

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50098576 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19770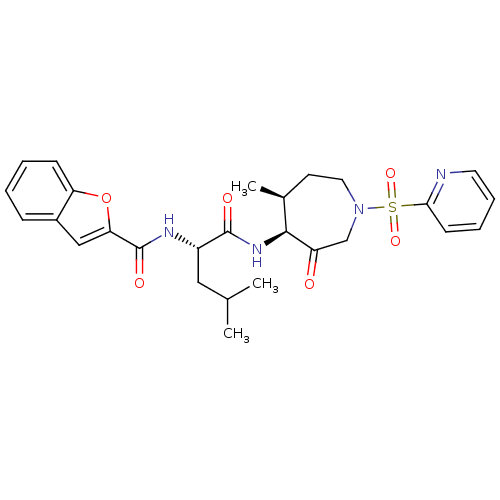 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19770 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0410 | -58.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0680 | -57.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19775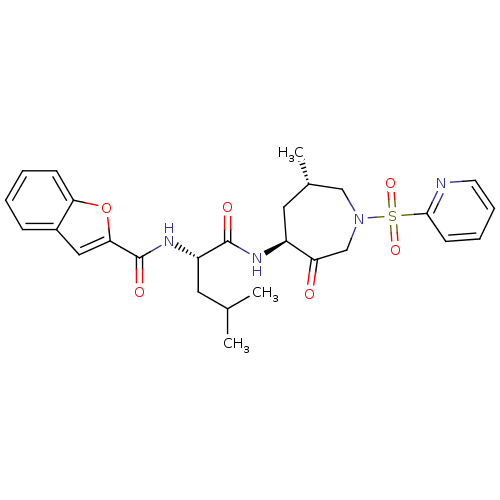 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464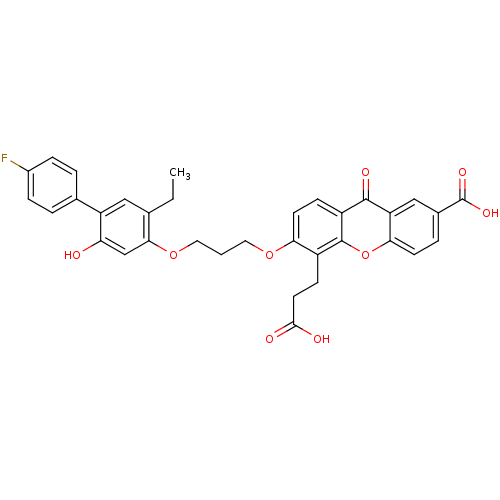 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50098576 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Human cathepsin L | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19775 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | -52.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083761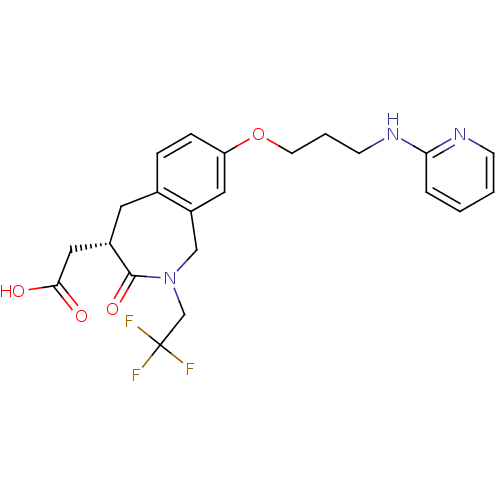 (CHEMBL87429 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288109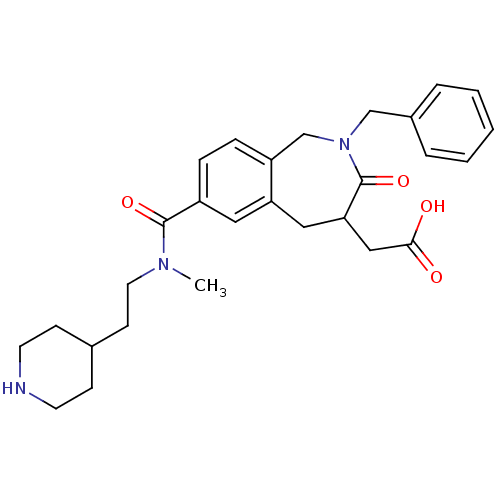 (CHEMBL82123 | {2-Benzyl-7-[methyl-(2-piperidin-4-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037391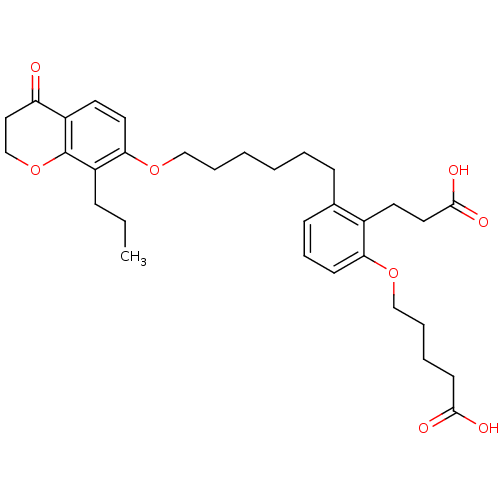 (5-{2-(2-Carboxy-ethyl)-3-[6-(4-oxo-8-propyl-chroma...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083763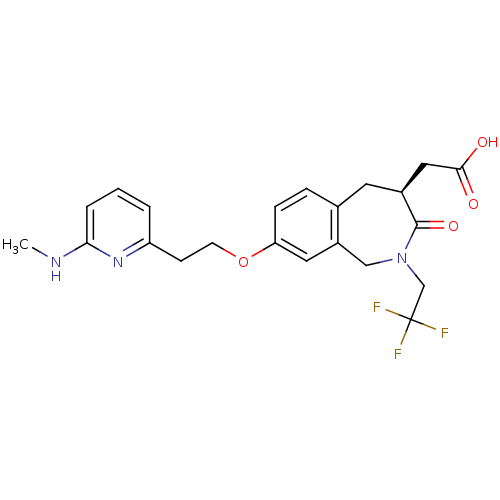 (CHEMBL86992 | [(S)-8-[2-(6-Methylamino-pyridin-2-y...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037390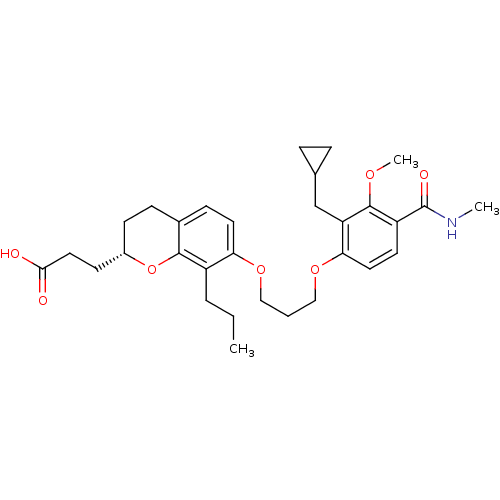 (3-{(S)-2-(2-Carboxy-ethyl)-7-[3-(2-cyclopropylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288120 (CHEMBL313768 | {2-Cyclohexyl-7-[methyl-(2-piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50078714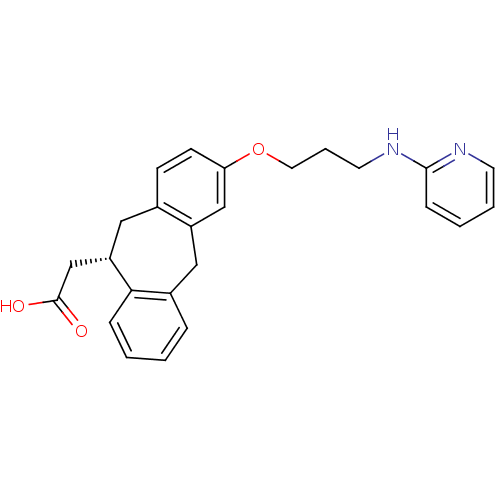 (CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for non-peptide Vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 9: 1807-12 (1999) BindingDB Entry DOI: 10.7270/Q2Q23ZFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083764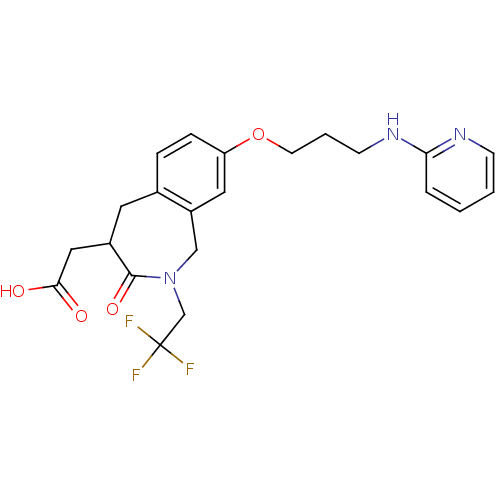 (CHEMBL421533 | [3-Oxo-8-[3-(pyridin-2-ylamino)-pro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19780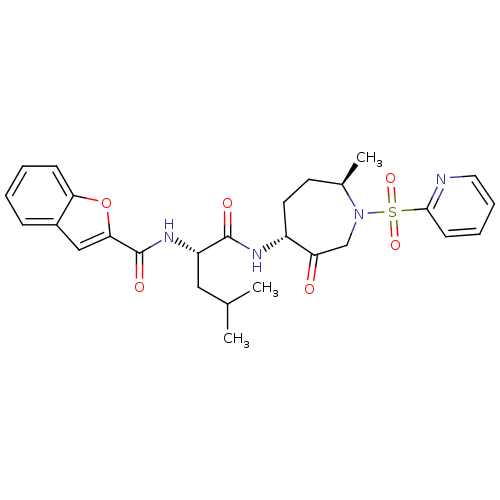 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288118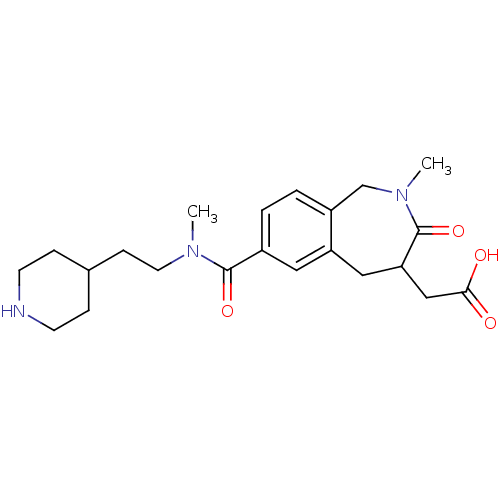 (CHEMBL312122 | {2-Methyl-7-[methyl-(2-piperidin-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288115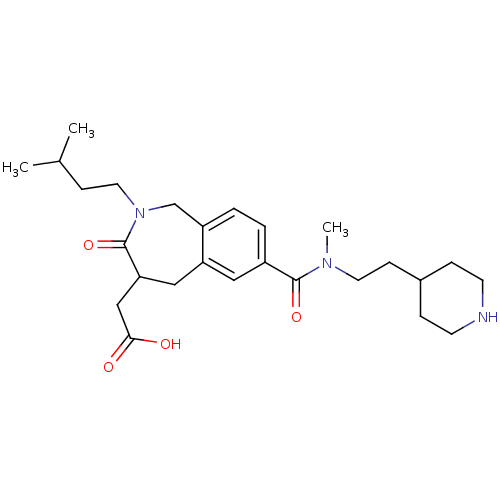 (CHEMBL82746 | {2-(3-Methyl-butyl)-7-[methyl-(2-pip...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288119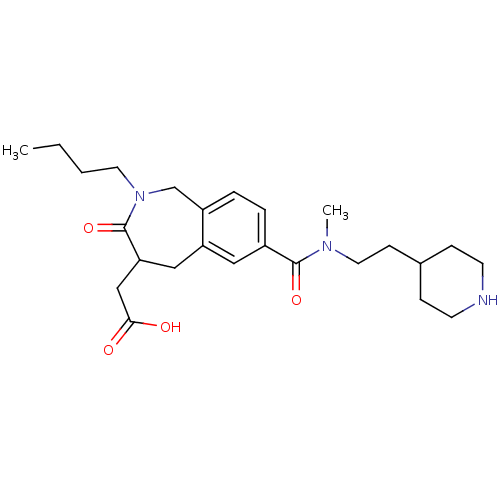 (CHEMBL84073 | {2-Butyl-7-[methyl-(2-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50074035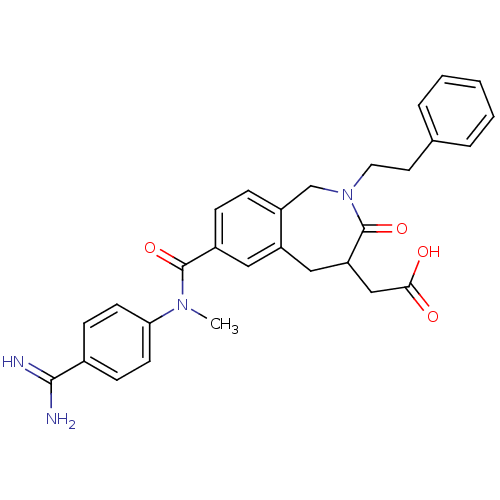 (CHEMBL82980 | {7-[(4-Carbamimidoyl-phenyl)-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Fibrinogen receptor binding affinity was determined by assaying for inhibition of [3H]-1 binding to purified Fibrinogen Receptor isolated from human... | J Med Chem 42: 545-59 (1999) Article DOI: 10.1021/jm980166z BindingDB Entry DOI: 10.7270/Q2QZ295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19771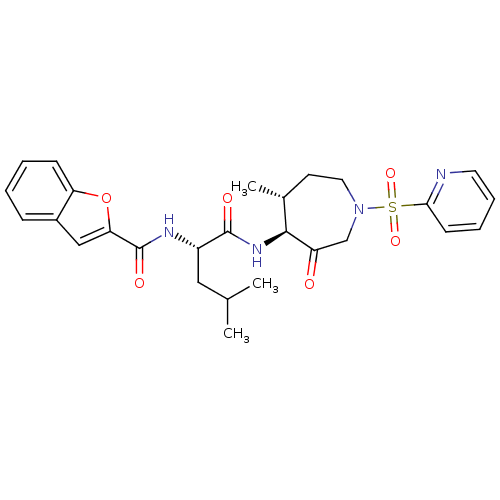 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50074035 (CHEMBL82980 | {7-[(4-Carbamimidoyl-phenyl)-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50054827 (CHEMBL85094 | SB-208651 | {8-[(4-Carbamimidoyl-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Fibrinogen