 Found 226 hits with Last Name = 'hammond' and Initial = 'b'
Found 226 hits with Last Name = 'hammond' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772
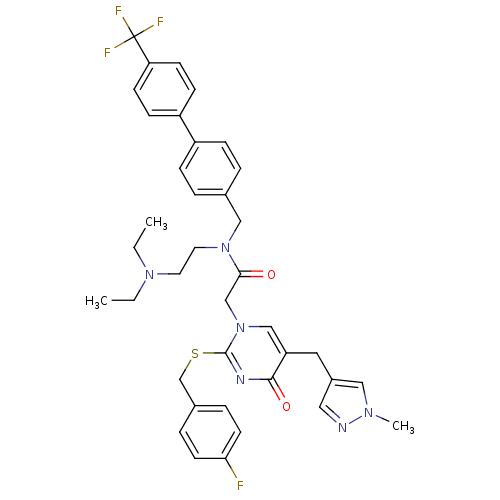
(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Steady state and transient kinetics to a freely reversible, non-covalently bound, human recombinant Phospholipase A2 (rhLp-PLA2) was determined |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265
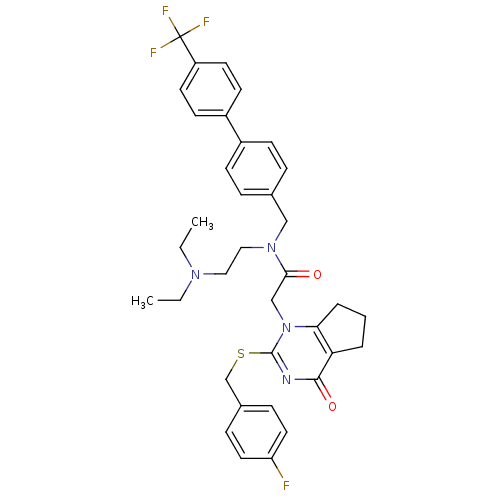
(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
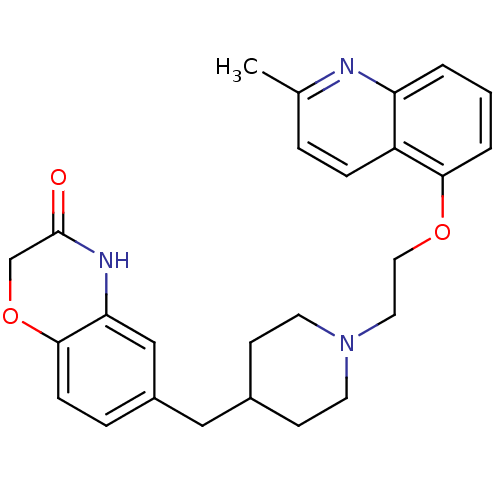
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474976

(CHEMBL180557)Show SMILES O=C1COc2ccc(CC3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C25H27N3O3/c29-25-17-31-24-7-6-19(16-22(24)27-25)15-18-8-11-28(12-9-18)13-14-30-23-5-1-4-21-20(23)3-2-10-26-21/h1-7,10,16,18H,8-9,11-15,17H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474983
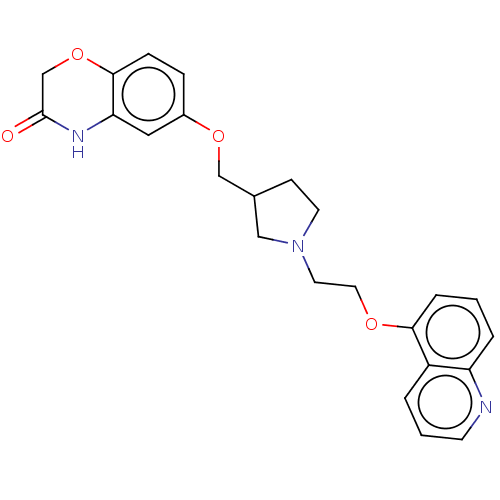
(CHEMBL183034)Show SMILES O=C1COc2ccc(OCC3CCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-31-23-7-6-18(13-21(23)26-24)30-15-17-8-10-27(14-17)11-12-29-22-5-1-4-20-19(22)3-2-9-25-20/h1-7,9,13,17H,8,10-12,14-16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474975
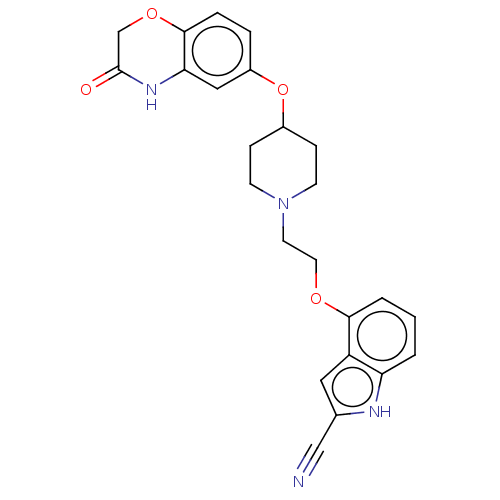
(CHEMBL3706797)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5[nH]c(cc45)C#N)CC3)cc2N1 Show InChI InChI=1S/C24H24N4O4/c25-14-16-12-19-20(26-16)2-1-3-22(19)30-11-10-28-8-6-17(7-9-28)32-18-4-5-23-21(13-18)27-24(29)15-31-23/h1-5,12-13,17,26H,6-11,15H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474977

