10-9-12-40(38)41)66(109)84-37(7)60(103)95-58(36(5)6)70(113)82-32-57(101)86-50(27-39-30-78-33-83-39)68(111)93-48(25-35(3)4)67(110)88-42(59(75)102)21-23-114-8/h9-10,12-13,29-30,33-37,42-51,58,80H,11,14-28,31-32H2,1-8H3,(H2,72,96)(H2,73,97)(H2,74,98)(H2,75,102)(H,78,83)(H,81,104)(H,82,113)(H,84,109)(H,85,99)(H,86,101)(H,87,100)(H,88,110)(H,89,107)(H,90,106)(H,91,112)(H,92,105)(H,93,111)(H,94,108)(H,95,103)(H4,76,77,79)/t37-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,58-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against [125 I][4Tyr]-bombesin labeled cloned human GRP(gastrin releasing peptide) receptors stably expressed in CHO cells |
Bioorg Med Chem Lett 6: 2617-2622 (1996)
Article DOI: 10.1016/0960-894X(96)00481-7
BindingDB Entry DOI: 10.7270/Q2NC61QD |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659

((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II |
Bioorg Med Chem Lett 13: 2097-100 (2003)
BindingDB Entry DOI: 10.7270/Q2Z60NF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338
((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583640
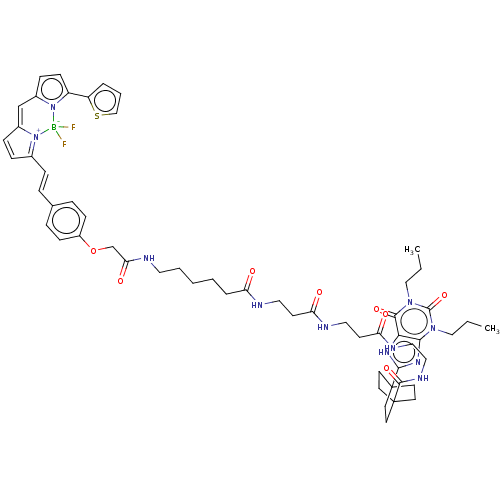
(CHEMBL5075285)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCNC(=O)CCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\C2=[N+]3C(C=C2)=Cc2ccc(-c4cccs4)n2[B-]3(F)F)cc1 |c:66,68,t:63| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50436546
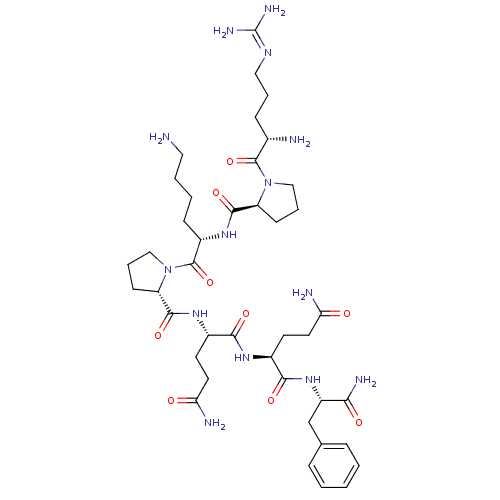
(CHEMBL2397481)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#7])=O |r| Show InChI InChI=1S/C41H66N14O9/c42-19-5-4-12-28(52-38(62)30-13-7-21-54(30)39(63)25(43)11-6-20-49-41(47)48)40(64)55-22-8-14-31(55)37(61)51-27(16-18-33(45)57)35(59)50-26(15-17-32(44)56)36(60)53-29(34(46)58)23-24-9-2-1-3-10-24/h1-3,9-10,25-31H,4-8,11-23,42-43H2,(H2,44,56)(H2,45,57)(H2,46,58)(H,50,59)(H,51,61)(H,52,62)(H,53,60)(H4,47,48,49)/t25-,26-,27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to substance P receptor (1 to 7 amino acids) binding site in rat spinal cord membranes |
J Med Chem 56: 4953-65 (2013)
Article DOI: 10.1021/jm400209h
BindingDB Entry DOI: 10.7270/Q2K075PK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50436546

