

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334988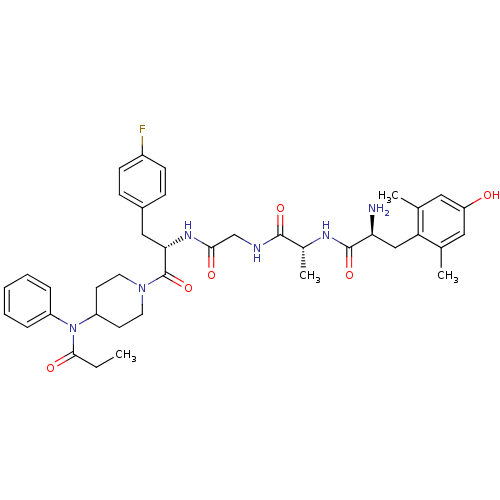 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-fluorophenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334995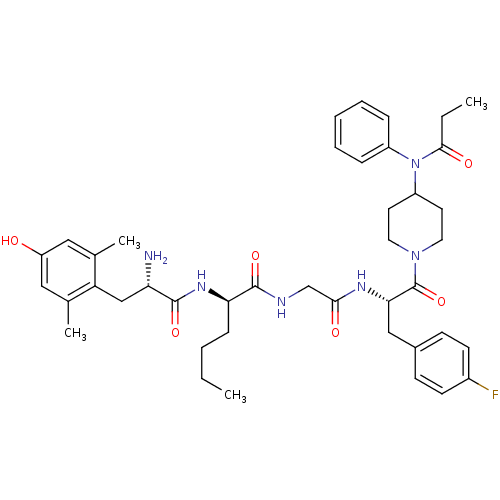 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334988 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-fluorophenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334994 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334994 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334990 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334996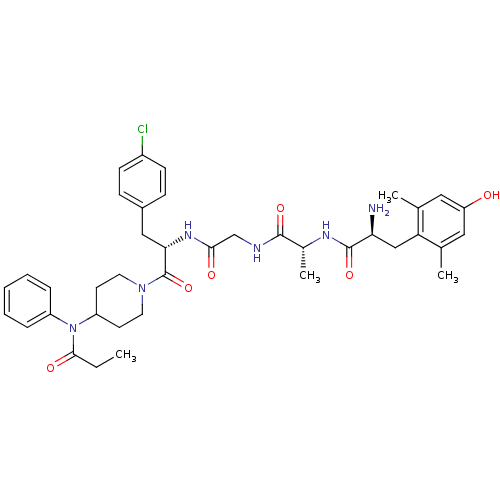 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-chlorophenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334990 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334991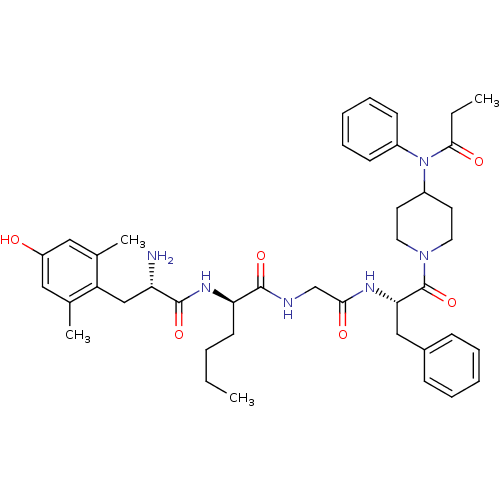 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334987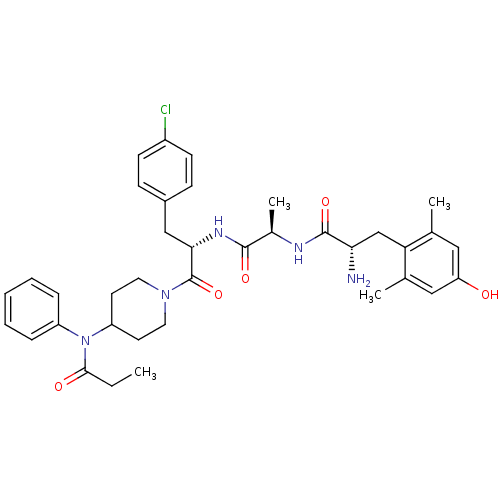 ((S)-2-amino-N-((R)-1-((S)-3-(4-chlorophenyl)-1-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334992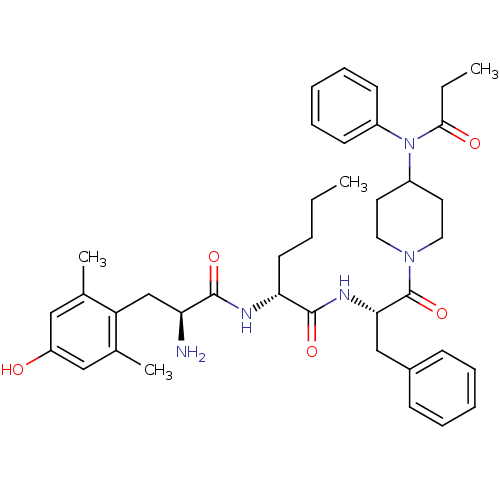 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21121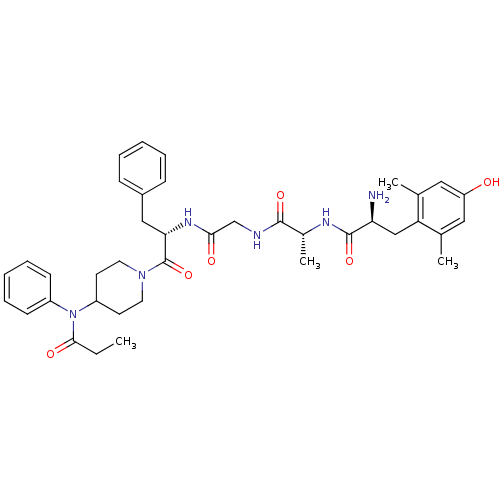 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21121 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21121 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334991 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334995 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334989 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334992 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334987 ((S)-2-amino-N-((R)-1-((S)-3-(4-chlorophenyl)-1-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334993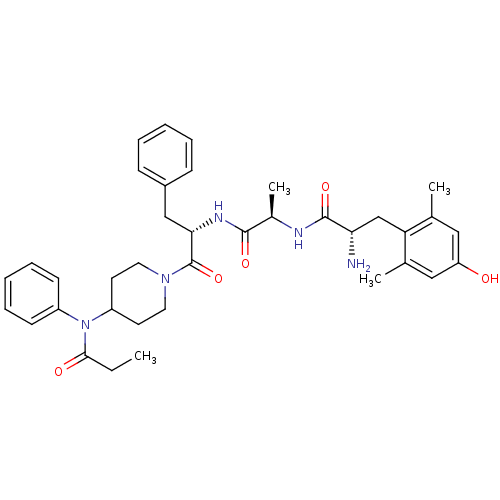 ((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50334997 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334989 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334990 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334995 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334993 ((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.339 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334995 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334996 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-chlorophenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.389 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334991 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334996 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-chlorophenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334989 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334989 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334988 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-fluorophenyl)-1-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334994 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334991 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334988 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-fluorophenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM21121 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334994 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334996 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-chlorophenyl)-1-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334993 ((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334992 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334990 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21123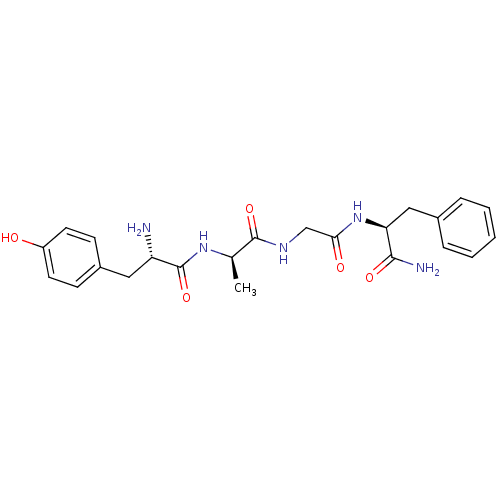 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334992 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21121 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334997 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334993 ((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21123 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50334987 ((S)-2-amino-N-((R)-1-((S)-3-(4-chlorophenyl)-1-oxo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM21123 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50334987 ((S)-2-amino-N-((R)-1-((S)-3-(4-chlorophenyl)-1-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |