 Found 155 hits with Last Name = 'hansson' and Initial = 'k'
Found 155 hits with Last Name = 'hansson' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447515
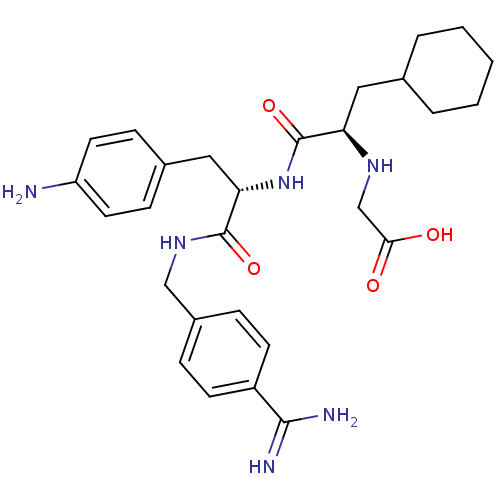
(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447528
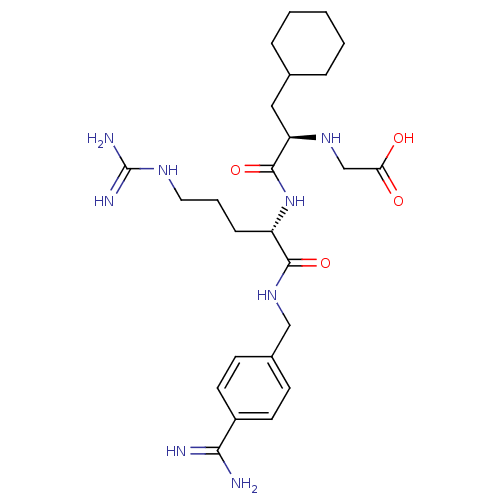
(CHEMBL3115904)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C25H40N8O4/c26-22(27)18-10-8-17(9-11-18)14-32-23(36)19(7-4-12-30-25(28)29)33-24(37)20(31-15-21(34)35)13-16-5-2-1-3-6-16/h8-11,16,19-20,31H,1-7,12-15H2,(H3,26,27)(H,32,36)(H,33,37)(H,34,35)(H4,28,29,30)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447516
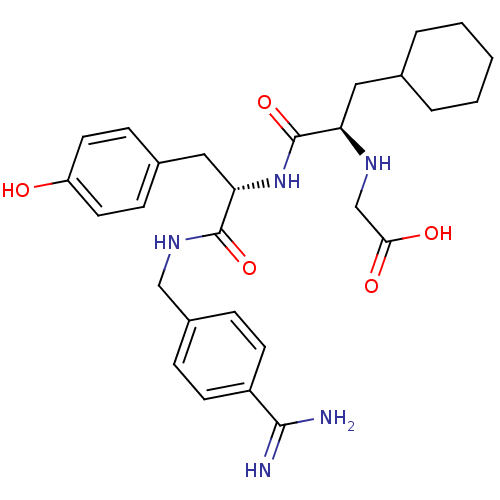
(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447523
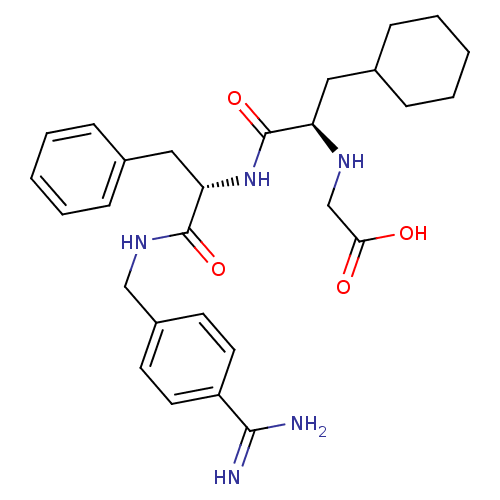
(CHEMBL3115897)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O4/c29-26(30)22-13-11-21(12-14-22)17-32-27(36)24(16-20-9-5-2-6-10-20)33-28(37)23(31-18-25(34)35)15-19-7-3-1-4-8-19/h2,5-6,9-14,19,23-24,31H,1,3-4,7-8,15-18H2,(H3,29,30)(H,32,36)(H,33,37)(H,34,35)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447515

