 Found 47499 hits with Last Name = 'han' and Initial = 's'
Found 47499 hits with Last Name = 'han' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605126

(CHEMBL5187340)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCC2CCNCC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
M17 leucyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM50497553
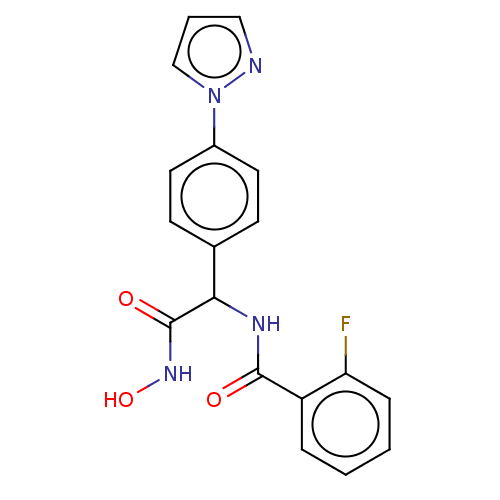
(CHEMBL3359696)Show SMILES ONC(=O)C(NC(=O)c1ccccc1F)c1ccc(cc1)-n1cccn1 Show InChI InChI=1S/C18H15FN4O3/c19-15-5-2-1-4-14(15)17(24)21-16(18(25)22-26)12-6-8-13(9-7-12)23-11-3-10-20-23/h1-11,16,26H,(H,21,24)(H,22,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay |
J Med Chem 57: 9168-83 (2014)
Article DOI: 10.1021/jm501323a
BindingDB Entry DOI: 10.7270/Q2QN69RJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50589137

(CHEMBL5196050)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)N[C@H]1OC[C@H](O)[C@H](O)[C@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50570996

(CHEMBL4864797)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)N[C@@H]1OC[C@@H](O)[C@H](O)[C@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [491-589,Q498K,D521N,V555I,N579D]
(Human immunodeficiency virus type 1) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School
| Assay Description
HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an... |
J Med Chem 50: 4316-28 (2007)
Article DOI: 10.1021/jm070284z
BindingDB Entry DOI: 10.7270/Q2W37TKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50589138

(CHEMBL5201718)Show SMILES C[C@@H]1O[C@H](NC(=O)c2ccc(cc2)S(N)(=O)=O)[C@H](O)[C@H](O)[C@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50589139

(CHEMBL5176489)Show SMILES COC(=O)[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@]1(O)S(=O)(=O)Nc1ccccc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50571000

(CHEMBL4865818)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50570994

(CHEMBL4863999)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)N[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50570993

(CHEMBL4849226)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50570997

(CHEMBL4849396)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50082842
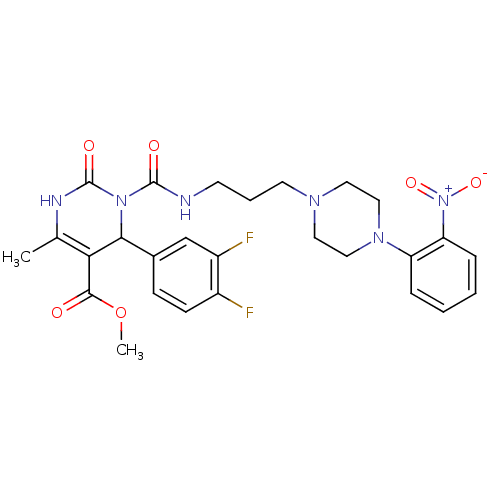
(4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[4-(2-nitro-...)Show SMILES COC(=O)C1=C(C)NC(=O)N(C1c1ccc(F)c(F)c1)C(=O)NCCCN1CCN(CC1)c1ccccc1[N+]([O-])=O |c:4| Show InChI InChI=1S/C27H30F2N6O6/c1-17-23(25(36)41-2)24(18-8-9-19(28)20(29)16-18)34(27(38)31-17)26(37)30-10-5-11-32-12-14-33(15-13-32)21-6-3-4-7-22(21)35(39)40/h3-4,6-9,16,24H,5,10-15H2,1-2H3,(H,30,37)(H,31,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. |
J Med Chem 42: 4794-803 (1999)
BindingDB Entry DOI: 10.7270/Q25B01P0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156449
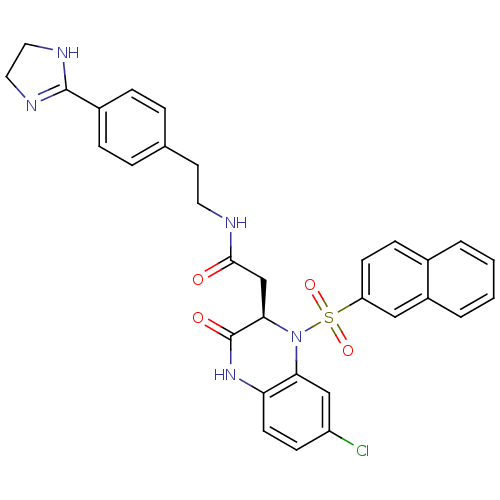
(2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...)Show SMILES Clc1ccc2NC(=O)[C@@H](CC(=O)NCCc3ccc(cc3)C3=NCCN3)N(c2c1)S(=O)(=O)c1ccc2ccccc2c1 |t:22| Show InChI InChI=1S/C31H28ClN5O4S/c32-24-10-12-26-27(18-24)37(42(40,41)25-11-9-21-3-1-2-4-23(21)17-25)28(31(39)36-26)19-29(38)33-14-13-20-5-7-22(8-6-20)30-34-15-16-35-30/h1-12,17-18,28H,13-16,19H2,(H,33,38)(H,34,35)(H,36,39)/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144258

(4-[(8-Benzo[1,3]dioxol-5-ylmethyl-8-aza-bicyclo[3....)Show SMILES [#6]-[#6]-[#7](-[#6]-[#6])-[#6](=O)-c1ccc(cc1)-[#6](=[#6]-1/[#6]-[#6]-2-[#6]-[#6]-[#6](-[#6]-1)-[#7]-2-[#6]-c1ccc2-[#8]-[#6]-[#8]-c2c1)\c1ccccc1 Show InChI InChI=1S/C33H36N2O3/c1-3-34(4-2)33(36)26-13-11-25(12-14-26)32(24-8-6-5-7-9-24)27-19-28-15-16-29(20-27)35(28)21-23-10-17-30-31(18-23)38-22-37-30/h5-14,17-18,28-29H,3-4,15-16,19-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for delta opioid receptor |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
M17 leucyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM50497551
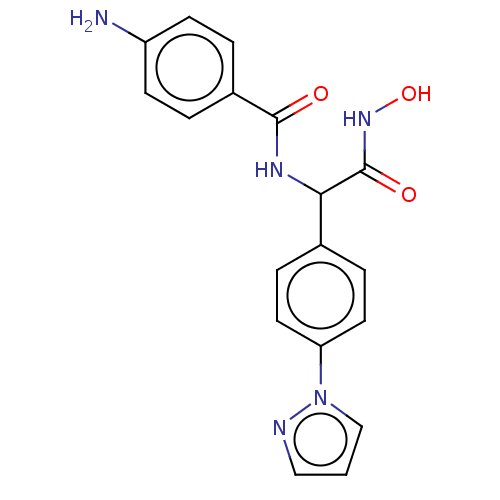
(CHEMBL3359700)Show SMILES Nc1ccc(cc1)C(=O)NC(C(=O)NO)c1ccc(cc1)-n1cccn1 Show InChI InChI=1S/C18H17N5O3/c19-14-6-2-13(3-7-14)17(24)21-16(18(25)22-26)12-4-8-15(9-5-12)23-11-1-10-20-23/h1-11,16,26H,19H2,(H,21,24)(H,22,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay |
J Med Chem 57: 9168-83 (2014)
Article DOI: 10.1021/jm501323a
BindingDB Entry DOI: 10.7270/Q2QN69RJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
M17 leucyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM50497552

(CHEMBL3359698)Show SMILES ONC(=O)C(NC(=O)c1ccc(F)cc1)c1ccc(cc1)-n1cccn1 Show InChI InChI=1S/C18H15FN4O3/c19-14-6-2-13(3-7-14)17(24)21-16(18(25)22-26)12-4-8-15(9-5-12)23-11-1-10-20-23/h1-11,16,26H,(H,21,24)(H,22,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay |
J Med Chem 57: 9168-83 (2014)
Article DOI: 10.1021/jm501323a
BindingDB Entry DOI: 10.7270/Q2QN69RJ |
More data for this
Ligand-Target Pair | |
M17 leucyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM50497556

(CHEMBL3359699)Show SMILES Nc1cccc(c1)C(=O)NC(C(=O)NO)c1ccc(cc1)-n1cccn1 Show InChI InChI=1S/C18H17N5O3/c19-14-4-1-3-13(11-14)17(24)21-16(18(25)22-26)12-5-7-15(8-6-12)23-10-2-9-20-23/h1-11,16,26H,19H2,(H,21,24)(H,22,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay |
J Med Chem 57: 9168-83 (2014)
Article DOI: 10.1021/jm501323a
BindingDB Entry DOI: 10.7270/Q2QN69RJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
M17 leucyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM50497548
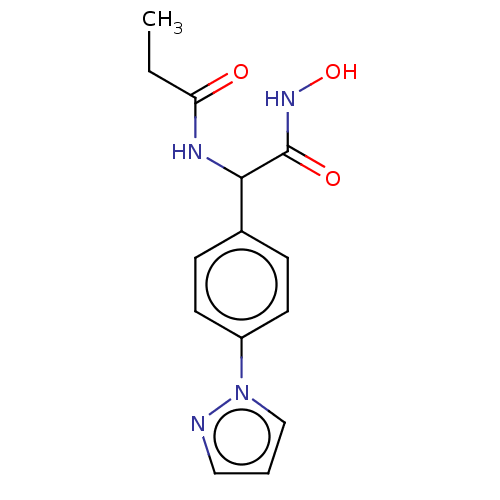
(CHEMBL3359691)Show InChI InChI=1S/C14H16N4O3/c1-2-12(19)16-13(14(20)17-21)10-4-6-11(7-5-10)18-9-3-8-15-18/h3-9,13,21H,2H2,1H3,(H,16,19)(H,17,20) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay |
J Med Chem 57: 9168-83 (2014)
Article DOI: 10.1021/jm501323a
BindingDB Entry DOI: 10.7270/Q2QN69RJ |
More data for this
Ligand-Target Pair | |
M17 leucyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM50497549

