

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50448583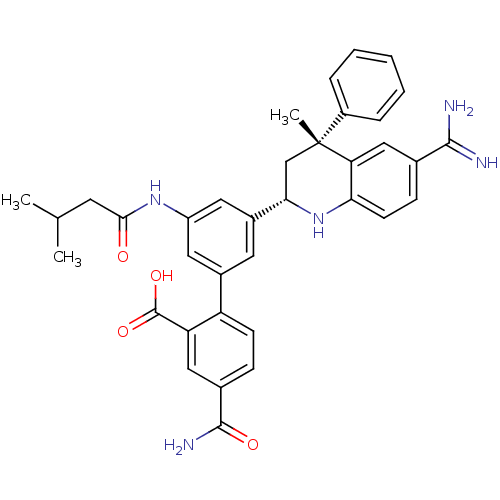 (CHEMBL3127491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032873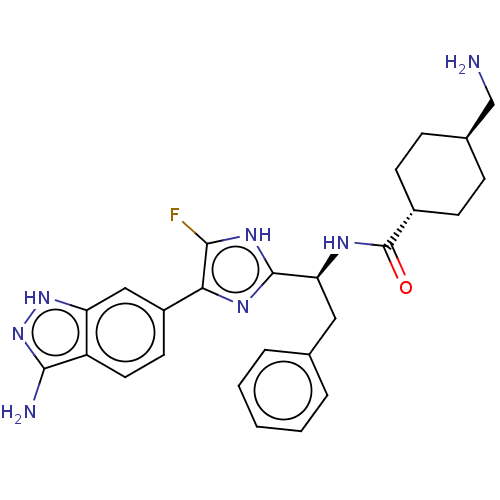 (CHEMBL3355684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032874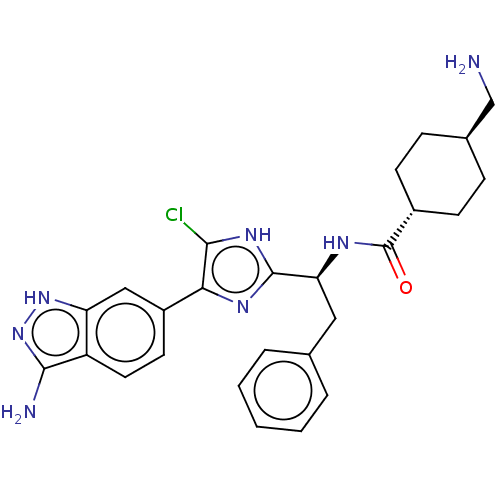 (CHEMBL3355683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032874 (CHEMBL3355683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis at 37 degC by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50032873 (CHEMBL3355684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50448583 (CHEMBL3127491) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214244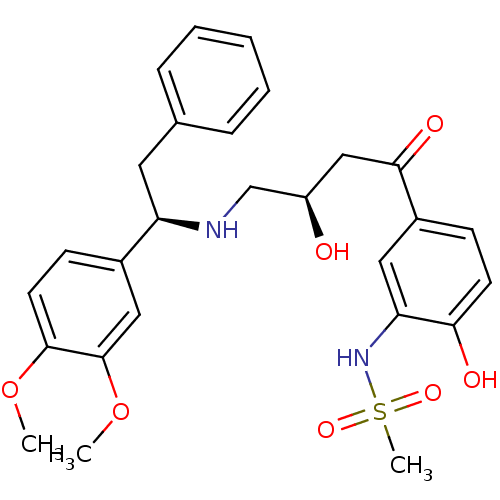 (CHEMBL250978 | N-(5-((R)-4-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032875 (CHEMBL3355682) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032876 (CHEMBL3355681) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50103208 (CHEMBL3393381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a after 10 to 120 mins | Bioorg Med Chem Lett 25: 925-30 (2015) Article DOI: 