(CHEMBL2397481)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#7])=O |r| Show InChI InChI=1S/C41H66N14O9/c42-19-5-4-12-28(52-38(62)30-13-7-21-54(30)39(63)25(43)11-6-20-49-41(47)48)40(64)55-22-8-14-31(55)37(61)51-27(16-18-33(45)57)35(59)50-26(15-17-32(44)56)36(60)53-29(34(46)58)23-24-9-2-1-3-10-24/h1-3,9-10,25-31H,4-8,11-23,42-43H2,(H2,44,56)(H2,45,57)(H2,46,58)(H,50,59)(H,51,61)(H,52,62)(H,53,60)(H4,47,48,49)/t25-,26-,27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 2446-2450 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.009
BindingDB Entry DOI: 10.7270/Q2QF8WCB |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558711
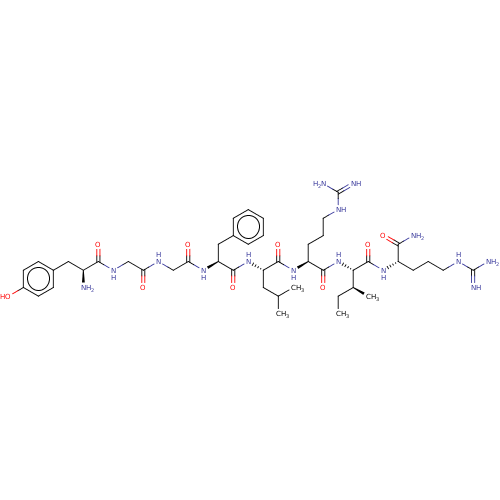
(CHEMBL4754961)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009338

((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...)Show SMILES CNCC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |r,wU:60.63,wD:24.23,36.36,43.43,56.59,17.16,6.5,(6.84,-31.4,;5.3,-31.4,;4.53,-30.07,;2.99,-30.07,;2.22,-31.4,;2.22,-28.73,;2.99,-27.4,;4.53,-27.4,;5.3,-26.07,;4.53,-24.73,;5.3,-23.4,;6.84,-23.4,;7.61,-24.73,;7.61,-22.07,;2.22,-26.07,;2.99,-24.73,;.68,-26.07,;-.09,-24.73,;.68,-23.4,;2.22,-23.4,;-.09,-22.07,;-1.63,-24.73,;-2.4,-23.4,;-2.4,-26.07,;-3.94,-26.07,;-4.71,-24.73,;-6.25,-24.73,;-7.02,-23.4,;-8.56,-23.4,;-9.33,-24.73,;-10.87,-24.73,;-8.56,-26.07,;-7.02,-26.07,;-4.71,-27.4,;-6.25,-27.4,;-3.94,-28.73,;-4.71,-30.07,;-6.25,-30.07,;-7.02,-28.73,;-7.02,-31.4,;-3.94,-31.4,;-2.4,-31.4,;-4.71,-32.73,;-3.94,-34.07,;-4.71,-35.4,;-6.25,-35.4,;-7.15,-34.16,;-8.62,-34.63,;-8.62,-36.17,;-7.15,-36.65,;-2.4,-34.07,;-1.63,-32.73,;-1.63,-35.4,;-2.26,-36.81,;-1.12,-37.84,;.22,-37.07,;-.1,-35.56,;.93,-34.42,;.45,-32.95,;2.42,-34.82,;3.5,-33.73,;3.11,-32.24,;5,-34.13,;6.09,-33.04,;5.39,-35.61,)| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT2 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558723
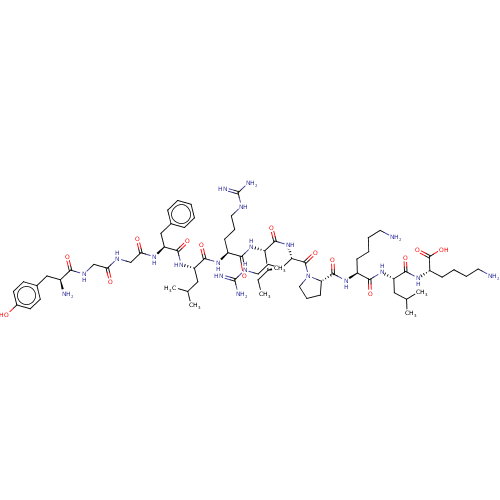
(CHEMBL4760958)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Sus scrofa) | BDBM50156173
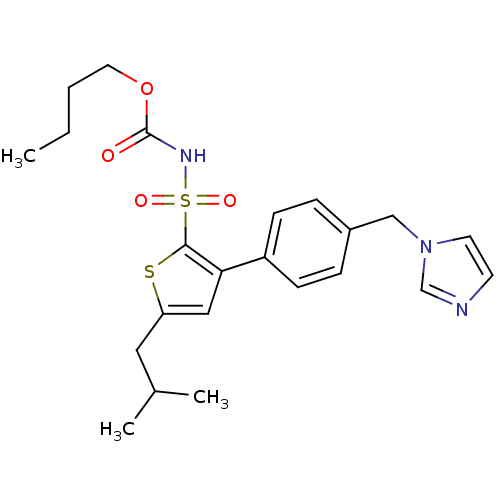
((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2ccnc2)cc1 Show InChI InChI=1S/C23H29N3O4S2/c1-4-5-12-30-23(27)25-32(28,29)22-21(14-20(31-22)13-17(2)3)19-8-6-18(7-9-19)15-26-11-10-24-16-26/h6-11,14,16-17H,4-5,12-13,15H2,1-3H3,(H,25,27) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116790
BindingDB Entry DOI: 10.7270/Q2ZW1QXF |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50156173

((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2ccnc2)cc1 Show InChI InChI=1S/C23H29N3O4S2/c1-4-5-12-30-23(27)25-32(28,29)22-21(14-20(31-22)13-17(2)3)19-8-6-18(7-9-19)15-26-11-10-24-16-26/h6-11,14,16-17H,4-5,12-13,15H2,1-3H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT2 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50558722
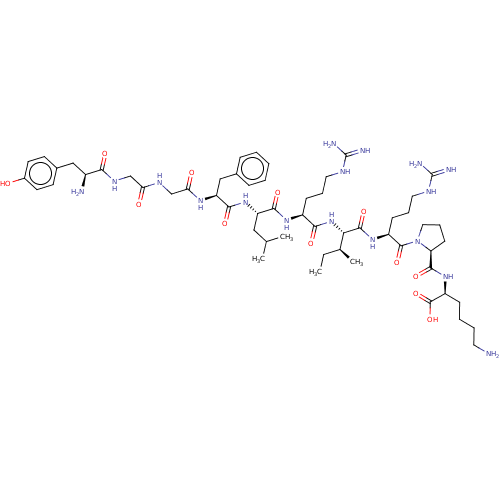
(CHEMBL4757601)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50199311

(CHEMBL215160 | N-butyloxycarbonyl-2-(4-imidazol-1-...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccc(OCCCC)cc1-c1ccc(Cn2ccnc2)cc1 Show InChI InChI=1S/C25H31N3O5S/c1-3-5-15-32-22-11-12-24(34(30,31)27-25(29)33-16-6-4-2)23(17-22)21-9-7-20(8-10-21)18-28-14-13-26-19-28/h7-14,17,19H,3-6,15-16,18H2,1-2H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang2 from AT2 receptor in pig uterus membrane |
J Med Chem 49: 7160-8 (2006)
Article DOI: 10.1021/jm0606185
BindingDB Entry DOI: 10.7270/Q21C1WHK |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50199326
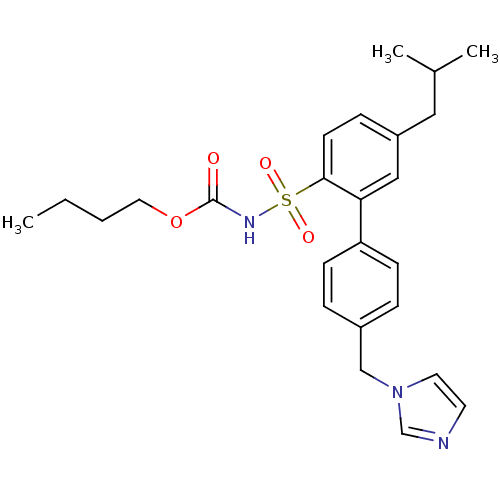
(CHEMBL217673 | N-butyloxycarbonyl-2-(4-imidazol-1-...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccc(CC(C)C)cc1-c1ccc(Cn2ccnc2)cc1 Show InChI InChI=1S/C25H31N3O4S/c1-4-5-14-32-25(29)27-33(30,31)24-11-8-21(15-19(2)3)16-23(24)22-9-6-20(7-10-22)17-28-13-12-26-18-28/h6-13,16,18-19H,4-5,14-15,17H2,1-3H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang2 from AT2 receptor in pig uterus membrane |
J Med Chem 49: 7160-8 (2006)
Article DOI: 10.1021/jm0606185
BindingDB Entry DOI: 10.7270/Q21C1WHK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030815