(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447521
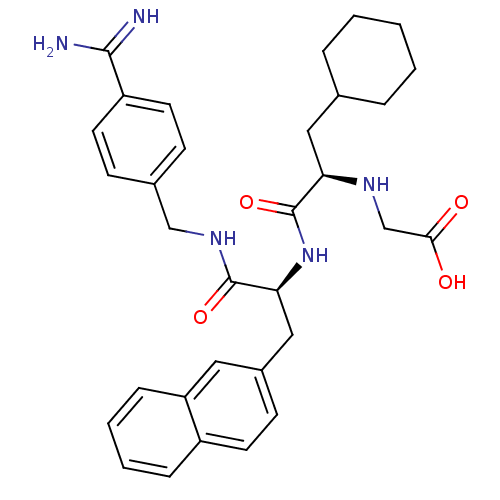
(CHEMBL3115899)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C32H39N5O4/c33-30(34)25-14-10-22(11-15-25)19-36-31(40)28(18-23-12-13-24-8-4-5-9-26(24)16-23)37-32(41)27(35-20-29(38)39)17-21-6-2-1-3-7-21/h4-5,8-16,21,27-28,35H,1-3,6-7,17-20H2,(H3,33,34)(H,36,40)(H,37,41)(H,38,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447514
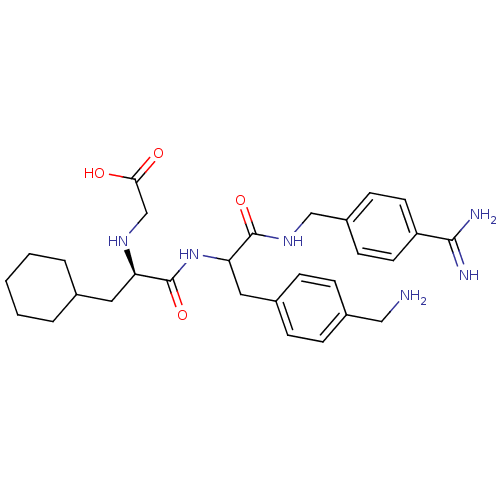
(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447522