(CHEMBL3359690)Show InChI InChI=1S/C13H14N4O3/c1-9(18)15-12(13(19)16-20)10-3-5-11(6-4-10)17-8-2-7-14-17/h2-8,12,20H,1H3,(H,15,18)(H,16,19) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay |
J Med Chem 57: 9168-83 (2014)
Article DOI: 10.1021/jm501323a
BindingDB Entry DOI: 10.7270/Q2QN69RJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308541
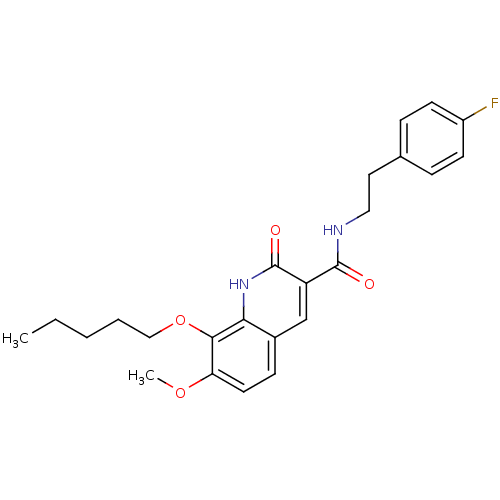
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H27FN2O4/c1-3-4-5-14-31-22-20(30-2)11-8-17-15-19(24(29)27-21(17)22)23(28)26-13-12-16-6-9-18(25)10-7-16/h6-11,15H,3-5,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163573

((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-2...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCCc2c1)c1ncc(cn1)-c1ccccn1 Show InChI InChI=1S/C28H21N5O2/c34-27-20-5-1-2-7-23(20)32-25-21(27)16-33(26(25)18-8-9-24-17(13-18)10-12-35-24)28-30-14-19(15-31-28)22-6-3-4-11-29-22/h1-9,11,13-15,26H,10,12,16H2,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308541

(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H27FN2O4/c1-3-4-5-14-31-22-20(30-2)11-8-17-15-19(24(29)27-21(17)22)23(28)26-13-12-16-6-9-18(25)10-7-16/h6-11,15H,3-5,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353091

(CHEMBL1822944)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCCc3ccncc3)c(=O)[nH]c12 Show InChI InChI=1S/C20H21N3O4/c1-3-27-18-16(26-2)5-4-14-12-15(20(25)23-17(14)18)19(24)22-11-8-13-6-9-21-10-7-13/h4-7,9-10,12H,3,8,11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Scytalone dehydratase
(Magnaporthe grisea) | BDBM50078280

((R)-2-Cyano-N-[(R)-2-(2,5-difluoro-phenoxy)-1-meth...)Show SMILES C[C@H](COc1cc(F)ccc1F)NC(=O)[C@@H](C#N)C(C)(C)C Show InChI InChI=1S/C16H20F2N2O2/c1-10(20-15(21)12(8-19)16(2,3)4)9-22-14-7-11(17)5-6-13(14)18/h5-7,10,12H,9H2,1-4H3,(H,20,21)/t10-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine-Haskell Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against scytalone dehydratase enzyme obtained from Magnaporthe grisea |
Bioorg Med Chem Lett 9: 1607-12 (1999)
BindingDB Entry DOI: 10.7270/Q2JH3KBD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378812