(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Binding affinity to AT1 receptor (unknown origin) |
ACS Med Chem Lett 5: 1129-32 (2014)
Article DOI: 10.1021/ml500278g
BindingDB Entry DOI: 10.7270/Q2BV7J7R |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50288252
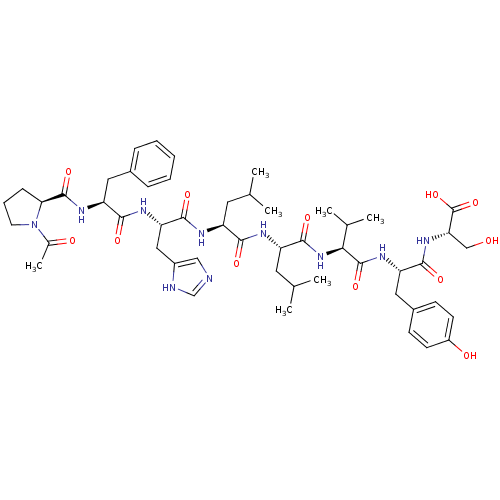
(Bombesin analogue | CHEMBL269432)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(O)=O Show InChI InChI=1S/C51H72N10O12/c1-28(2)20-36(54-47(68)40(24-34-25-52-27-53-34)56-45(66)38(22-32-12-9-8-10-13-32)57-49(70)42-14-11-19-61(42)31(7)63)44(65)55-37(21-29(3)4)48(69)60-43(30(5)6)50(71)58-39(23-33-15-17-35(64)18-16-33)46(67)59-41(26-62)51(72)73/h8-10,12-13,15-18,25,27-30,36-43,62,64H,11,14,19-24,26H2,1-7H3,(H,52,53)(H,54,68)(H,55,65)(H,56,66)(H,57,70)(H,58,71)(H,59,67)(H,60,69)(H,72,73)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against [125 I][4Tyr]-bombesin labeled cloned human GRP(gastrin releasing peptide) receptors stably expressed in CHO cells |
Bioorg Med Chem Lett 6: 2617-2622 (1996)
Article DOI: 10.1016/0960-894X(96)00481-7
BindingDB Entry DOI: 10.7270/Q2NC61QD |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583641
(CHEMBL5086197)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCNC(=O)CCNC(=O)CCCCC[N+]1=C(\C=C\C=C\C=C2\N(C)c3ccc(cc3C2(C)C)S(O)(=O)=O)C(C)(C)c2cc(ccc12)S([O-])(=O)=O |c:52| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of SLV320 by NanoBRET competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50558721

(CHEMBL4784791)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in mouse NG108-15 cell membranes incubated for 2 hrs by liquid scintillation counting base... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZG6WWT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes & Digestive & Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity of specific [3H]-R-PIA binding to rat Adenosine A1 receptor in HEK-293 cells |
J Med Chem 45: 2131-8 (2002)
BindingDB Entry DOI: 10.7270/Q2V1243G |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50254115

(CHEMBL4080589)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCCCCN(C)[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C25H39N3O6S/c1-27(2)20-12-8-11-18-17(20)10-9-13-21(18)35(33,34)26-14-6-4-5-7-15-28(3)22-19(16-29)23(30)25(32)24(22)31/h8-13,19,22-26,29-32H,4-7,14-16H2,1-3H3/t19-,22+,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant beta-glucocerebrosidase (unknown origin) using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubate... |
Bioorg Med Chem Lett 27: 3431-3435 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.086
BindingDB Entry DOI: 10.7270/Q29889D9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581950