(CHEMBL3115898)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C32H39N5O4/c33-30(34)24-15-13-22(14-16-24)19-36-31(40)28(18-25-11-6-10-23-9-4-5-12-26(23)25)37-32(41)27(35-20-29(38)39)17-21-7-2-1-3-8-21/h4-6,9-16,21,27-28,35H,1-3,7-8,17-20H2,(H3,33,34)(H,36,40)(H,37,41)(H,38,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447517
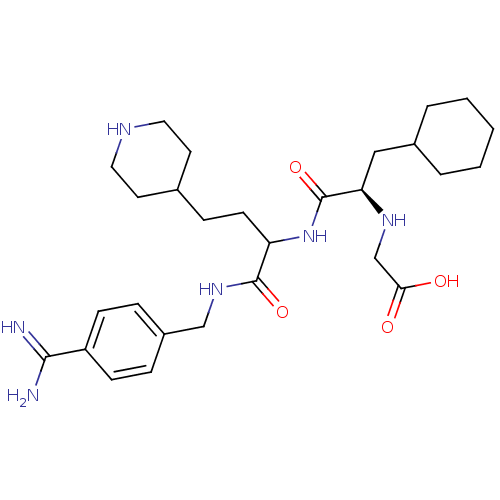
(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447525
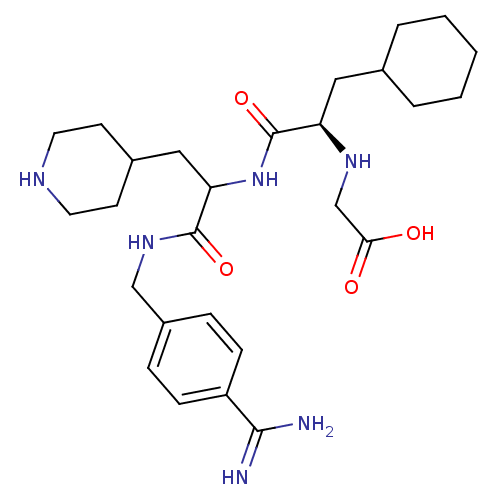
(CHEMBL3115893)Show SMILES NC(=N)c1ccc(CNC(=O)C(CC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C27H42N6O4/c28-25(29)21-8-6-20(7-9-21)16-32-26(36)23(15-19-10-12-30-13-11-19)33-27(37)22(31-17-24(34)35)14-18-4-2-1-3-5-18/h6-9,18-19,22-23,30-31H,1-5,10-17H2,(H3,28,29)(H,32,36)(H,33,37)(H,34,35)/t22-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447527
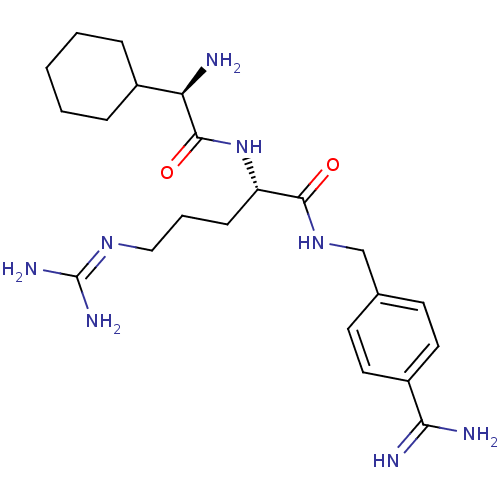
(CHEMBL3115905)Show SMILES [#7]-[#6@H](-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C22H36N8O2/c23-18(15-5-2-1-3-6-15)21(32)30-17(7-4-12-28-22(26)27)20(31)29-13-14-8-10-16(11-9-14)19(24)25/h8-11,15,17-18H,1-7,12-13,23H2,(H3,24,25)(H,29,31)(H,30,32)(H4,26,27,28)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50193243
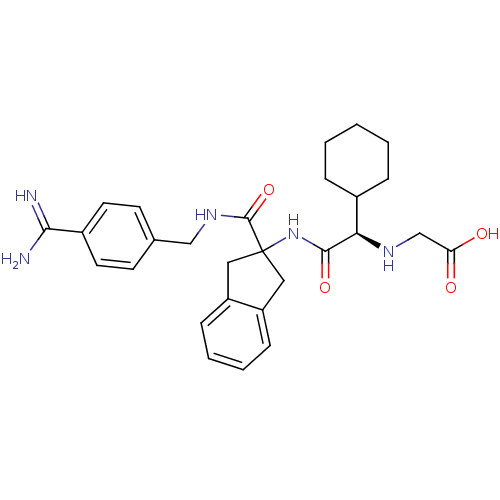
(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447519
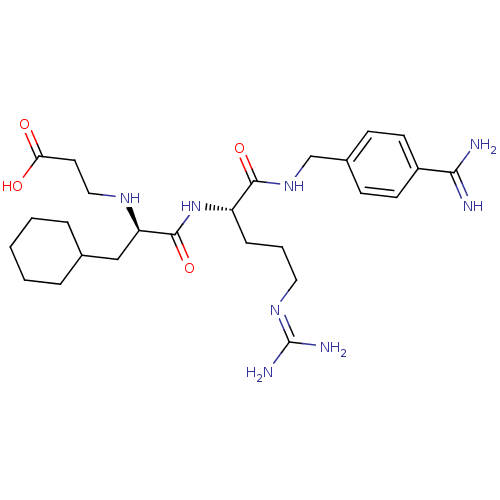
(CHEMBL3115906)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#7]-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C26H42N8O4/c27-23(28)19-10-8-18(9-11-19)16-33-24(37)20(7-4-13-32-26(29)30)34-25(38)21(31-14-12-22(35)36)15-17-5-2-1-3-6-17/h8-11,17,20-21,31H,1-7,12-16H2,(H3,27,28)(H,33,37)(H,34,38)(H,35,36)(H4,29,30,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447516