(CHEMBL1221411)Show SMILES Cn1c2nc(Nc3cccc(NC(=O)CN)c3)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(4.47,-45.1,;4.48,-43.56,;3.15,-42.79,;1.82,-43.56,;.49,-42.79,;-.84,-43.57,;-2.18,-42.8,;-2.18,-41.25,;-3.52,-40.49,;-4.85,-41.26,;-4.85,-42.8,;-6.18,-43.57,;-7.51,-42.8,;-7.51,-41.26,;-8.85,-43.57,;-10.18,-42.8,;-3.51,-43.57,;.48,-41.26,;1.8,-40.48,;3.15,-41.25,;4.48,-40.47,;5.81,-41.25,;7.14,-40.49,;7.14,-38.95,;5.81,-38.18,;8.47,-38.18,;9.81,-38.95,;9.81,-40.49,;8.48,-41.26,;8.47,-42.8,;5.81,-42.79,;7.14,-43.56,)| Show InChI InChI=1S/C22H18Cl2N6O2/c1-30-20-12(8-15(21(30)32)19-16(23)6-3-7-17(19)24)11-26-22(29-20)28-14-5-2-4-13(9-14)27-18(31)10-25/h2-9,11H,10,25H2,1H3,(H,27,31)(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50303233
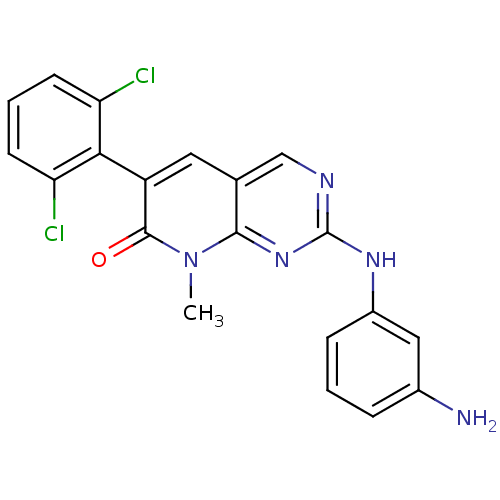
(2-(3-aminophenylamino)-6-(2,6-dichlorophenyl)-8-me...)Show SMILES Cn1c2nc(Nc3cccc(N)c3)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(1.69,-30.93,;1.69,-29.39,;.36,-28.62,;-.97,-29.39,;-2.3,-28.62,;-3.63,-29.39,;-4.96,-28.63,;-4.97,-27.08,;-6.3,-26.31,;-7.63,-27.08,;-7.63,-28.63,;-8.97,-29.4,;-6.3,-29.4,;-2.31,-27.09,;-.98,-26.31,;.36,-27.08,;1.69,-26.31,;3.02,-27.07,;4.34,-26.3,;4.33,-24.76,;3,-24,;5.66,-23.99,;7,-24.75,;7.01,-26.29,;5.68,-27.07,;5.68,-28.61,;3.03,-28.62,;4.36,-29.39,)| Show InChI InChI=1S/C20H15Cl2N5O/c1-27-18-11(8-14(19(27)28)17-15(21)6-3-7-16(17)22)10-24-20(26-18)25-13-5-2-4-12(23)9-13/h2-10H,23H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353091

(CHEMBL1822944)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCCc3ccncc3)c(=O)[nH]c12 Show InChI InChI=1S/C20H21N3O4/c1-3-27-18-16(26-2)5-4-14-12-15(20(25)23-17(14)18)19(24)22-11-8-13-6-9-21-10-7-13/h4-7,9-10,12H,3,8,11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50029257

((3-((4-(2-isopropoxyphenyl)piperazin-1-yl)methyl)p...)Show SMILES CC(C)Oc1ccccc1N1CCN(Cc2cccc(c2)C(=O)N2CCCCC2)CC1 Show InChI InChI=1S/C26H35N3O2/c1-21(2)31-25-12-5-4-11-24(25)28-17-15-27(16-18-28)20-22-9-8-10-23(19-22)26(30)29-13-6-3-7-14-29/h4-5,8-12,19,21H,3,6-7,13-18,20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sinhgad College of Pharmacy
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha1A receptor (unknown origin) |
Bioorg Med Chem 16: 4759-800 (2008)
Article DOI: 10.1016/j.bmc.2008.02.091
BindingDB Entry DOI: 10.7270/Q2DV1JPX |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156451
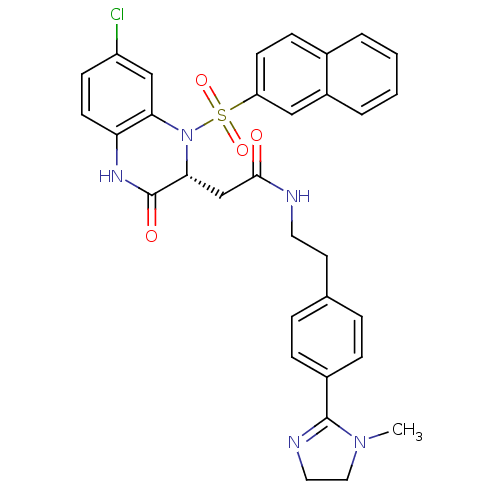
(2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...)Show SMILES CN1CCN=C1c1ccc(CCNC(=O)C[C@H]2N(c3cc(Cl)ccc3NC2=O)S(=O)(=O)c2ccc3ccccc3c2)cc1 |c:4| Show InChI InChI=1S/C32H30ClN5O4S/c1-37-17-16-35-31(37)23-8-6-21(7-9-23)14-15-34-30(39)20-29-32(40)36-27-13-11-25(33)19-28(27)38(29)43(41,42)26-12-10-22-4-2-3-5-24(22)18-26/h2-13,18-19,29H,14-17,20H2,1H3,(H,34,39)(H,36,40)/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156446
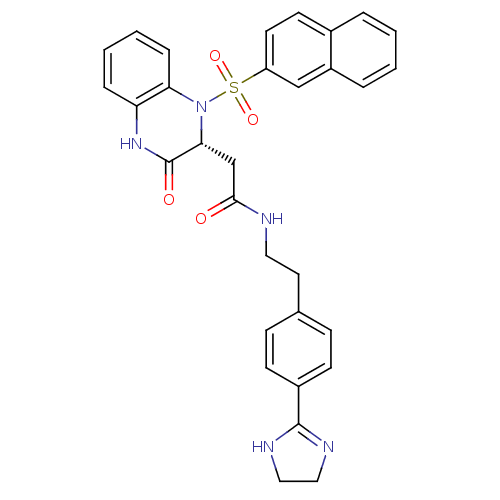
(CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...)Show SMILES O=C(C[C@H]1N(c2ccccc2NC1=O)S(=O)(=O)c1ccc2ccccc2c1)NCCc1ccc(cc1)C1=NCCN1 |t:41| Show InChI InChI=1S/C31H29N5O4S/c37-29(32-16-15-21-9-11-23(12-10-21)30-33-17-18-34-30)20-28-31(38)35-26-7-3-4-8-27(26)36(28)41(39,40)25-14-13-22-5-1-2-6-24(22)19-25/h1-14,19,28H,15-18,20H2,(H,32,37)(H,33,34)(H,35,38)/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the [35S]- radiolabelled compound to rhesus monkey Bradykinin receptor B1 |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50082839
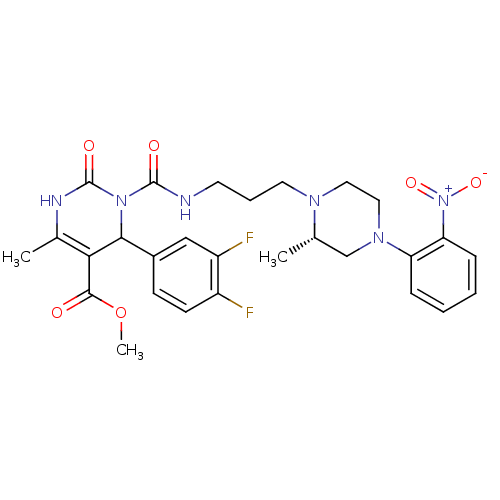
(4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[(S)-2-methy...)Show SMILES COC(=O)C1=C(C)NC(=O)N(C1c1ccc(F)c(F)c1)C(=O)NCCCN1CCN(C[C@@H]1C)c1ccccc1[N+]([O-])=O |c:4| Show InChI InChI=1S/C28H32F2N6O6/c1-17-16-34(22-7-4-5-8-23(22)36(40)41)14-13-33(17)12-6-11-31-27(38)35-25(19-9-10-20(29)21(30)15-19)24(26(37)42-3)18(2)32-28(35)39/h4-5,7-10,15,17,25H,6,11-14,16H2,1-3H3,(H,31,38)(H,32,39)/t17-,25?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. |
J Med Chem 42: 4794-803 (1999)
BindingDB Entry DOI: 10.7270/Q25B01P0 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156446

(CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...)Show SMILES O=C(C[C@H]1N(c2ccccc2NC1=O)S(=O)(=O)c1ccc2ccccc2c1)NCCc1ccc(cc1)C1=NCCN1 |t:41| Show InChI InChI=1S/C31H29N5O4S/c37-29(32-16-15-21-9-11-23(12-10-21)30-33-17-18-34-30)20-28-31(38)35-26-7-3-4-8-27(26)36(28)41(39,40)25-14-13-22-5-1-2-6-24(22)19-25/h1-14,19,28H,15-18,20H2,(H,32,37)(H,33,34)(H,35,38)/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the [35S]- radiolabelled compound to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo rec... |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073061
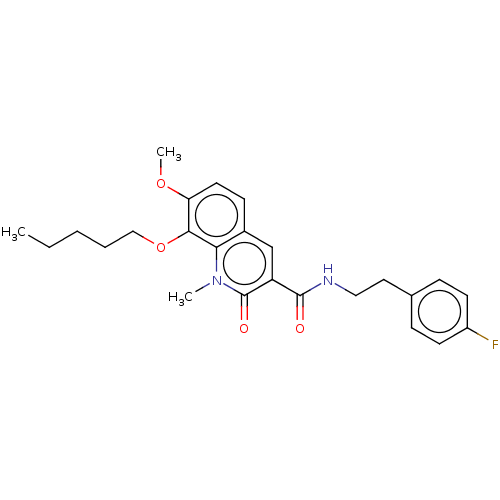
(CHEMBL3410832)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)n(C)c12 Show InChI InChI=1S/C25H29FN2O4/c1-4-5-6-15-32-23-21(31-3)12-9-18-16-20(25(30)28(2)22(18)23)24(29)27-14-13-17-7-10-19(26)11-8-17/h7-12,16H,4-6,13-15H2,1-3H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073061

(CHEMBL3410832)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)n(C)c12 Show InChI InChI=1S/C25H29FN2O4/c1-4-5-6-15-32-23-21(31-3)12-9-18-16-20(25(30)28(2)22(18)23)24(29)27-14-13-17-7-10-19(26)11-8-17/h7-12,16H,4-6,13-15H2,1-3H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144236