(CHEMBL4204703)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(nc2[nH]ccc12)-c1ccccc1 |r,wU:4.7,wD:1.0,(5.09,-4.45,;6.42,-5.22,;6.43,-6.76,;7.77,-7.53,;9.09,-6.74,;9.09,-5.21,;7.76,-4.45,;10.43,-7.5,;10.44,-9.04,;9.12,-9.82,;9.13,-11.35,;10.47,-12.12,;11.8,-11.34,;13.26,-11.81,;14.16,-10.57,;13.25,-9.33,;11.79,-9.81,;7.8,-12.13,;6.47,-11.36,;5.14,-12.13,;5.14,-13.68,;6.47,-14.45,;7.81,-13.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50199331
(CHEMBL216250 | N-(1-butyloxy-methylcarbonyl)-3-(4-...)Show SMILES CCCCOCC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2ccnc2)cc1 Show InChI InChI=1S/C24H31N3O4S2/c1-4-5-12-31-16-23(28)26-33(29,30)24-22(14-21(32-24)13-18(2)3)20-8-6-19(7-9-20)15-27-11-10-25-17-27/h6-11,14,17-18H,4-5,12-13,15-16H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ang2 from AT2 receptor in pig uterus membrane |
J Med Chem 49: 7160-8 (2006)
Article DOI: 10.1021/jm0606185
BindingDB Entry DOI: 10.7270/Q21C1WHK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049189
(3-[4-(2-Ethyl-57-dimethyl-imidazo[45-b]pyridin-3-y...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2c(CC)nc3c(C)cc(C)nc23)cc1 Show InChI InChI=1S/C30H38N4O4S2/c1-7-9-14-38-30(35)33-40(36,37)29-25(17-24(39-29)15-19(3)4)23-12-10-22(11-13-23)18-34-26(8-2)32-27-20(5)16-21(6)31-28(27)34/h10-13,16-17,19H,7-9,14-15,18H2,1-6H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116790
BindingDB Entry DOI: 10.7270/Q2ZW1QXF |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50594171
(CHEMBL5186237)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CCC)nc1-c1ccc(Cn2ccnc2)cc1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116790
BindingDB Entry DOI: 10.7270/Q2ZW1QXF |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50594186
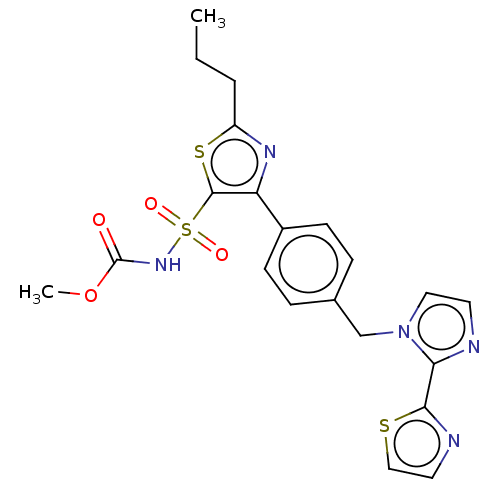
(CHEMBL5174659)Show SMILES CCCc1nc(c(s1)S(=O)(=O)NC(=O)OC)-c1ccc(Cn2ccnc2-c2nccs2)cc1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116790
BindingDB Entry DOI: 10.7270/Q2ZW1QXF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583644
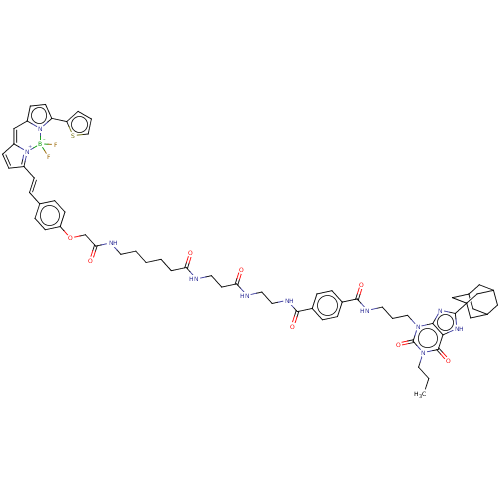
(CHEMBL5080679)Show SMILES CCCn1c(=O)n(CCCNC(=O)c2ccc(cc2)C(=O)NCCNC(=O)CCNC(=O)CCCCCNC(=O)COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)c2nc([nH]c2c1=O)C12CC3CC(CC(C3)C1)C2 |c:52,54,t:49,TLB:79:80:77.78.83:84,THB:81:80:77:83.82.84,81:82:77:85.79.80,79:78:84:85.80.81| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of SLV320 by NanoBRET competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50188489
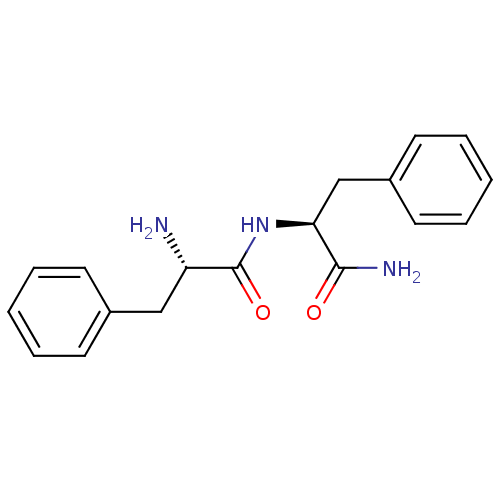
((S)-2-Amino-N-((S)-1-carbamoyl-2-phenyl-ethyl)-3-p...)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C18H21N3O2/c19-15(11-13-7-3-1-4-8-13)18(23)21-16(17(20)22)12-14-9-5-2-6-10-14/h1-10,15-16H,11-12,19H2,(H2,20,22)(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to NK1 receptor (unknown origin) |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50188489