(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447524
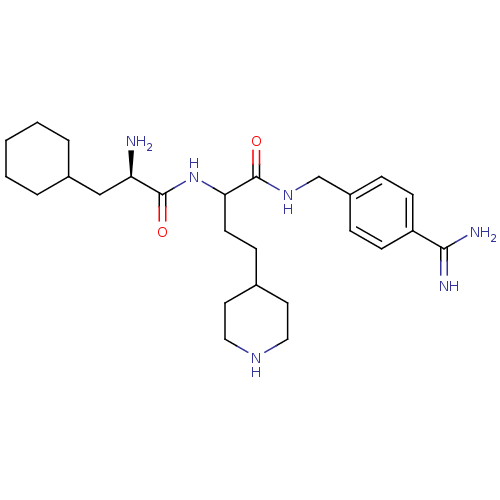
(CHEMBL3115896)Show SMILES N[C@H](CC1CCCCC1)C(=O)NC(CCC1CCNCC1)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C26H42N6O2/c27-22(16-19-4-2-1-3-5-19)25(33)32-23(11-8-18-12-14-30-15-13-18)26(34)31-17-20-6-9-21(10-7-20)24(28)29/h6-7,9-10,18-19,22-23,30H,1-5,8,11-17,27H2,(H3,28,29)(H,31,34)(H,32,33)/t22-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447518
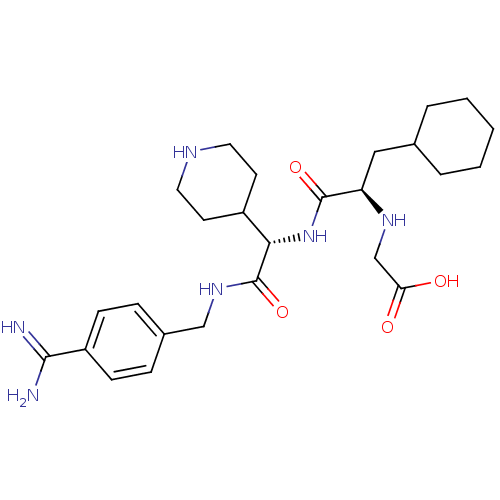
(CHEMBL3115892)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H](NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C2CCNCC2)cc1 |r| Show InChI InChI=1S/C26H40N6O4/c27-24(28)20-8-6-18(7-9-20)15-31-26(36)23(19-10-12-29-13-11-19)32-25(35)21(30-16-22(33)34)14-17-4-2-1-3-5-17/h6-9,17,19,21,23,29-30H,1-5,10-16H2,(H3,27,28)(H,31,36)(H,32,35)(H,33,34)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447520
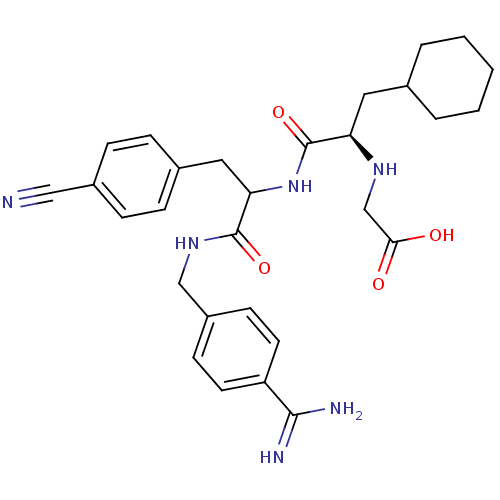
(CHEMBL3115902)Show SMILES NC(=N)c1ccc(CNC(=O)C(Cc2ccc(cc2)C#N)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C29H36N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-15,17-18H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447526
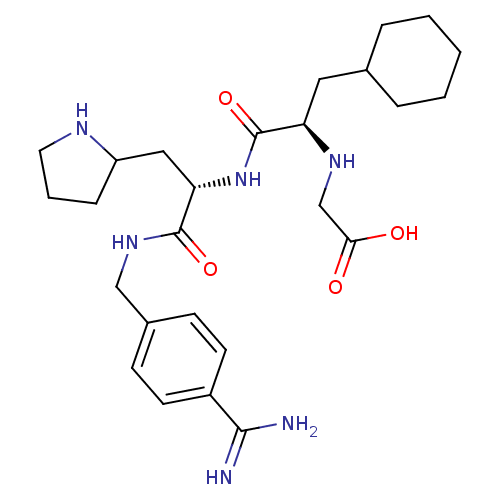
(CHEMBL3115907)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CC2CCCN2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C26H40N6O4/c27-24(28)19-10-8-18(9-11-19)15-31-25(35)22(14-20-7-4-12-29-20)32-26(36)21(30-16-23(33)34)13-17-5-2-1-3-6-17/h8-11,17,20-22,29-30H,1-7,12-16H2,(H3,27,28)(H,31,35)(H,32,36)(H,33,34)/t20?,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447516

(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447514

(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447515

(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193243

(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447514

(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447519

(CHEMBL3115906)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#7]-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C26H42N8O4/c27-23(28)19-10-8-18(9-11-19)16-33-24(37)20(7-4-13-32-26(29)30)34-25(38)21(31-14-12-22(35)36)15-17-5-2-1-3-6-17/h8-11,17,20-21,31H,1-7,12-16H2,(H3,27,28)(H,33,37)(H,34,38)(H,35,36)(H4,29,30,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447518

(CHEMBL3115892)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H](NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C2CCNCC2)cc1 |r| Show InChI InChI=1S/C26H40N6O4/c27-24(28)20-8-6-18(7-9-20)15-31-26(36)23(19-10-12-29-13-11-19)32-25(35)21(30-16-22(33)34)14-17-4-2-1-3-5-17/h6-9,17,19,21,23,29-30H,1-5,10-16H2,(H3,27,28)(H,31,36)(H,32,35)(H,33,34)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447517

(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50193243

(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447517

(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365495
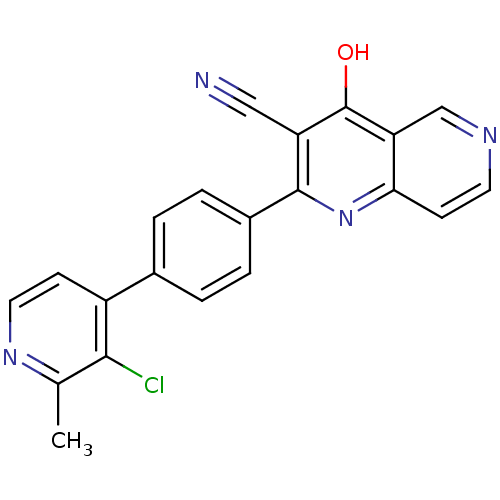
(CHEMBL1957460)Show SMILES Cc1nccc(-c2ccc(cc2)-c2nc3ccncc3c(O)c2C#N)c1Cl Show InChI InChI=1S/C21H13ClN4O/c1-12-19(22)15(6-9-25-12)13-2-4-14(5-3-13)20-16(10-23)21(27)17-11-24-8-7-18(17)26-20/h2-9,11H,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365480
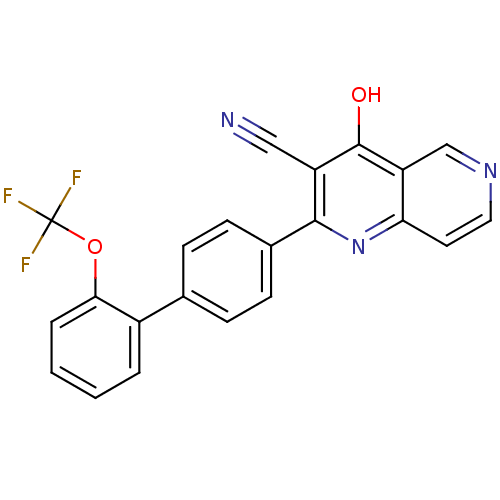
(CHEMBL1957366)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1OC(F)(F)F Show InChI InChI=1S/C22H12F3N3O2/c23-22(24,25)30-19-4-2-1-3-15(19)13-5-7-14(8-6-13)20-16(11-26)21(29)17-12-27-10-9-18(17)28-20/h1-10,12H,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365481

(CHEMBL1957367)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1OCC#N Show InChI InChI=1S/C23H14N4O2/c24-10-12-29-21-4-2-1-3-17(21)15-5-7-16(8-6-15)22-18(13-25)23(28)19-14-26-11-9-20(19)27-22/h1-9,11,14H,12H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365484

(CHEMBL1955881)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C22H12F3N3O/c23-22(24,25)18-4-2-1-3-15(18)13-5-7-14(8-6-13)20-16(11-26)21(29)17-12-27-10-9-19(17)28-20/h1-10,12H,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365487
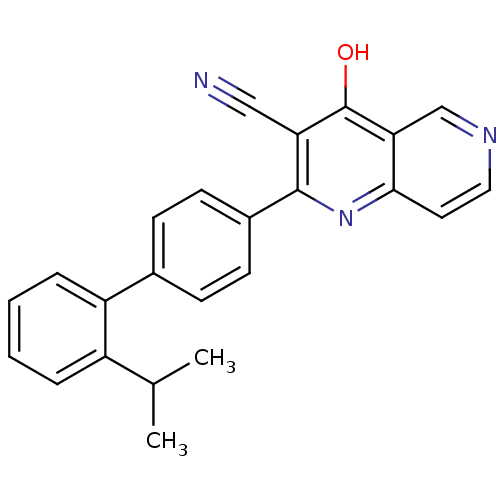
(CHEMBL1957375)Show SMILES CC(C)c1ccccc1-c1ccc(cc1)-c1nc2ccncc2c(O)c1C#N Show InChI InChI=1S/C24H19N3O/c1-15(2)18-5-3-4-6-19(18)16-7-9-17(10-8-16)23-20(13-25)24(28)21-14-26-12-11-22(21)27-23/h3-12,14-15H,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365483

(CHEMBL1957369)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1Cl Show InChI InChI=1S/C21H12ClN3O/c22-18-4-2-1-3-15(18)13-5-7-14(8-6-13)20-16(11-23)21(26)17-12-24-10-9-19(17)25-20/h1-10,12H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50209531
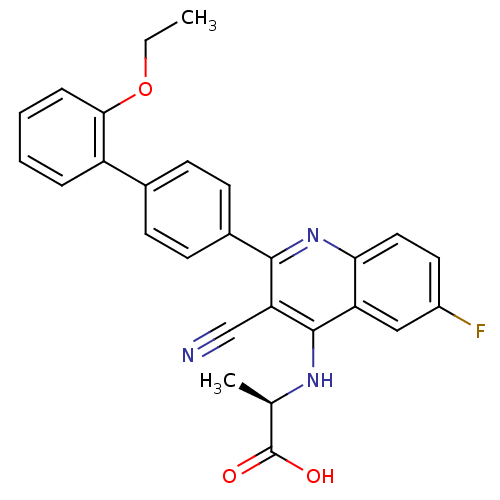
((R)-2-[3-cyano-2-(2'-ethoxy-biphenyl-4-yl)-6-fluor...)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2ccc(F)cc2c(N[C@H](C)C(O)=O)c1C#N Show InChI InChI=1S/C27H22FN3O3/c1-3-34-24-7-5-4-6-20(24)17-8-10-18(11-9-17)25-22(15-29)26(30-16(2)27(32)33)21-14-19(28)12-13-23(21)31-25/h4-14,16H,3H2,1-2H3,(H,30,31)(H,32,33)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365486
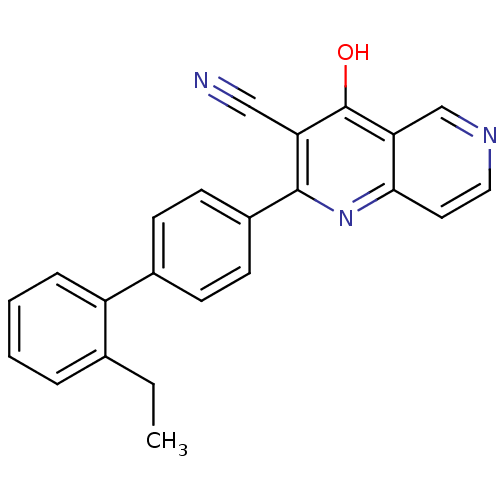
(CHEMBL1957374)Show InChI InChI=1S/C23H17N3O/c1-2-15-5-3-4-6-18(15)16-7-9-17(10-8-16)22-19(13-24)23(27)20-14-25-12-11-21(20)26-22/h3-12,14H,2H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365476
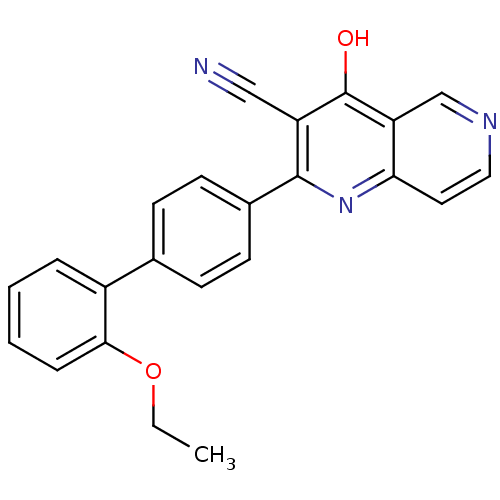
(CHEMBL1957361)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2ccncc2c(O)c1C#N Show InChI InChI=1S/C23H17N3O2/c1-2-28-21-6-4-3-5-17(21)15-7-9-16(10-8-15)22-18(13-24)23(27)19-14-25-12-11-20(19)26-22/h3-12,14H,2H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pyroglutamylated RF-amide peptide receptor
(Homo sapiens (Human)) | BDBM50020015
(CHEMBL3287814)Show InChI InChI=1S/C20H23ClN2OS/c1-24-19-11-10-17(12-15(19)13-25-18-4-2-3-5-18)23-20(22)14-6-8-16(21)9-7-14/h6-12,18H,2-5,13H2,1H3,(H2,22,23) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR103 receptor assessed as inhibition of inositol-1-phosphate production by cell-based assay |
ACS Med Chem Lett 5: 527-32 (2014)
Article DOI: 10.1021/ml400519h
BindingDB Entry DOI: 10.7270/Q2V989NR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365500
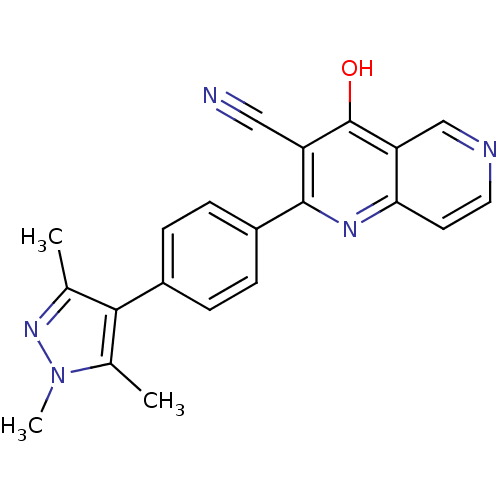
(CHEMBL1957462)Show SMILES Cc1nn(C)c(C)c1-c1ccc(cc1)-c1nc2ccncc2c(O)c1C#N Show InChI InChI=1S/C21H17N5O/c1-12-19(13(2)26(3)25-12)14-4-6-15(7-5-14)20-16(10-22)21(27)17-11-23-9-8-18(17)24-20/h4-9,11H,1-3H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365479
(CHEMBL1957364)Show InChI InChI=1S/C22H15N3O2/c1-27-20-5-3-2-4-16(20)14-6-8-15(9-7-14)21-17(12-23)22(26)18-13-24-11-10-19(18)25-21/h2-11,13H,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Pyroglutamylated RF-amide peptide receptor
(Homo sapiens (Human)) | BDBM50020011
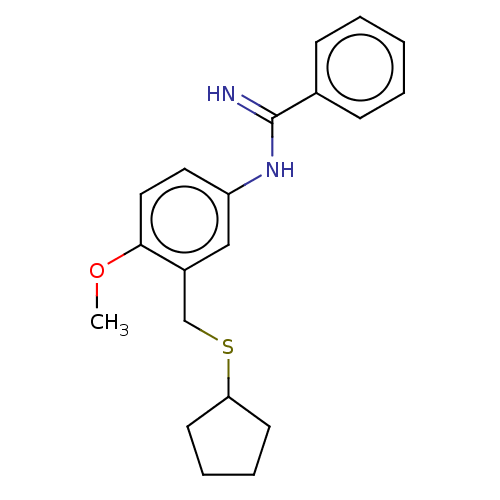
(CHEMBL3287810)Show InChI InChI=1S/C20H24N2OS/c1-23-19-12-11-17(22-20(21)15-7-3-2-4-8-15)13-16(19)14-24-18-9-5-6-10-18/h2-4,7-8,11-13,18H,5-6,9-10,14H2,1H3,(H2,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR103 receptor assessed as inhibition of inositol-1-phosphate production by cell-based assay |
ACS Med Chem Lett 5: 527-32 (2014)
Article DOI: 10.1021/ml400519h
BindingDB Entry DOI: 10.7270/Q2V989NR |
More data for this
Ligand-Target Pair | |
Pyroglutamylated RF-amide peptide receptor
(Homo sapiens (Human)) | BDBM50020014
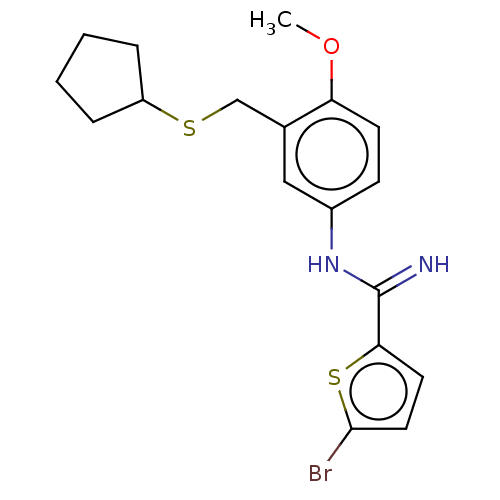
(CHEMBL3287813)Show InChI InChI=1S/C18H21BrN2OS2/c1-22-15-7-6-13(21-18(20)16-8-9-17(19)24-16)10-12(15)11-23-14-4-2-3-5-14/h6-10,14H,2-5,11H2,1H3,(H2,20,21) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR103 receptor assessed as inhibition of inositol-1-phosphate production by cell-based assay |
ACS Med Chem Lett 5: 527-32 (2014)
Article DOI: 10.1021/ml400519h
BindingDB Entry DOI: 10.7270/Q2V989NR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365485