10.1016/j.bmcl.2014.12.050 BindingDB Entry DOI: 10.7270/Q2CF9RWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50103201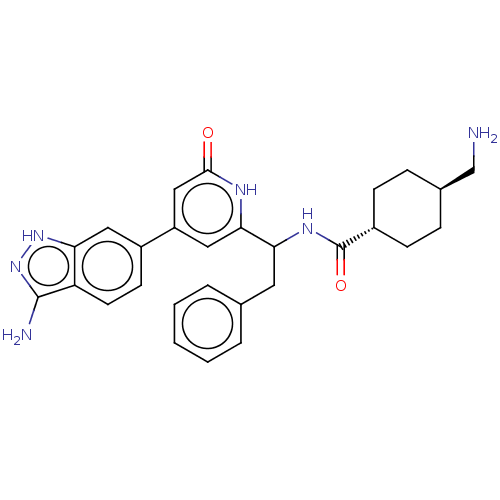 (CHEMBL3393388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a after 10 to 120 mins | Bioorg Med Chem Lett 25: 925-30 (2015) Article DOI: 10.1016/j.bmcl.2014.12.050 BindingDB Entry DOI: 10.7270/Q2CF9RWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032877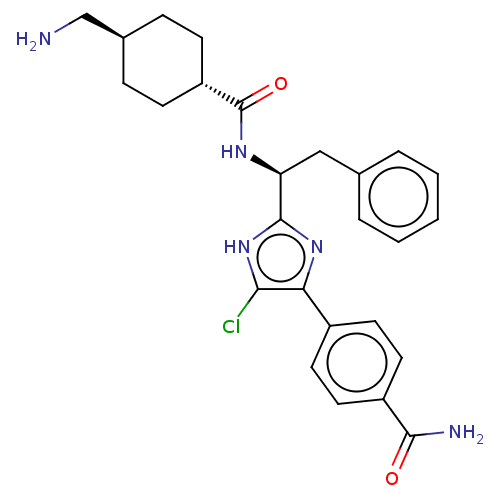 (CHEMBL3355680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50032874 (CHEMBL3355683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214246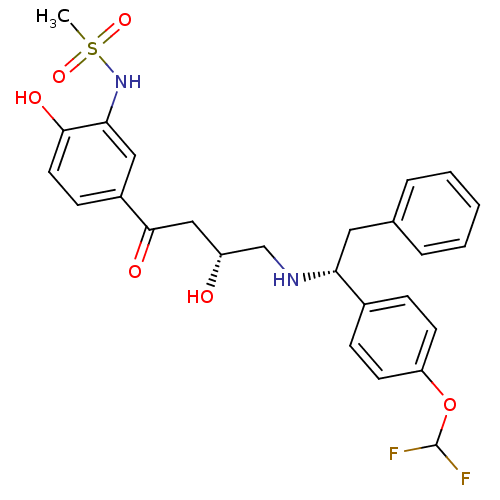 (CHEMBL401135 | N-(5-((R)-4-((R)-1-(4-(difluorometh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214234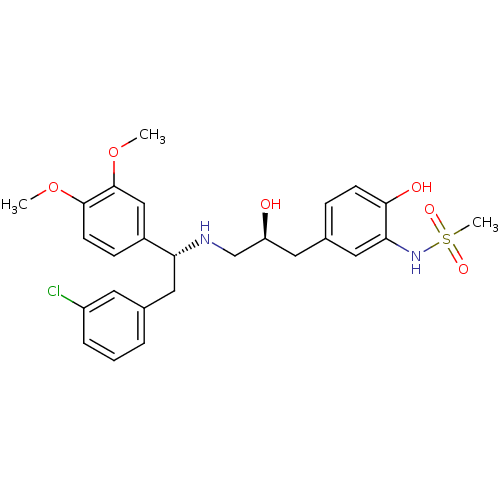 (CHEMBL250755 | N-(5-((S)-3-((R)-2-(3-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214213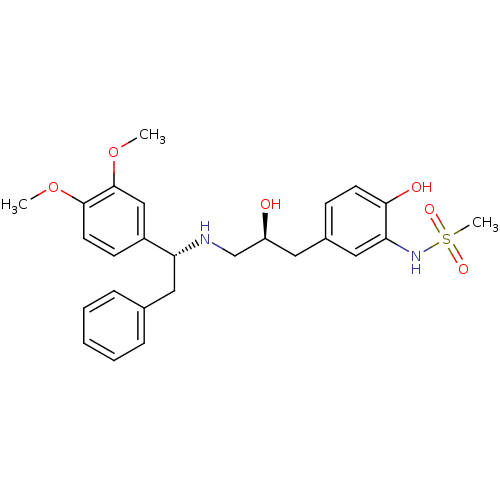 (CHEMBL399329 | N-(5-((S)-3-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214242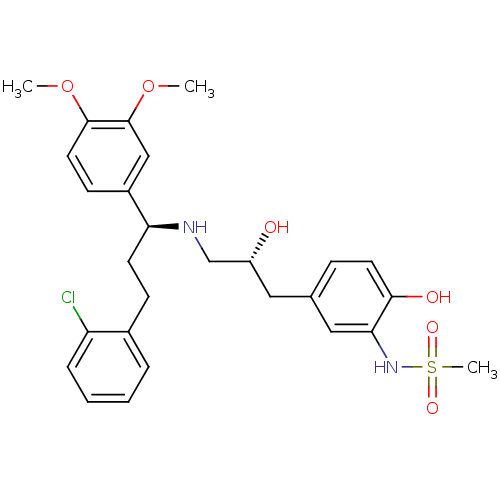 (CHEMBL447786 | N-(5-((R)-3-((S)-3-(2-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214248 (CHEMBL437578 | N-(5-((S)-3-((R)-2-(2-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214240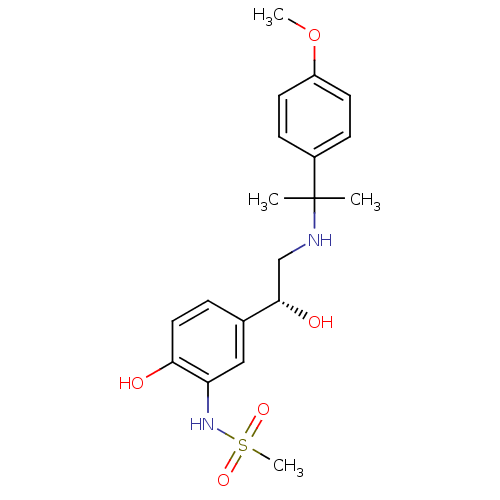 ((R)-N-(2-hydroxy-5-(1-hydroxy-2-(2-(4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214237 (CHEMBL400947 | N-(2-hydroxy-5-((S)-2-hydroxy-3-((R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50032876 (CHEMBL3355681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50103200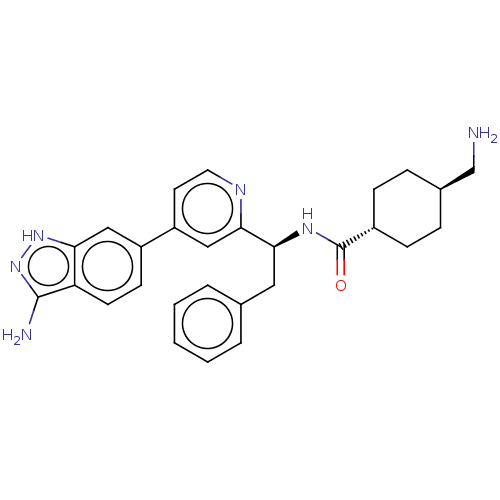 (CHEMBL3393386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a after 10 to 120 mins | Bioorg Med Chem Lett 25: 925-30 (2015) Article DOI: 10.1016/j.bmcl.2014.12.050 BindingDB Entry DOI: 10.7270/Q2CF9RWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032878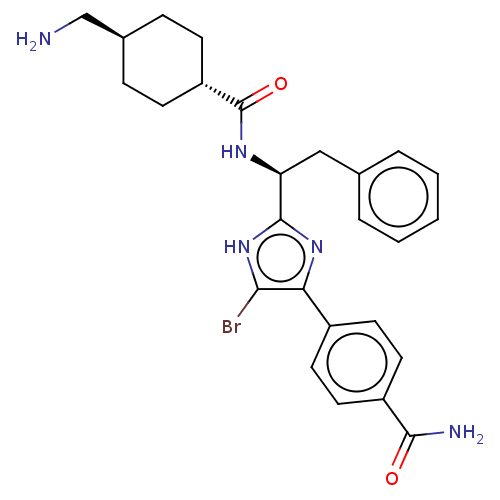 (CHEMBL3355679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214225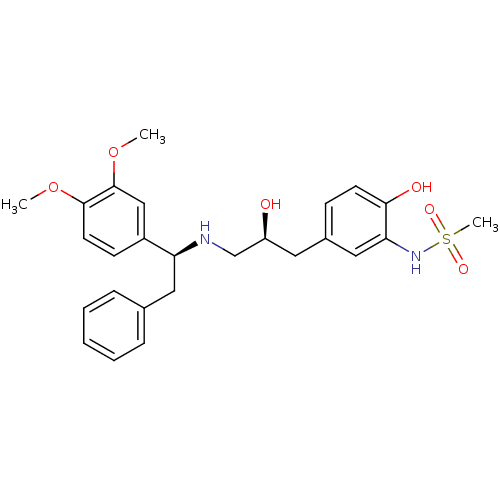 (CHEMBL398557 | N-(5-((S)-3-((S)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214228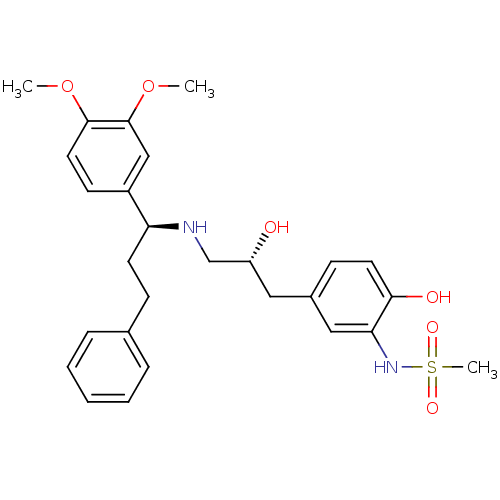 (CHEMBL400067 | N-(5-((R)-3-((S)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106810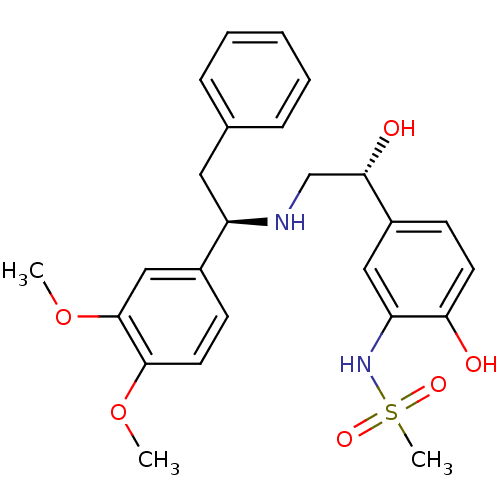 (CHEMBL317621 | N-(5-((R)-2-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214227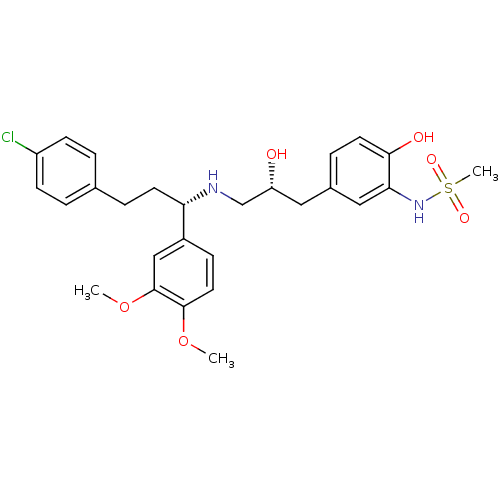 (CHEMBL251764 | N-(5-((R)-3-((S)-3-(4-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50103216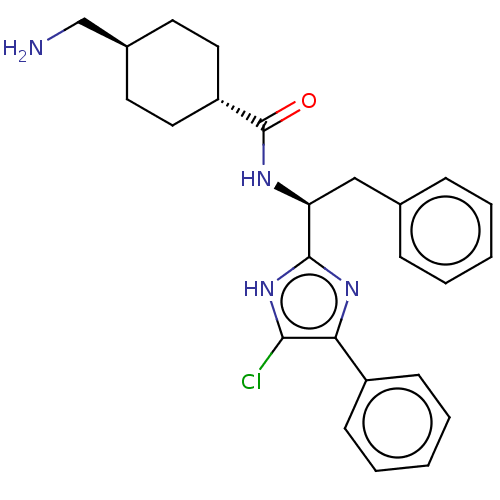 (CHEMBL3393373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a after 10 to 120 mins | Bioorg Med Chem Lett 25: 925-30 (2015) Article DOI: 10.1016/j.bmcl.2014.12.050 BindingDB Entry DOI: 10.7270/Q2CF9RWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50103204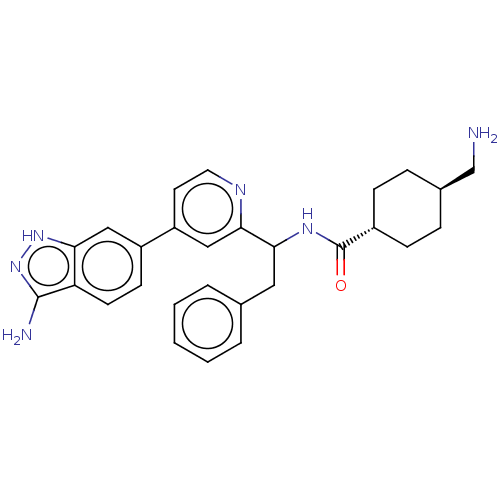 (CHEMBL3393385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a after 10 to 120 mins | Bioorg Med Chem Lett 25: 925-30 (2015) Article DOI: 10.1016/j.bmcl.2014.12.050 BindingDB Entry DOI: 10.7270/Q2CF9RWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214233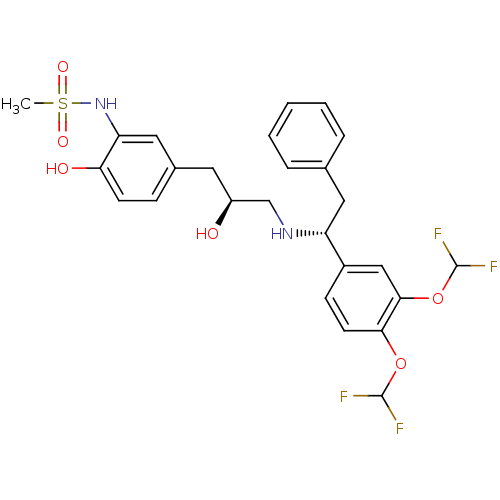 (CHEMBL250553 | N-(5-((S)-3-((R)-1-(3,4-bis(difluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50032877 (CHEMBL3355680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50103206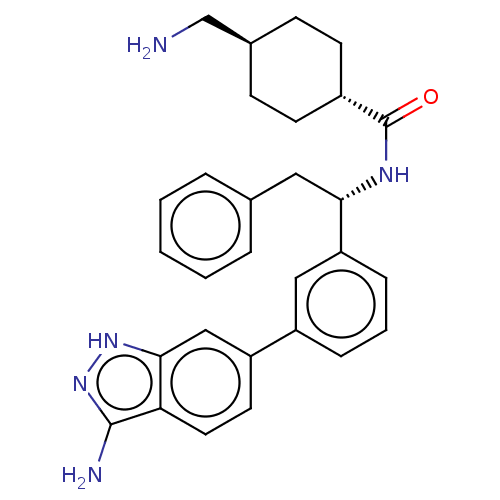 (CHEMBL3393383) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a after 10 to 120 mins | Bioorg Med Chem Lett 25: 925-30 (2015) Article DOI: 10.1016/j.bmcl.2014.12.050 BindingDB Entry DOI: 10.7270/Q2CF9RWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106829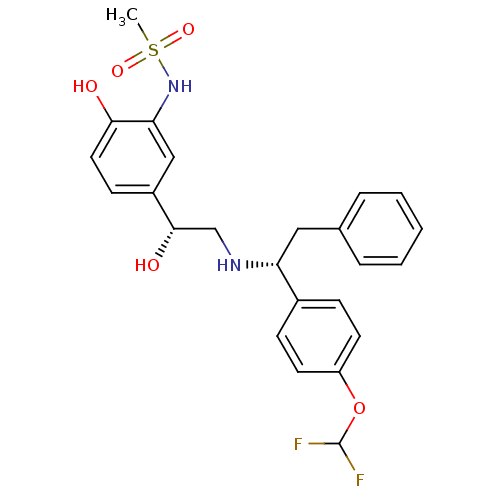 (BMS-196085 | CHEMBL322862 | N-(5-((R)-2-((R)-1-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50032874 (CHEMBL3355683) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation trypsin using N-benzoyl-Ile-Glu-(OH, OMe)-Gly-Arg-pNA as substrate by spectrophotometric analysis | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214245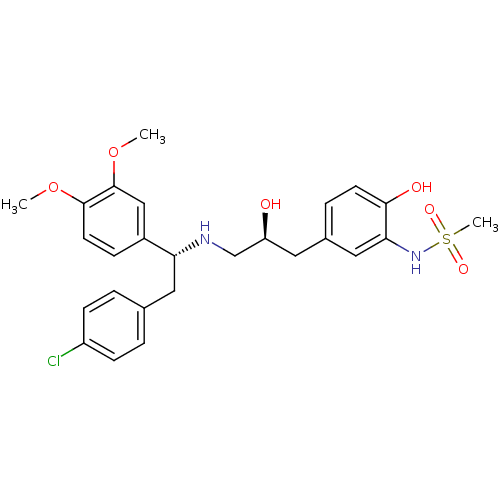 (CHEMBL429665 | N-(5-((S)-3-((R)-2-(4-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50103200 (CHEMBL3393386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein | Bioorg Med Chem Lett 25: 925-30 (2015) Article DOI: 10.1016/j.bmcl.2014.12.050 BindingDB Entry DOI: 10.7270/Q2CF9RWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50214225 (CHEMBL398557 | N-(5-((S)-3-((S)-1-(3,4-dimethoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human cloned adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214212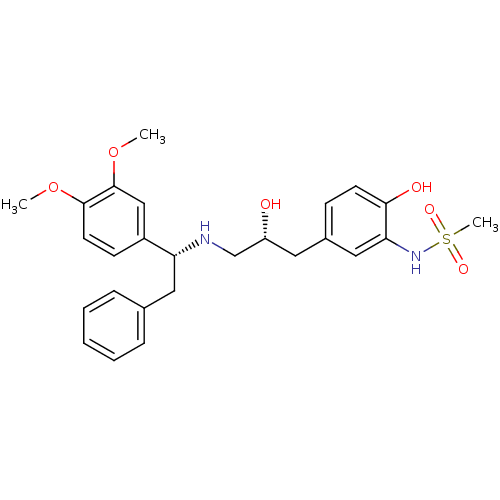 (CHEMBL248548 | N-(5-((R)-3-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50214224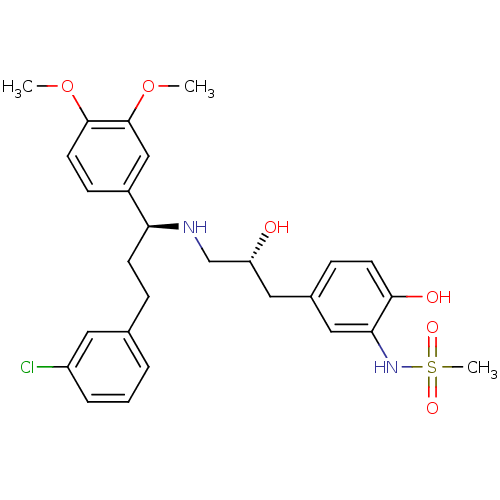 (CHEMBL398241 | N-(5-((R)-3-((S)-3-(3-chlorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human cloned adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214224 (CHEMBL398241 | N-(5-((R)-3-((S)-3-(3-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50032858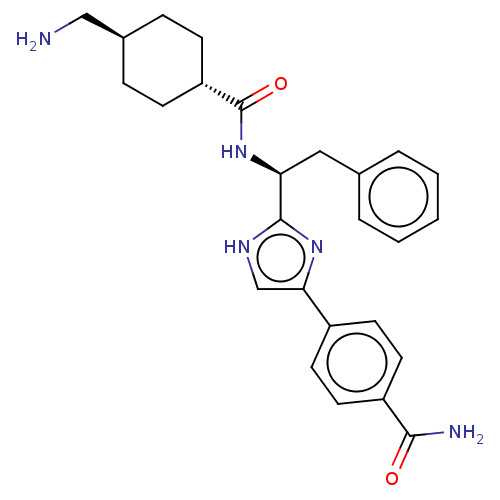 (CHEMBL3355670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214223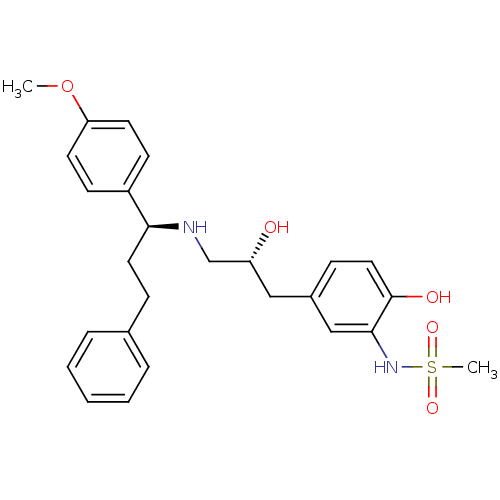 (CHEMBL251347 | N-(2-hydroxy-5-((R)-2-hydroxy-3-((S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50214231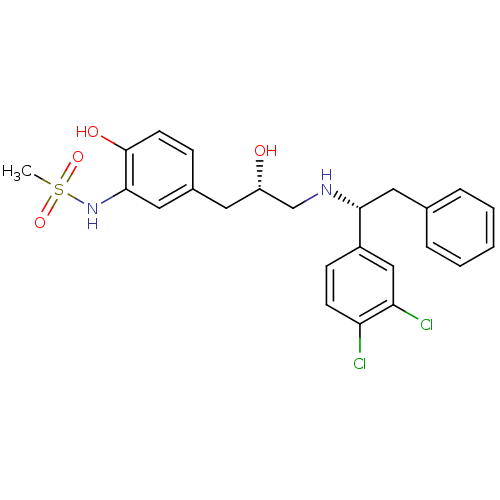 (CHEMBL249359 | N-(5-((S)-3-((R)-1-(3,4-dichlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human cloned adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50103207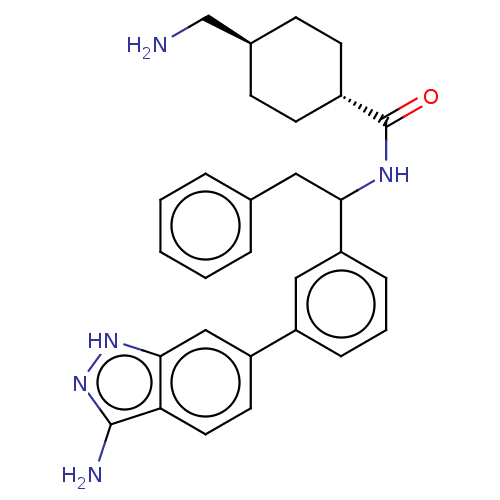 (CHEMBL3393382) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a after 10 to 120 mins | Bioorg Med Chem Lett 25: 925-30 (2015) Article DOI: 10.1016/j.bmcl.2014.12.050 BindingDB Entry DOI: 10.7270/Q2CF9RWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50032878 (CHEMBL3355679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50032875 (CHEMBL3355682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214215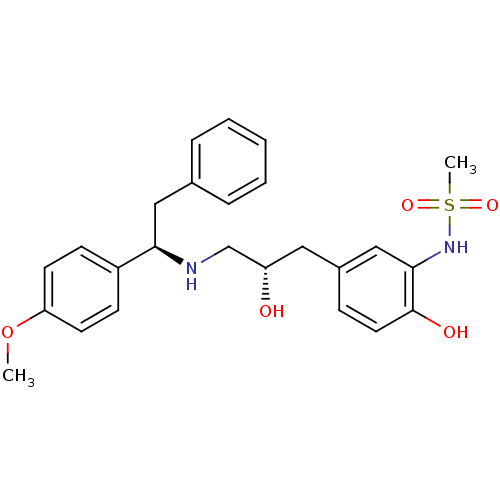 (CHEMBL251581 | N-(2-hydroxy-5-((S)-2-hydroxy-3-((R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50214242 (CHEMBL447786 | N-(5-((R)-3-((S)-3-(2-chlorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human cloned adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214220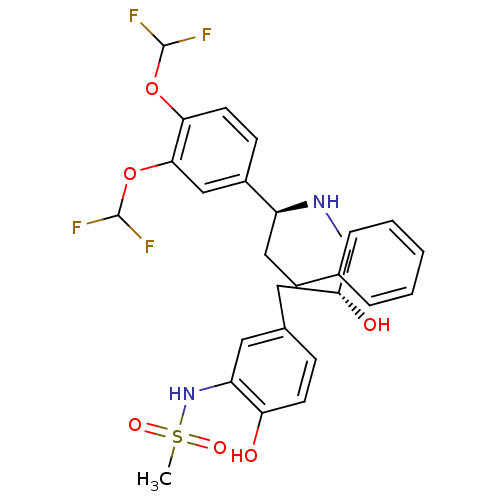 (CHEMBL251555 | N-(5-((R)-3-((S)-1-(3,4-bis(difluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50032858 (CHEMBL3355670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate | J Med Chem 57: 9915-32 (2014) Article DOI: 10.1021/jm5010607 BindingDB Entry DOI: 10.7270/Q2RV0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1121 total ) | Next | Last >> |