receptor binding affinity was determined by assaying for inhibition of [3H]-1 binding to purified Fibrinogen Receptor isolated from human... | J Med Chem 42: 545-59 (1999) Article DOI: 10.1021/jm980166z BindingDB Entry DOI: 10.7270/Q2QZ295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50054827 (CHEMBL85094 | SB-208651 | {8-[(4-Carbamimidoyl-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50098580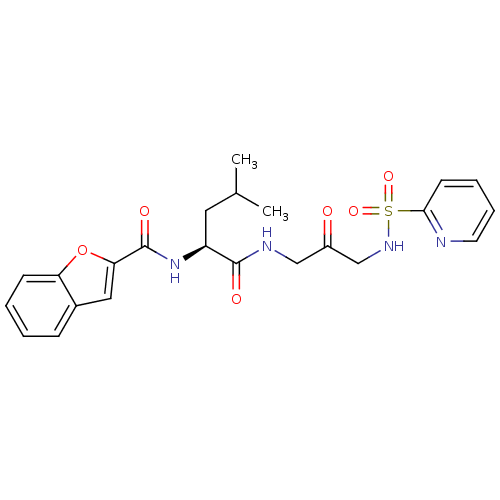 (Benzofuran-2-carboxylic acid {3-methyl-1-[2-oxo-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288117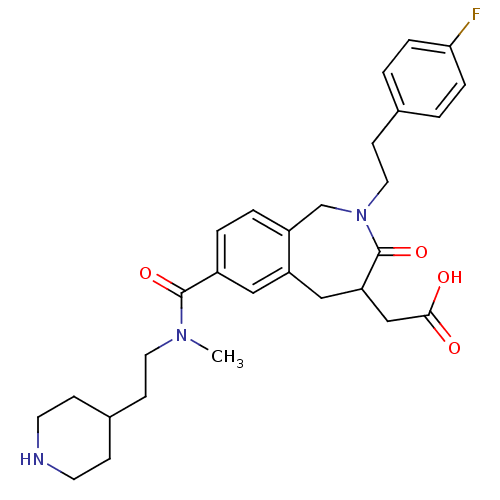 (CHEMBL83756 | {2-[2-(4-Fluoro-phenyl)-ethyl]-7-[me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50364972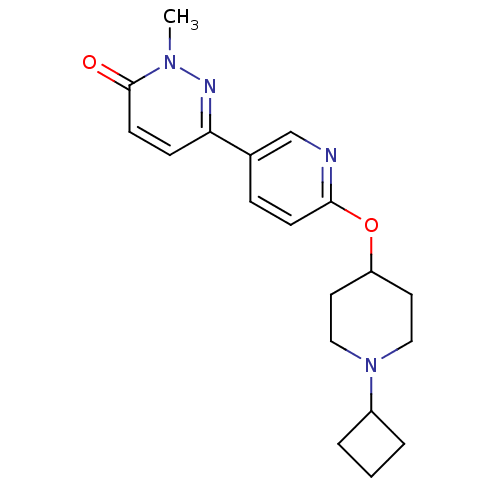 (CHEMBL1950738) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 22: 1504-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.026 BindingDB Entry DOI: 10.7270/Q2W37WSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50364957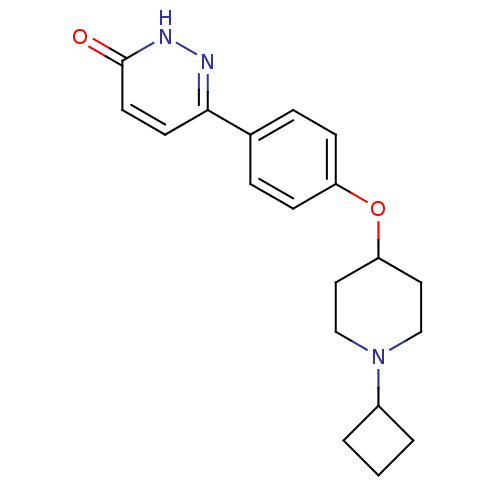 (CHEMBL1950643) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells | Bioorg Med Chem Lett 22: 1504-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.026 BindingDB Entry DOI: 10.7270/Q2W37WSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50074030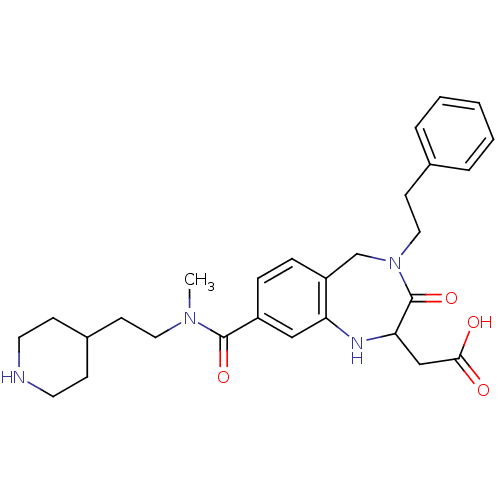 (CHEMBL356020 | {8-[Methyl-(2-piperidin-4-yl-ethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Fibrinogen receptor binding affinity was determined by assaying for inhibition of [3H]-1 binding to purified Fibrinogen Receptor isolated from human... | J Med Chem 42: 545-59 (1999) Article DOI: 10.1021/jm980166z BindingDB Entry DOI: 10.7270/Q2QZ295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083762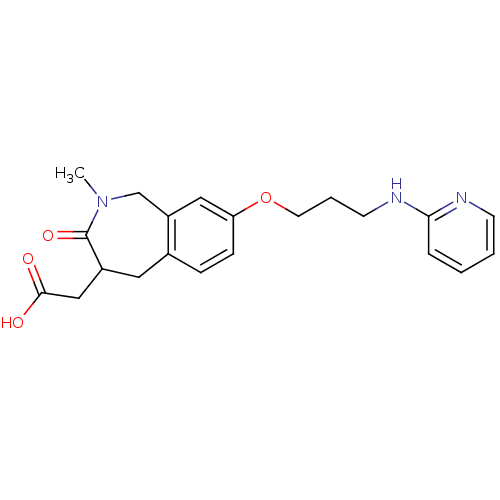 (CHEMBL314022 | {2-Methyl-3-oxo-8-[3-(pyridin-2-yla...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288113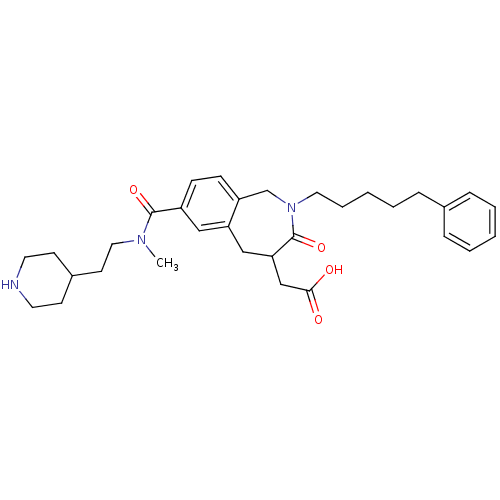 (CHEMBL311696 | [7-[Methyl-(2-piperidin-4-yl-ethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609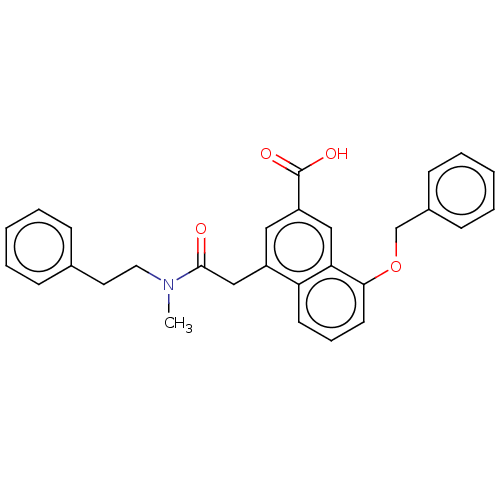 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50059133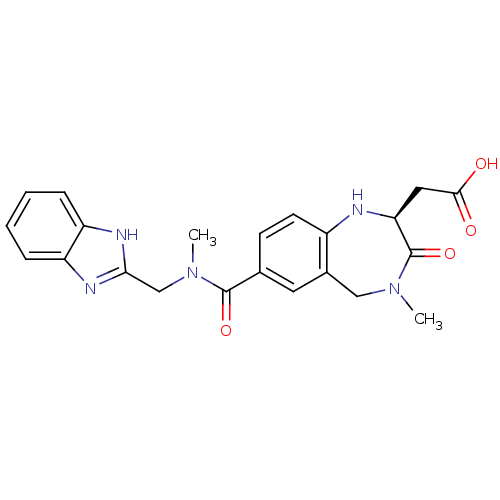 (CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HEK 