(CHEMBL1957373)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1C#N Show InChI InChI=1S/C22H12N4O/c23-11-16-3-1-2-4-17(16)14-5-7-15(8-6-14)21-18(12-24)22(27)19-13-25-10-9-20(19)26-21/h1-10,13H,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365482
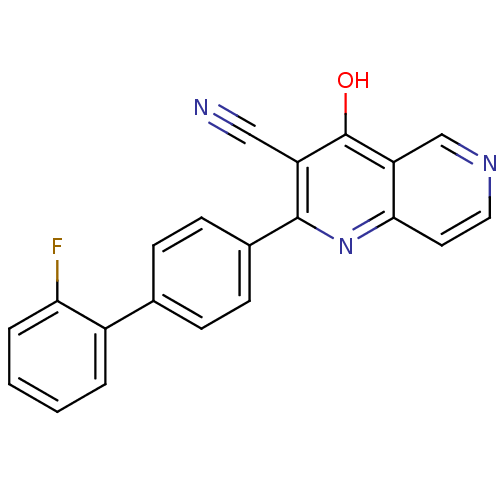
(CHEMBL1957368)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1F Show InChI InChI=1S/C21H12FN3O/c22-18-4-2-1-3-15(18)13-5-7-14(8-6-13)20-16(11-23)21(26)17-12-24-10-9-19(17)25-20/h1-10,12H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Pyroglutamylated RF-amide peptide receptor
(Homo sapiens (Human)) | BDBM50020011