(CHEMBL183901)Show SMILES O=C1COc2ccc(OCC3CCCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C25H27N3O4/c29-25-17-32-24-9-8-19(14-22(24)27-25)31-16-18-4-3-11-28(15-18)12-13-30-23-7-1-6-21-20(23)5-2-10-26-21/h1-2,5-10,14,18H,3-4,11-13,15-17H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027184

(CHEMBL180700)Show InChI InChI=1S/C21H23N3O4/c25-21-14-28-20-6-5-15(13-18(20)24-21)26-12-10-22-8-2-11-27-19-4-1-3-17-16(19)7-9-23-17/h1,3-7,9,13,22-23H,2,8,10-12,14H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474980

(CHEMBL180405)Show SMILES CC1(C)OC2C=CC=CC2=C1OCCN1CCC(CC1)Oc1ccc2OCC(=O)Nc2c1 |c:5,7,10| Show InChI InChI=1S/C25H30N2O5/c1-25(2)24(19-5-3-4-6-21(19)32-25)29-14-13-27-11-9-17(10-12-27)31-18-7-8-22-20(15-18)26-23(28)16-30-22/h3-8,15,17,21H,9-14,16H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474979

(CHEMBL180445)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C25H26N2O4/c28-25-17-30-24-9-8-20(16-22(24)26-25)31-19-10-12-27(13-11-19)14-15-29-23-7-3-5-18-4-1-2-6-21(18)23/h1-9,16,19H,10-15,17H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474981
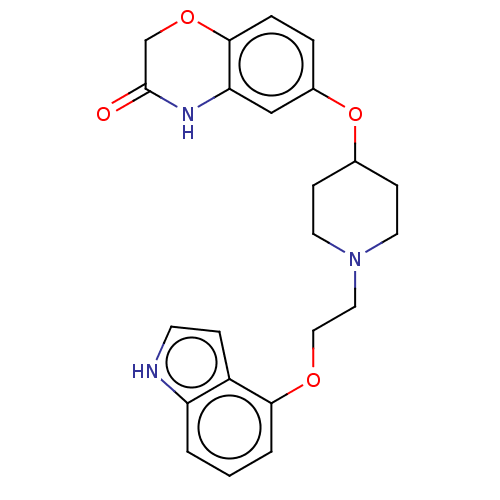
(CHEMBL2113021)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5[nH]ccc45)CC3)cc2N1 Show InChI InChI=1S/C23H25N3O4/c27-23-15-29-22-5-4-17(14-20(22)25-23)30-16-7-10-26(11-8-16)12-13-28-21-3-1-2-19-18(21)6-9-24-19/h1-6,9,14,16,24H,7-8,10-13,15H2,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474981

(CHEMBL2113021)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5[nH]ccc45)CC3)cc2N1 Show InChI InChI=1S/C23H25N3O4/c27-23-15-29-22-5-4-17(14-20(22)25-23)30-16-7-10-26(11-8-16)12-13-28-21-3-1-2-19-18(21)6-9-24-19/h1-6,9,14,16,24H,7-8,10-13,15H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474982

(CHEMBL181186)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5cccnc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-23-16-30-21-7-6-19(15-20(21)26-23)31-18-8-11-27(12-9-18)13-14-29-22-5-1-3-17-4-2-10-25-24(17)22/h1-7,10,15,18H,8-9,11-14,16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50412114

