 Found 3545 hits with Last Name = 'hara' and Initial = 'a'
Found 3545 hits with Last Name = 'hara' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50295693
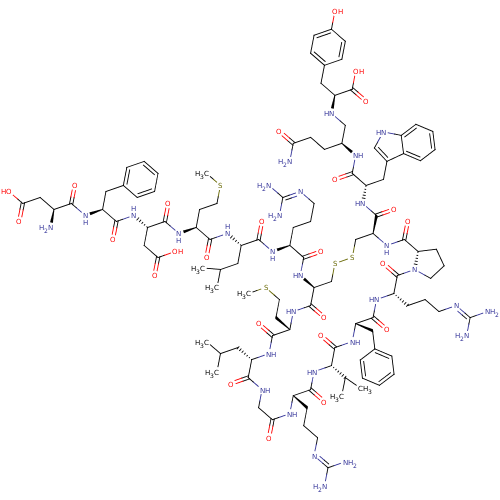
(CHEMBL557629)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)CN[C@@H](Cc1ccc(O)cc1)C(O)=O |r,wU:27.28,36.36,71.73,148.153,55.55,60.134,8.12,wD:112.115,4.4,97.100,93.131,82.84,16.24,134.137,44.44,64.69,156.161,120.123,(5.27,-20.83,;6.6,-20.06,;6.6,-18.52,;5.27,-17.75,;5.27,-16.21,;3.93,-15.44,;2.6,-16.21,;2.6,-17.75,;1.27,-15.44,;1.27,-13.9,;2.6,-13.13,;3.93,-13.9,;2.6,-11.59,;-.07,-16.21,;-1.4,-15.44,;-1.4,-13.9,;-2.73,-16.21,;-2.73,-17.75,;-1.4,-18.52,;-.07,-17.74,;1.27,-18.51,;1.27,-20.05,;-.08,-20.82,;-1.4,-20.05,;-4.07,-15.44,;-5.4,-16.21,;-5.4,-17.75,;-6.74,-15.44,;-8.07,-16.21,;-6.74,-13.9,;-8.07,-13.13,;-8.07,-11.59,;-9.4,-13.9,;6.6,-15.44,;6.6,-13.9,;7.93,-16.21,;9.27,-15.44,;9.27,-13.9,;10.6,-13.13,;10.6,-11.59,;11.94,-13.9,;10.6,-16.21,;10.6,-17.75,;11.94,-15.44,;13.27,-16.21,;13.27,-17.75,;11.94,-18.52,;11.94,-20.06,;10.6,-20.83,;10.6,-22.37,;9.27,-23.14,;11.94,-23.14,;14.6,-15.44,;14.6,-13.9,;15.94,-16.21,;17.27,-15.44,;17.27,-13.9,;15.94,-13.13,;15.71,-11.29,;14.63,-10.2,;15.04,-8.71,;16.59,-8.74,;17.38,-7.41,;18.92,-7.43,;16.62,-6.07,;15.22,-6.04,;14.79,-4.38,;16.14,-3.62,;17.27,-4.66,;18.6,-3.89,;18.6,-2.35,;19.94,-4.66,;19.94,-6.2,;21.27,-6.97,;21.27,-8.51,;19.94,-9.28,;19.94,-10.82,;18.6,-11.59,;21.27,-11.59,;21.27,-3.89,;22.61,-4.66,;22.61,-6.2,;23.94,-3.89,;23.94,-2.35,;22.61,-1.58,;22.61,-.04,;21.28,.73,;19.95,-.04,;19.95,-1.59,;21.27,-2.35,;25.27,-4.66,;26.61,-3.89,;26.61,-2.35,;27.94,-4.66,;27.94,-6.2,;26.61,-6.97,;25.27,-6.2,;26.61,-8.51,;27.94,-9.28,;27.94,-10.82,;29.27,-11.59,;29.27,-13.13,;30.61,-13.9,;31.94,-13.13,;30.61,-15.44,;25.27,-9.28,;25.27,-10.82,;23.94,-11.59,;26.61,-11.59,;26.61,-13.13,;25.27,-13.9,;23.94,-13.13,;25.27,-15.44,;26.61,-16.21,;26.61,-17.75,;27.94,-18.52,;25.27,-18.52,;23.94,-16.21,;22.61,-15.44,;22.61,-13.9,;21.27,-16.21,;21.27,-17.75,;19.94,-18.52,;19.94,-20.06,;18.6,-20.83,;19.94,-15.44,;18.6,