((S)-2-Amino-N-((S)-1-carbamoyl-2-phenyl-ethyl)-3-p...)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C18H21N3O2/c19-15(11-13-7-3-1-4-8-13)18(23)21-16(17(20)22)12-14-9-5-2-6-10-14/h1-10,15-16H,11-12,19H2,(H2,20,22)(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50188489

((S)-2-Amino-N-((S)-1-carbamoyl-2-phenyl-ethyl)-3-p...)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C18H21N3O2/c19-15(11-13-7-3-1-4-8-13)18(23)21-16(17(20)22)12-14-9-5-2-6-10-14/h1-10,15-16H,11-12,19H2,(H2,20,22)(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SP1-7 from NK1 receptor (unknown origin) by scintillation counting analysis |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50188489

((S)-2-Amino-N-((S)-1-carbamoyl-2-phenyl-ethyl)-3-p...)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C18H21N3O2/c19-15(11-13-7-3-1-4-8-13)18(23)21-16(17(20)22)12-14-9-5-2-6-10-14/h1-10,15-16H,11-12,19H2,(H2,20,22)(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 2446-2450 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.009
BindingDB Entry DOI: 10.7270/Q2QF8WCB |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50308381
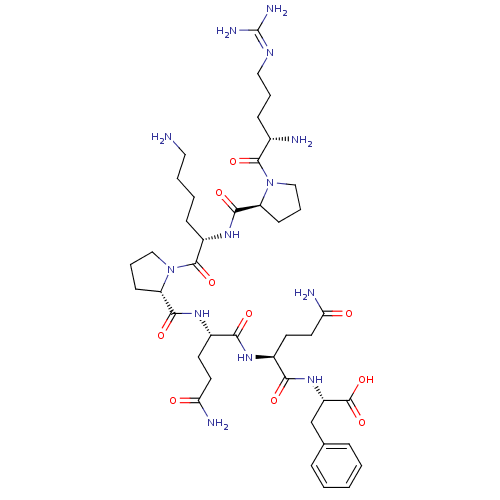
(CHEMBL589979 | H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-OH)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C41H65N13O10/c42-19-5-4-12-28(51-37(60)30-13-7-21-53(30)38(61)25(43)11-6-20-48-41(46)47)39(62)54-22-8-14-31(54)36(59)50-27(16-18-33(45)56)34(57)49-26(15-17-32(44)55)35(58)52-29(40(63)64)23-24-9-2-1-3-10-24/h1-3,9-10,25-31H,4-8,11-23,42-43H2,(H2,44,55)(H2,45,56)(H,49,57)(H,50,59)(H,51,60)(H,52,58)(H,63,64)(H4,46,47,48)/t25-,26-,27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 2446-2450 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.009
BindingDB Entry DOI: 10.7270/Q2QF8WCB |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50308381