(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474976

(CHEMBL180557)Show SMILES O=C1COc2ccc(CC3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C25H27N3O3/c29-25-17-31-24-7-6-19(16-22(24)27-25)15-18-8-11-28(12-9-18)13-14-30-23-5-1-4-21-20(23)3-2-10-26-21/h1-7,10,16,18H,8-9,11-15,17H2,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474978
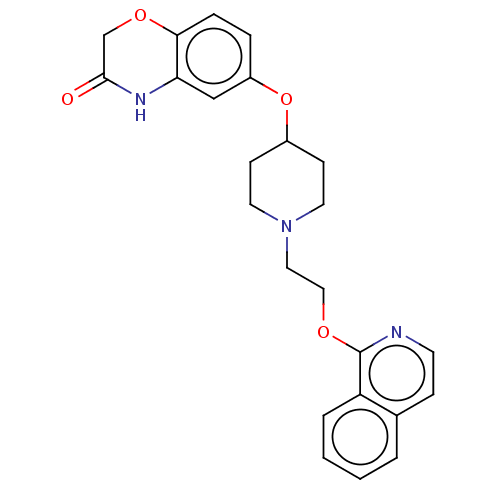
(CHEMBL183551)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4nccc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-23-16-30-22-6-5-19(15-21(22)26-23)31-18-8-11-27(12-9-18)13-14-29-24-20-4-2-1-3-17(20)7-10-25-24/h1-7,10,15,18H,8-9,11-14,16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474975

(CHEMBL3706797)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5[nH]c(cc45)C#N)CC3)cc2N1 Show InChI InChI=1S/C24H24N4O4/c25-14-16-12-19-20(26-16)2-1-3-22(19)30-11-10-28-8-6-17(7-9-28)32-18-4-5-23-21(13-18)27-24(29)15-31-23/h1-5,12-13,17,26H,6-11,15H2,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474985

(CHEMBL180182)Show SMILES O=C1COc2ccc(CN3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C24H26N4O3/c29-24-17-31-23-7-6-18(15-21(23)26-24)16-28-11-9-27(10-12-28)13-14-30-22-5-1-4-20-19(22)3-2-8-25-20/h1-8,15H,9-14,16-17H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474974

(CHEMBL183332)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-30-23-7-6-18(15-21(23)26-24)31-17-8-11-27(12-9-17)13-14-29-22-5-1-4-20-19(22)3-2-10-25-20/h1-7,10,15,17H,8-9,11-14,16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474980

(CHEMBL180405)Show SMILES CC1(C)OC2C=CC=CC2=C1OCCN1CCC(CC1)Oc1ccc2OCC(=O)Nc2c1 |c:5,7,10| Show InChI InChI=1S/C25H30N2O5/c1-25(2)24(19-5-3-4-6-21(19)32-25)29-14-13-27-11-9-17(10-12-27)31-18-7-8-22-20(15-18)26-23(28)16-30-22/h3-8,15,17,21H,9-14,16H2,1-2H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474974

(CHEMBL183332)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-30-23-7-6-18(15-21(23)26-24)31-17-8-11-27(12-9-17)13-14-29-22-5-1-4-20-19(22)3-2-10-25-20/h1-7,10,15,17H,8-9,11-14,16H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474985

(CHEMBL180182)Show SMILES O=C1COc2ccc(CN3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C24H26N4O3/c29-24-17-31-23-7-6-18(15-21(23)26-24)16-28-11-9-27(10-12-28)13-14-30-22-5-1-4-20-19(22)3-2-8-25-20/h1-8,15H,9-14,16-17H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474982

(CHEMBL181186)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5cccnc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-23-16-30-21-7-6-19(15-20(21)26-23)31-18-8-11-27(12-9-18)13-14-29-22-5-1-3-17-4-2-10-25-24(17)22/h1-7,10,15,18H,8-9,11-14,16H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474983

(CHEMBL183034)Show SMILES O=C1COc2ccc(OCC3CCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-31-23-7-6-18(13-21(23)26-24)30-15-17-8-10-27(14-17)11-12-29-22-5-1-4-20-19(22)3-2-9-25-20/h1-7,9,13,17H,8,10-12,14-16H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474978

(CHEMBL183551)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4nccc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-23-16-30-22-6-5-19(15-21(22)26-23)31-18-8-11-27(12-9-18)13-14-29-24-20-4-2-1-3-17(20)7-10-25-24/h1-7,10,15,18H,8-9,11-14,16H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027184

(CHEMBL180700)Show InChI InChI=1S/C21H23N3O4/c25-21-14-28-20-6-5-15(13-18(20)24-21)26-12-10-22-8-2-11-27-19-4-1-3-17-16(19)7-9-23-17/h1,3-7,9,13,22-23H,2,8,10-12,14H2,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474977

(CHEMBL183901)Show SMILES O=C1COc2ccc(OCC3CCCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C25H27N3O4/c29-25-17-32-24-9-8-19(14-22(24)27-25)31-16-18-4-3-11-28(15-18)12-13-30-23-7-1-6-21-20(23)5-2-10-26-21/h1-2,5-10,14,18H,3-4,11-13,15-17H2,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474979

(CHEMBL180445)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C25H26N2O4/c28-25-17-30-24-9-8-20(16-22(24)26-25)31-19-10-12-27(13-11-19)14-15-29-23-7-3-5-18-4-1-2-6-21(18)23/h1-9,16,19H,10-15,17H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474984

(CHEMBL181143)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4ccnc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-30-23-6-5-18(15-21(23)26-24)31-17-8-11-27(12-9-17)13-14-29-22-7-10-25-20-4-2-1-3-19(20)22/h1-7,10,15,17H,8-9,11-14,16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027184

(CHEMBL180700)Show InChI InChI=1S/C21H23N3O4/c25-21-14-28-20-6-5-15(13-18(20)24-21)26-12-10-22-8-2-11-27-19-4-1-3-17-16(19)7-9-23-17/h1,3-7,9,13,22-23H,2,8,10-12,14H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772

(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of the Phospholipase A2 (Lp-PLA2) enzyme in whole human plasma |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772

(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125266
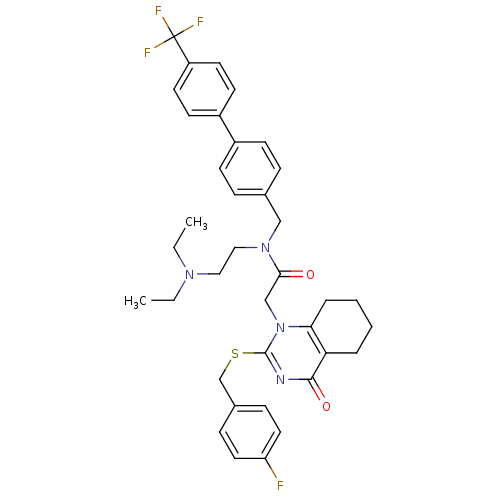
(CHEMBL10441 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(SCc2ccc(F)cc2)nc(=O)c2CCCCc12 Show InChI InChI=1S/C37H40F4N4O2S/c1-3-43(4-2)21-22-44(23-26-9-13-28(14-10-26)29-15-17-30(18-16-29)37(39,40)41)34(46)24-45-33-8-6-5-7-32(33)35(47)42-36(45)48-25-27-11-19-31(38)20-12-27/h9-20H,3-8,21-25H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117785
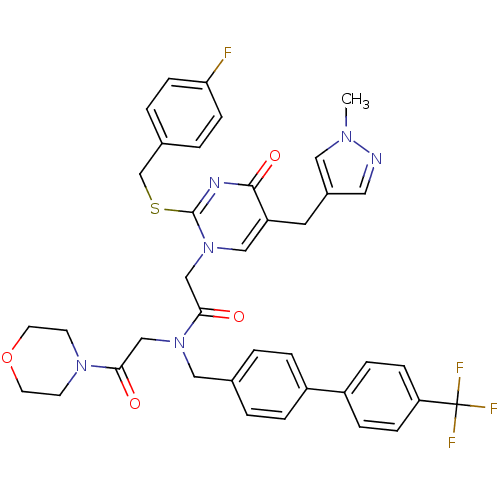
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES Cn1cc(Cc2cn(CC(=O)N(CC(=O)N3CCOCC3)Cc3ccc(cc3)-c3ccc(cc3)C(F)(F)F)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C38H36F4N6O4S/c1-45-20-28(19-43-45)18-31-22-48(37(44-36(31)51)53-25-27-4-12-33(39)13-5-27)24-35(50)47(23-34(49)46-14-16-52-17-15-46)21-26-2-6-29(7-3-26)30-8-10-32(11-9-30)38(40,41)42/h2-13,19-20,22H,14-18,21,23-25H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117802
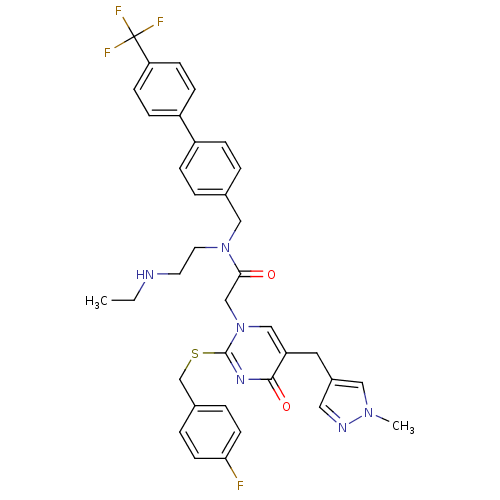
(CHEMBL87100 | N-(2-Ethylamino-ethyl)-2-[2-(4-fluor...)Show SMILES CCNCCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H36F4N6O2S/c1-3-41-16-17-45(21-25-4-8-28(9-5-25)29-10-12-31(13-11-29)36(38,39)40)33(47)23-46-22-30(18-27-19-42-44(2)20-27)34(48)43-35(46)49-24-26-6-14-32(37)15-7-26/h4-15,19-20,22,41H,3,16-18,21,23-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data