-16.21,;18.6,-17.75,;29.27,-3.89,;30.61,-4.66,;29.27,-2.35,;13.71,-7.94,;13.71,-6.4,;12.38,-8.71,;11.04,-7.94,;11.05,-6.4,;9.71,-5.63,;8.29,-6.29,;7.28,-5.11,;8.05,-3.78,;7.58,-2.33,;8.6,-1.2,;10.11,-1.52,;10.57,-2.98,;9.54,-4.11,;9.71,-8.71,;9.71,-10.25,;8.38,-7.93,;7.04,-8.7,;7.04,-10.24,;5.71,-11.01,;5.7,-12.55,;7.19,-12.94,;5.3,-14.03,;5.71,-7.93,;4.38,-8.7,;3.04,-7.93,;3.04,-6.39,;1.71,-5.62,;1.72,-4.07,;.39,-3.3,;-.95,-4.07,;-2.28,-3.3,;-.94,-5.62,;.39,-6.38,;1.71,-8.7,;.38,-7.92,;1.71,-10.24,)| Show InChI InChI=1S/C109H162N30O25S4/c1-58(2)45-75-91(148)123-55-86(142)125-70(27-17-39-118-107(112)113)95(152)138-89(60(5)6)104(161)135-78(48-62-23-13-10-14-24-62)98(155)129-74(29-19-41-120-109(116)117)105(162)139-42-20-30-84(139)103(160)137-83(102(159)133-79(50-64-53-121-69-26-16-15-25-67(64)69)96(153)124-65(33-36-85(111)141)54-122-81(106(163)164)49-63-31-34-66(140)35-32-63)57-168-167-56-82(101(158)128-73(38-44-166-8)93(150)131-75)136-92(149)71(28-18-40-119-108(114)115)126-97(154)76(46-59(3)4)132-94(151)72(37-43-165-7)127-100(157)80(52-88(145)146)134-99(156)77(47-61-21-11-9-12-22-61)130-90(147)68(110)51-87(143)144/h9-16,21-26,31-32,34-35,53,58-60,65,68,70-84,89,121-122,140H,17-20,27-30,33,36-52,54-57,110H2,1-8H3,(H2,111,141)(H,123,148)(H,124,153)(H,125,142)(H,126,154)(H,127,157)(H,128,158)(H,129,155)(H,130,147)(H,131,150)(H,132,151)(H,133,159)(H,134,156)(H,135,161)(H,136,149)(H,137,160)(H,138,152)(H,143,144)(H,145,146)(H,163,164)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120)/t65-,68-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,89-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Mus musculus) | BDBM50295693

(CHEMBL557629)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC1=O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)CN[C@@H](Cc1ccc(O)cc1)C(O)=O |r,wU:27.28,36.36,71.73,148.153,55.55,60.134,8.12,wD:112.115,4.4,97.100,93.131,82.84,16.24,134.137,44.44,64.69,156.161,120.123,(5.27,-20.83,;6.6,-20.06,;6.6,-18.52,;5.27,-17.75,;5.27,-16.21,;3.93,-15.44,;2.6,-16.21,;2.6,-17.75,;1.27,-15.44,;1.27,-13.9,;2.6,-13.13,;3.93,-13.9,;2.6,-11.59,;-.07,-16.21,;-1.4,-15.44,;-1.4,-13.9,;-2.73,-16.21,;-2.73,-17.75,;-1.4,-18.52,;-.07,-17.74,;1.27,-18.51,;1.27,-20.05,;-.08,-20.82,;-1.4,-20.05,;-4.07,-15.44,;-5.4,-16.21,;-5.4,-17.75,;-6.74,-15.44,;-8.07,-16.21,;-6.74,-13.9,;-8.07,-13.13,;-8.07,-11.59,;-9.4,-13.9,;6.6,-15.44,;6.6,-13.9,;7.93,-16.21,;9.27,-15.44,;9.27,-13.9,;10.6,-13.13,;10.6,-11.59,;11.94,-13.9,;10.6,-16.21,;10.6,-17.75,;11.94,-15.44,;13.27,-16.21,;13.27,-17.75,;11.94,-18.52,;11.94,-20.06,;10.6,-20.83,;10.6,-22.37,;9.27,-23.14,;11.94,-23.14,;14.6,-15.44,;14.6,-13.9,;15.94,-16.21,;17.27,-15.44,;17.27,-13.9,;15.94,-13.13,;15.71,-11.29,;14.63,-10.2,;15.04,-8.71,;16.59,-8.74,;17.38,-7.41,;18.92,-7.43,;16.62,-6.07,;15.22,-6.04,;14.79,-4.38,;16.14,-3.62,;17.27,-4.66,;18.6,-3.89,;18.6,-2.35,;19.94,-4.66,;19.94,-6.2,;21.27,-6.97,;21.27,-8.51,;19.94,-9.28,;19.94,-10.82,;18.6,-11.59,;21.27,-11.59,;21.27,-3.89,;22.61,-4.66,;22.61,-6.2,;23.94,-3.89,;23.94,-2.35,;22.61,-1.58,;22.61,-.04,;21.28,.73,;19.95,-.04,;19.95,-1.59,;21.27,-2.35,;25.27,-4.66,;26.61,-3.89,;26.61,-2.35,;27.94,-4.66,;27.94,-6.2,;26.61,-6.97,;25.27,-6.2,;26.61,-8.51,;27.94,-9.28,;27.94,-10.82,;29.27,-11.59,;29.27,-13.13,;30.61,-13.9,;31.94,-13.13,;30.61,-15.44,;25.27,-9.28,;25.27,-10.82,;23.94,-11.59,;26.61,-11.59,;26.61,-13.13,;25.27,-13.9,;23.94,-13.13,;25.27,-15.44,;26.61,-16.21,;26.61,-17.75,;27.94,-18.52,;25.27,-18.52,;23.94,-16.21,;22.61,-15.44,;22.61,-13.9,;21.27,-16.21,;21.27,-17.75,;19.94,-18.52,;19.94,-20.06,;18.6,-20.83,;19.94,-15.44,;18.6,-16.21,;18.6,-17.75,;29.27,-3.89,;30.61,-4.66,;29.27,-2.35,;13.71,-7.94,;13.71,-6.4,;12.38,-8.71,;11.04,-7.94,;11.05,-6.4,;9.71,-5.63,;8.29,-6.29,;7.28,-5.11,;8.05,-3.78,;7.58,-2.33,;8.6,-1.2,;10.11,-1.52,;10.57,-2.98,;9.54,-4.11,;9.71,-8.71,;9.71,-10.25,;8.38,-7.93,;7.04,-8.7,;7.04,-10.24,;5.71,-11.01,;5.7,-12.55,;7.19,-12.94,;5.3,-14.03,;5.71,-7.93,;4.38,-8.7,;3.04,-7.93,;3.04,-6.39,;1.71,-5.62,;1.72,-4.07,;.39,-3.3,;-.95,-4.07,;-2.28,-3.3,;-.94,-5.62,;.39,-6.38,;1.71,-8.7,;.38,-7.92,;1.71,-10.24,)| Show InChI