(CHEMBL589979 | H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-OH)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C41H65N13O10/c42-19-5-4-12-28(51-37(60)30-13-7-21-53(30)38(61)25(43)11-6-20-48-41(46)47)39(62)54-22-8-14-31(54)36(59)50-27(16-18-33(45)56)34(57)49-26(15-17-32(44)55)35(58)52-29(40(63)64)23-24-9-2-1-3-10-24/h1-3,9-10,25-31H,4-8,11-23,42-43H2,(H2,44,55)(H2,45,56)(H,49,57)(H,50,59)(H,51,60)(H,52,58)(H,63,64)(H4,46,47,48)/t25-,26-,27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to substance P receptor (1 to 7 amino acids) binding site in rat spinal cord membranes |
J Med Chem 56: 4953-65 (2013)
Article DOI: 10.1021/jm400209h
BindingDB Entry DOI: 10.7270/Q2K075PK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50308381

(CHEMBL589979 | H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-OH)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C41H65N13O10/c42-19-5-4-12-28(51-37(60)30-13-7-21-53(30)38(61)25(43)11-6-20-48-41(46)47)39(62)54-22-8-14-31(54)36(59)50-27(16-18-33(45)56)34(57)49-26(15-17-32(44)55)35(58)52-29(40(63)64)23-24-9-2-1-3-10-24/h1-3,9-10,25-31H,4-8,11-23,42-43H2,(H2,44,55)(H2,45,56)(H,49,57)(H,50,59)(H,51,60)(H,52,58)(H,63,64)(H4,46,47,48)/t25-,26-,27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane |
J Med Chem 53: 2383-9 (2010)
Article DOI: 10.1021/jm901352b
BindingDB Entry DOI: 10.7270/Q2542NQK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581950

(CHEMBL4204703)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(nc2[nH]ccc12)-c1ccccc1 |r,wU:4.7,wD:1.0,(5.09,-4.45,;6.42,-5.22,;6.43,-6.76,;7.77,-7.53,;9.09,-6.74,;9.09,-5.21,;7.76,-4.45,;10.43,-7.5,;10.44,-9.04,;9.12,-9.82,;9.13,-11.35,;10.47,-12.12,;11.8,-11.34,;13.26,-11.81,;14.16,-10.57,;13.25,-9.33,;11.79,-9.81,;7.8,-12.13,;6.47,-11.36,;5.14,-12.13,;5.14,-13.68,;6.47,-14.45,;7.81,-13.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Leucyl-cystinyl aminopeptidase
(Homo sapiens (Human)) | BDBM50346448

(2-(2-(((2S,6S,13S,E)-13-Amino-2-(4-hydroxybenzyl)-...)Show SMILES N[C@H]1CC\C=C\CC[C@H](NC(=O)C[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)NCc1ccccc1CC(O)=O |r,t:4| Show InChI InChI=1S/C29H36N4O6/c30-24-9-3-1-2-4-10-25(29(39)31-18-21-8-6-5-7-20(21)16-27(36)37)33-26(35)17-22(32-28(24)38)15-19-11-13-23(34)14-12-19/h1-2,5-8,11-14,22,24-25,34H,3-4,9-10,15-18,30H2,(H,31,39)(H,32,38)(H,33,35)(H,36,37)/b2-1+/t22-,24-,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of catalytic activity of recombinant human IRAP transfected in human HEK-293 cells assessed as clevage of substrate L-leucine-p-nitroanili... |
J Med Chem 54: 3779-92 (2011)
Article DOI: 10.1021/jm200036n
BindingDB Entry DOI: 10.7270/Q2348KPZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50276158
(CHEMBL4127872)Show SMILES NCCCC[C@H](NC(=O)[C@H]1CCCN1C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H65N13O10/c42-19-5-4-12-28(51-37(60)30-13-7-21-53(30)38(61)25(43)11-6-20-48-41(46)47)39(62)54-22-8-14-31(54)36(59)50-27(16-18-33(45)56)34(57)49-26(15-17-32(44)55)35(58)52-29(40(63)64)23-24-9-2-1-3-10-24/h1-3,9-10,25-31H,4-8,11-23,42-43H2,(H2,44,55)(H2,45,56)(H,49,57)(H,50,59)(H,51,60)(H,52,58)(H,63,64)(H4,46,47,48)/t25-,26-,27-,28-,29+,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 2446-2450 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.009
BindingDB Entry DOI: 10.7270/Q2QF8WCB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data