(CHEMBL3287810)Show InChI InChI=1S/C20H24N2OS/c1-23-19-12-11-17(22-20(21)15-7-3-2-4-8-15)13-16(19)14-24-18-9-5-6-10-18/h2-4,7-8,11-13,18H,5-6,9-10,14H2,1H3,(H2,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-QRFP43 from human GPR103 receptor overexpressed in HEK membranes after 90 mins by liquid scintillation counting |
ACS Med Chem Lett 5: 527-32 (2014)
Article DOI: 10.1021/ml400519h
BindingDB Entry DOI: 10.7270/Q2V989NR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365494
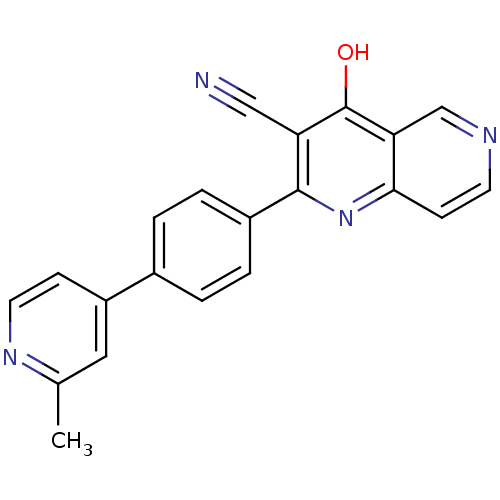
(CHEMBL1957381)Show SMILES Cc1cc(ccn1)-c1ccc(cc1)-c1nc2ccncc2c(O)c1C#N Show InChI InChI=1S/C21H14N4O/c1-13-10-16(6-9-24-13)14-2-4-15(5-3-14)20-17(11-22)21(26)18-12-23-8-7-19(18)25-20/h2-10,12H,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365496

(CHEMBL1957370)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccncc1Cl Show InChI InChI=1S/C20H11ClN4O/c21-17-11-24-7-5-14(17)12-1-3-13(4-2-12)19-15(9-22)20(26)16-10-23-8-6-18(16)25-19/h1-8,10-11H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM31589
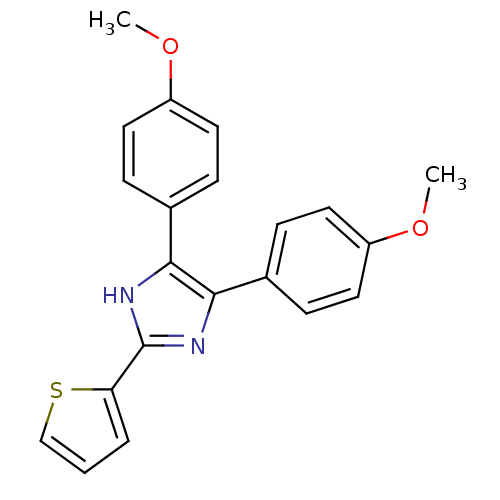
(triaryl imidazole, 1)Show SMILES COc1ccc(cc1)-c1nc([nH]c1-c1ccc(OC)cc1)-c1cccs1 Show InChI InChI=1S/C21H18N2O2S/c1-24-16-9-5-14(6-10-16)19-20(15-7-11-17(25-2)12-8-15)23-21(22-19)18-4-3-13-26-18/h3-13H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM14754

(1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-isoq...)Show InChI InChI=1S/C20H21NO4/c1-22-17-6-5-13(10-18(17)23-2)9-16-15-12-20(25-4)19(24-3)11-14(15)7-8-21-16/h5-8,10-12H,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pyroglutamylated RF-amide peptide receptor
(Homo sapiens (Human)) | BDBM50020015

(CHEMBL3287814)Show InChI InChI=1S/C20H23ClN2OS/c1-24-19-11-10-17(12-15(19)13-25-18-4-2-3-5-18)23-20(22)14-6-8-16(21)9-7-14/h6-12,18H,2-5,13H2,1H3,(H2,22,23) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-QRFP43 from human GPR103 receptor overexpressed in HEK membranes after 90 mins by liquid scintillation counting |
ACS Med Chem Lett 5: 527-32 (2014)
Article DOI: 10.1021/ml400519h
BindingDB Entry DOI: 10.7270/Q2V989NR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data