(4-[(R)-(S)-8-Aza-bicyclo[3.2.1]oct-(3Z)-ylidene-ph...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C(=C1\C[C@@H]2CC[C@H](C1)N2)\c1ccccc1 Show InChI InChI=1S/C25H30N2O/c1-3-27(4-2)25(28)20-12-10-19(11-13-20)24(18-8-6-5-7-9-18)21-16-22-14-15-23(17-21)26-22/h5-13,22-23,26H,3-4,14-17H2,1-2H3/b24-21-/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for delta opioid receptor |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50144229
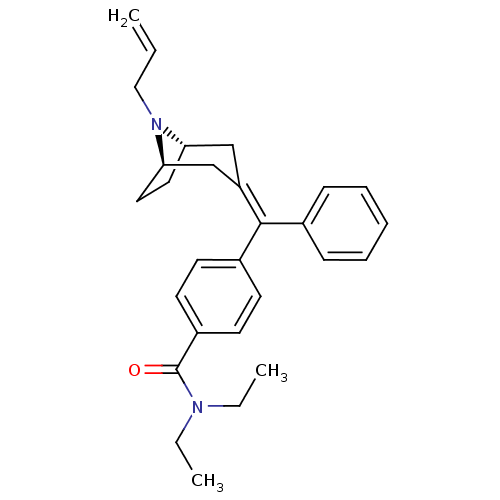
(4-{[(1S,5R)-8-Allyl-8-aza-bicyclo[3.2.1]oct-(3Z)-y...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C(=C1\C[C@@H]2CC[C@H](C1)N2CC=C)\c1ccccc1 |THB:22:21:14.15.20:17.18| Show InChI InChI=1S/C28H34N2O/c1-4-18-30-25-16-17-26(30)20-24(19-25)27(21-10-8-7-9-11-21)22-12-14-23(15-13-22)28(31)29(5-2)6-3/h4,7-15,25-26H,1,5-6,16-20H2,2-3H3/b27-24-/t25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration against stimulation of [35S]-GTP-gammaS, binding in CHO cells transfected with the human opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 2109-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.051
BindingDB Entry DOI: 10.7270/Q25B01X4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073068

(CHEMBL3410813)Show SMILES CCCCCOc1ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c2c1OCCCCC Show InChI InChI=1S/C28H35FN2O4/c1-3-5-7-17-34-24-14-11-21-19-23(27(32)30-16-15-20-9-12-22(29)13-10-20)28(33)31-25(21)26(24)35-18-8-6-4-2/h9-14,19H,3-8,15-18H2,1-2H3,(H,30,32)(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073068

(CHEMBL3410813)Show SMILES CCCCCOc1ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c2c1OCCCCC Show InChI InChI=1S/C28H35FN2O4/c1-3-5-7-17-34-24-14-11-21-19-23(27(32)30-16-15-20-9-12-22(29)13-10-20)28(33)31-25(21)26(24)35-18-8-6-4-2/h9-14,19H,3-8,15-18H2,1-2H3,(H,30,32)(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
M17 leucyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM50497543

(CHEMBL3359693)Show InChI InChI=1S/C16H20N4O3/c1-16(2,3)15(22)18-13(14(21)19-23)11-5-7-12(8-6-11)20-10-4-9-17-20/h4-10,13,23H,1-3H3,(H,18,22)(H,19,21) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay |
J Med Chem 57: 9168-83 (2014)
Article DOI: 10.1021/jm501323a
BindingDB Entry DOI: 10.7270/Q2QN69RJ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Plasmodium vivax (malaria parasite P. vivax)) | BDBM18788
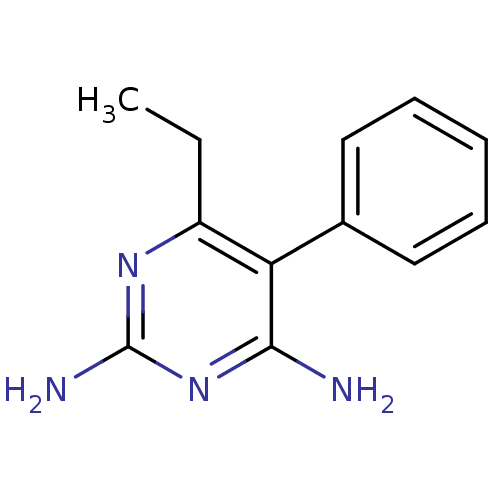
(6-ethyl-5-phenylpyrimidine-2,4-diamine | CHEMBL221...)Show InChI InChI=1S/C12H14N4/c1-2-9-10(8-6-4-3-5-7-8)11(13)16-12(14)15-9/h3-7H,2H2,1H3,(H4,13,14,15,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0300 | -60.1 | 1.82E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
National Center for Genetic Engineering and Biotechnology at Thailand
| Assay Description
Nineteen Pyr analogs were studied for their inhibition activity against cells expressing either WT or SP21 mutant PvDHFR-TS. The assays were conducte... |
Antimicrob Agents Chemother 50: 3631-7 (2006)
Article DOI: 10.1128/AAC.00448-06
BindingDB Entry DOI: 10.7270/Q2N8781P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50393719
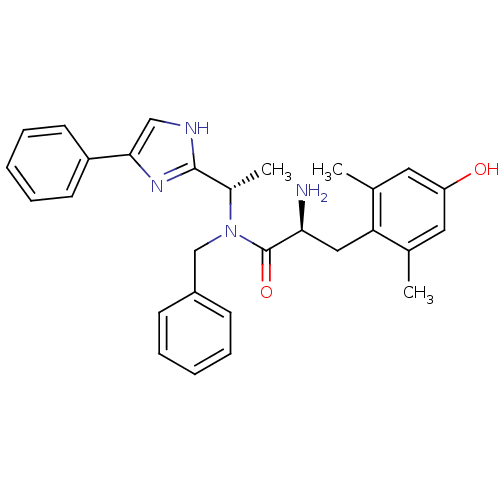
(CHEMBL2159118)Show SMILES C[C@H](N(Cc1ccccc1)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C29H32N4O2/c1-19-14-24(34)15-20(2)25(19)16-26(30)29(35)33(18-22-10-6-4-7-11-22)21(3)28-31-17-27(32-28)23-12-8-5-9-13-23/h4-15,17,21,26,34H,16,18,30H2,1-3H3,(H,31,32)/t21-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
M17 leucyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM50497544

(CHEMBL3359688)Show SMILES CC(C)(C)OC(=O)NC(C(=O)NO)c1ccc(cc1)-n1cccn1 Show InChI InChI=1S/C16H20N4O4/c1-16(2,3)24-15(22)18-13(14(21)19-23)11-5-7-12(8-6-11)20-10-4-9-17-20/h4-10,13,23H,1-3H3,(H,18,22)(H,19,21) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay |
J Med Chem 57: 9168-83 (2014)
Article DOI: 10.1021/jm501323a
BindingDB Entry DOI: 10.7270/Q2QN69RJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50393725
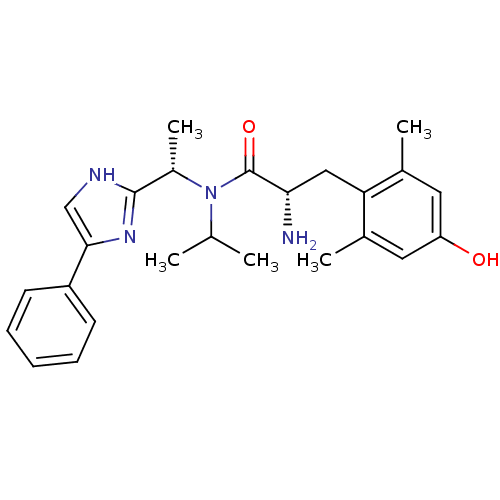
(CHEMBL2159117)Show SMILES CC(C)N([C@@H](C)c1nc(c[nH]1)-c1ccccc1)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C25H32N4O2/c1-15(2)29(18(5)24-27-14-23(28-24)19-9-7-6-8-10-19)25(31)22(26)13-21-16(3)11-20(30)12-17(21)4/h6-12,14-15,18,22,30H,13,26H2,1-5H3,(H,27,28)/t18-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156455
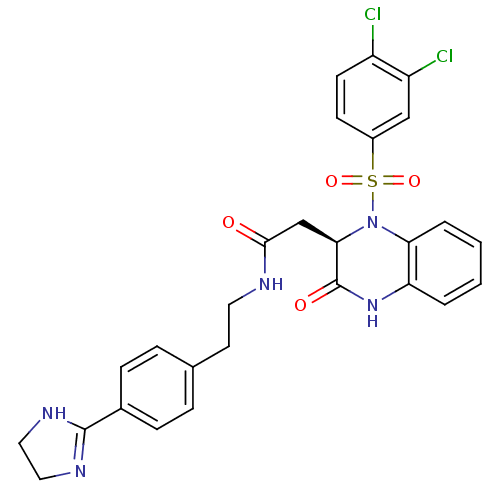
((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)NCCc2ccc(cc2)C2=NCCN2)C(=O)Nc2ccccc12 |r,t:27| Show InChI InChI=1S/C27H25Cl2N5O4S/c28-20-10-9-19(15-21(20)29)39(37,38)34-23-4-2-1-3-22(23)33-27(36)24(34)16-25(35)30-12-11-17-5-7-18(8-6-17)26-31-13-14-32-26/h1-10,15,24H,11-14,16H2,(H,30,35)(H,31,32)(H,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442922
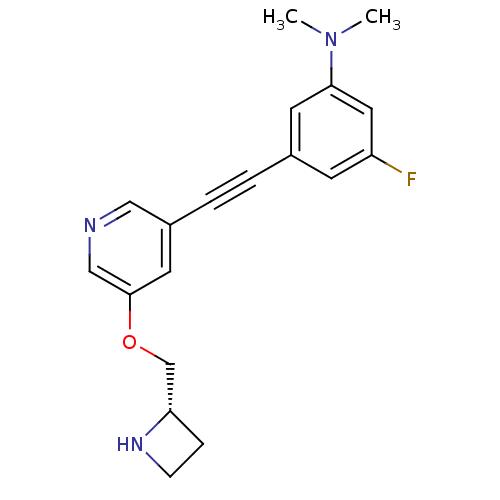
(CHEMBL3086984)Show SMILES CN(C)c1cc(F)cc(c1)C#Cc1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C19H20FN3O/c1-23(2)18-8-14(7-16(20)10-18)3-4-15-9-19(12-21-11-15)24-13-17-5-6-22-17/h7-12,17,22H,5-6,13H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442927