293 cell adhesion to vitronectin by alpha V beta 3 | Bioorg Med Chem Lett 9: 1807-12 (1999) BindingDB Entry DOI: 10.7270/Q2Q23ZFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288114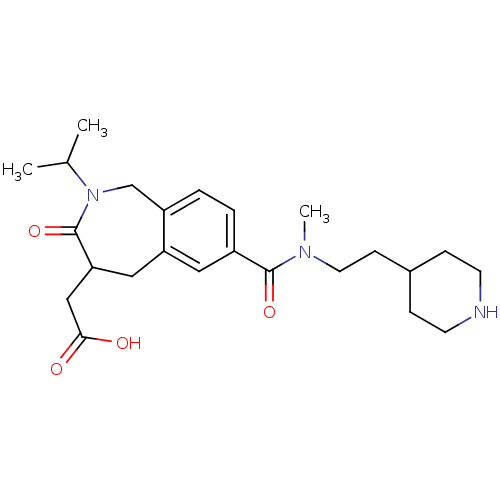 (CHEMBL312409 | {2-Isopropyl-7-[methyl-(2-piperidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288110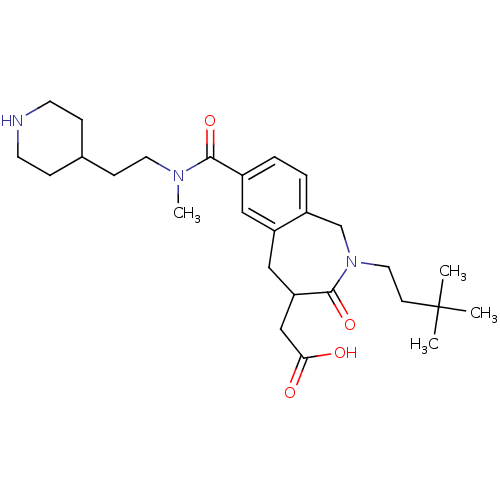 (CHEMBL84063 | {2-(3,3-Dimethyl-butyl)-7-[methyl-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50074044 (CHEMBL152156 | [(S)-8-(4-Carbamimidoyl-phenylcarba...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Fibrinogen receptor binding affinity was determined by assaying for inhibition of [3H]-1 binding to purified Fibrinogen Receptor isolated from human... | J Med Chem 42: 545-59 (1999) Article DOI: 10.1021/jm980166z BindingDB Entry DOI: 10.7270/Q2QZ295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50074029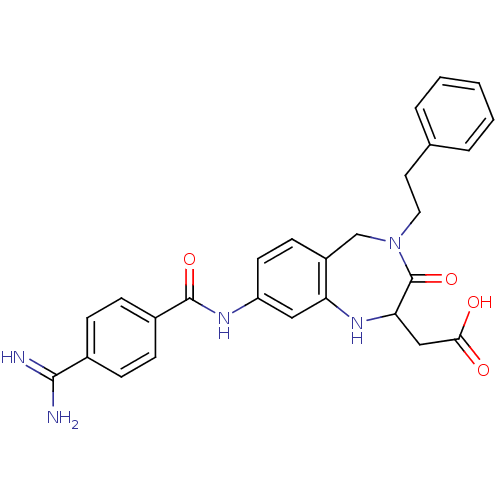 (CHEMBL153162 | [8-(4-Carbamimidoyl-benzoylamino)-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Fibrinogen receptor binding affinity was determined