(CHEMBL3086994)Show InChI InChI=1S/C17H15FN2O/c18-15-3-1-2-13(8-15)4-5-14-9-17(11-19-10-14)21-12-16-6-7-20-16/h1-3,8-11,16,20H,6-7,12H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006257
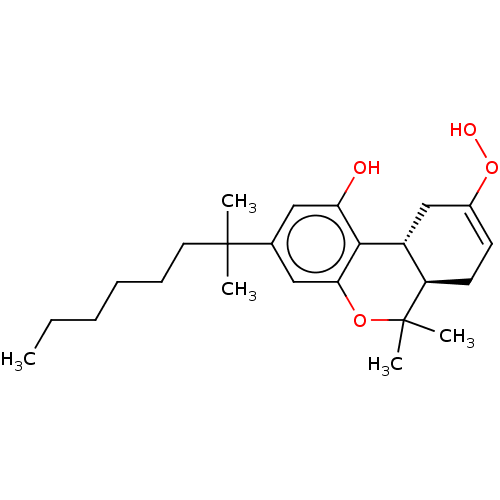
(CHEMBL3233404)Show SMILES [H][C@@]12CC(OO)=CC[C@@]1([H])C(C)(C)Oc1cc(cc(O)c21)C(C)(C)CCCCCC |r,c:5| Show InChI InChI=1S/C24H36O4/c1-6-7-8-9-12-23(2,3)16-13-20(25)22-18-15-17(28-26)10-11-19(18)24(4,5)27-21(22)14-16/h10,13-14,18-19,25-26H,6-9,11-12,15H2,1-5H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Cell Chem Biol 56: 8224-56 (2013)
Article DOI: 10.1021/jm4005626
BindingDB Entry DOI: 10.7270/Q2B859M5 |
More data for this
Ligand-Target Pair | |
M17 leucyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM50497545
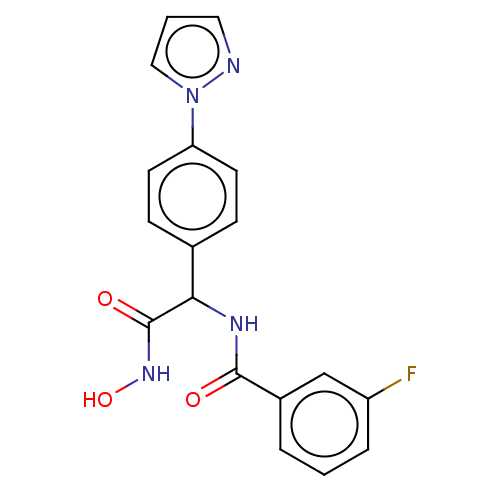
(CHEMBL3359697)Show SMILES ONC(=O)C(NC(=O)c1cccc(F)c1)c1ccc(cc1)-n1cccn1 Show InChI InChI=1S/C18H15FN4O3/c19-14-4-1-3-13(11-14)17(24)21-16(18(25)22-26)12-5-7-15(8-6-12)23-10-2-9-20-23/h1-11,16,26H,(H,21,24)(H,22,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum M17 incubated for 10 mins before H-Leu-NHMec substrate addition by fluorescence assay |
J Med Chem 57: 9168-83 (2014)
Article DOI: 10.1021/jm501323a
BindingDB Entry DOI: 10.7270/Q2QN69RJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200169
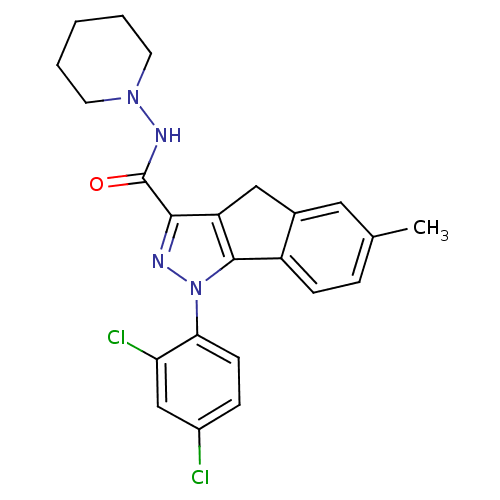
(6-methyl-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl...)Show SMILES Cc1ccc-2c(Cc3c(nn(c-23)-c2ccc(Cl)cc2Cl)C(=O)NN2CCCCC2)c1 Show InChI InChI=1S/C23H22Cl2N4O/c1-14-5-7-17-15(11-14)12-18-21(23(30)27-28-9-3-2-4-10-28)26-29(22(17)18)20-8-6-16(24)13-19(20)25/h5-8,11,13H,2-4,9-10,12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Cell Chem Biol 56: 8224-56 (2013)
Article DOI: 10.1021/jm4005626
BindingDB Entry DOI: 10.7270/Q2B859M5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data