by assaying for inhibition of [3H]-1 binding to purified Fibrinogen Receptor isolated from human... | J Med Chem 42: 545-59 (1999) Article DOI: 10.1021/jm980166z BindingDB Entry DOI: 10.7270/Q2QZ295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288108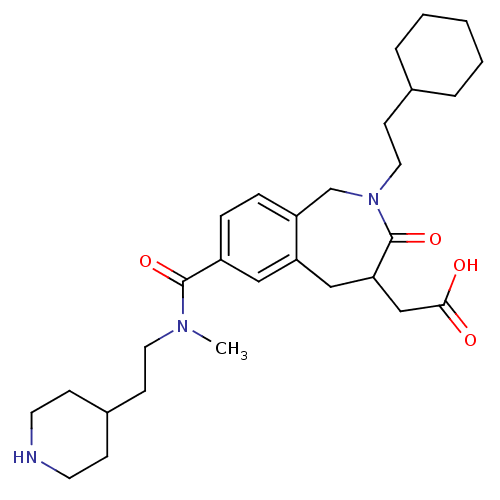 (CHEMBL82727 | {2-(2-Cyclohexyl-ethyl)-7-[methyl-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50350021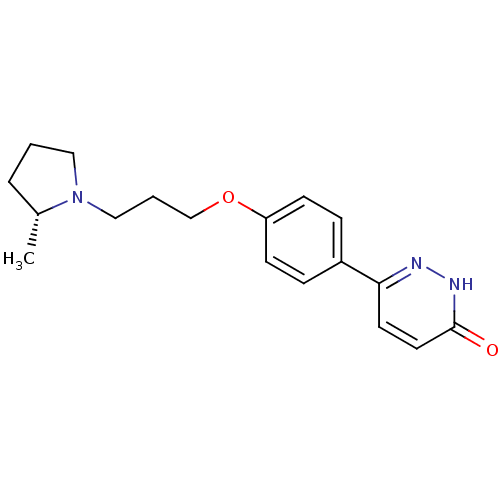 (CHEMBL1813067) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Global R&D. Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Eur J Med Chem 95: 349-56 (2015) Article DOI: 10.1016/j.ejmech.2015.03.054 BindingDB Entry DOI: 10.7270/Q2C24Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50098584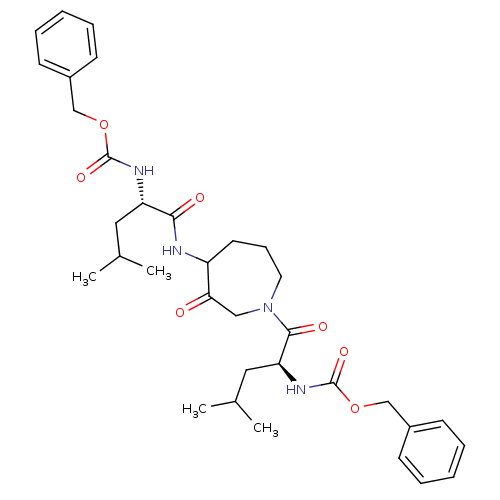 (CHEMBL31947 | {1-[4-(2-Benzyloxycarbonylamino-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50059133 (CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Human cathepsin L | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.20 | -48.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 514 total ) | Next | Last >> |