InChI=1S/C109H162N30O25S4/c1-58(2)45-75-91(148)123-55-86(142)125-70(27-17-39-118-107(112)113)95(152)138-89(60(5)6)104(161)135-78(48-62-23-13-10-14-24-62)98(155)129-74(29-19-41-120-109(116)117)105(162)139-42-20-30-84(139)103(160)137-83(102(159)133-79(50-64-53-121-69-26-16-15-25-67(64)69)96(153)124-65(33-36-85(111)141)54-122-81(106(163)164)49-63-31-34-66(140)35-32-63)57-168-167-56-82(101(158)128-73(38-44-166-8)93(150)131-75)136-92(149)71(28-18-40-119-108(114)115)126-97(154)76(46-59(3)4)132-94(151)72(37-43-165-7)127-100(157)80(52-88(145)146)134-99(156)77(47-61-21-11-9-12-22-61)130-90(147)68(110)51-87(143)144/h9-16,21-26,31-32,34-35,53,58-60,65,68,70-84,89,121-122,140H,17-20,27-30,33,36-52,54-57,110H2,1-8H3,(H2,111,141)(H,123,148)(H,124,153)(H,125,142)(H,126,154)(H,127,157)(H,128,158)(H,129,155)(H,130,147)(H,131,150)(H,132,151)(H,133,159)(H,134,156)(H,135,161)(H,136,149)(H,137,160)(H,138,152)(H,143,144)(H,145,146)(H,163,164)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120)/t65-,68-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,89-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50178975
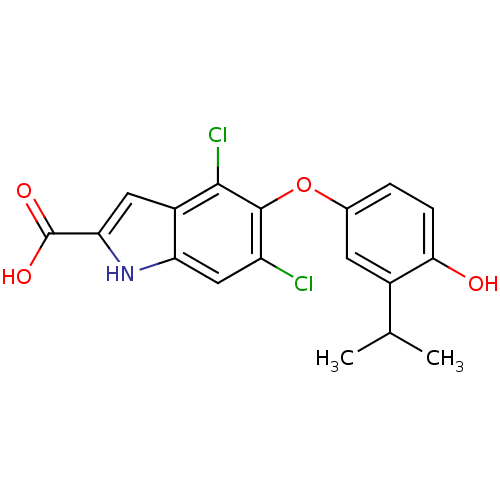
(4,6-dichloro-5-(4-hydroxy-3-isopropylphenoxy)-1H-i...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc3[nH]c(cc3c2Cl)C(O)=O)ccc1O Show InChI InChI=1S/C18H15Cl2NO4/c1-8(2)10-5-9(3-4-15(10)22)25-17-12(19)7-13-11(16(17)20)6-14(21-13)18(23)24/h3-8,21-22H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to TRbeta1 |
J Med Chem 55: 5649-75 (2012)
Article DOI: 10.1021/jm2004706
BindingDB Entry DOI: 10.7270/Q2DZ09FJ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108501
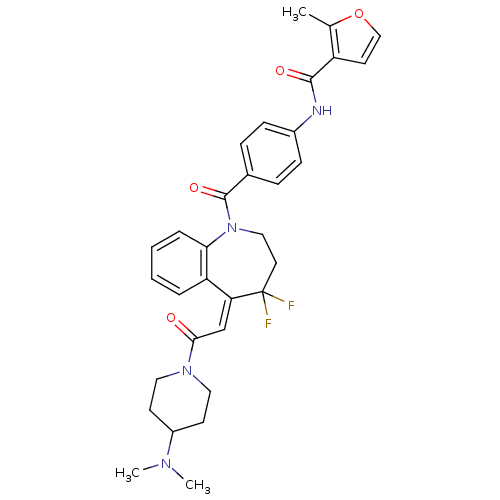
(2-Methyl-furan-3-carboxylic acid (4-{5-[2-(4-dimet...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1/c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccoc2C)cc1 Show InChI InChI=1S/C32H34F2N4O4/c1-21-25(14-19-42-21)30(40)35-23-10-8-22(9-11-23)31(41)38-18-15-32(33,34)27(26-6-4-5-7-28(26)38)20-29(39)37-16-12-24(13-17-37)36(2)3/h4-11,14,19-20,24H,12-13,15-18H2,1-3H3,(H,35,40)/b27-20+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108498
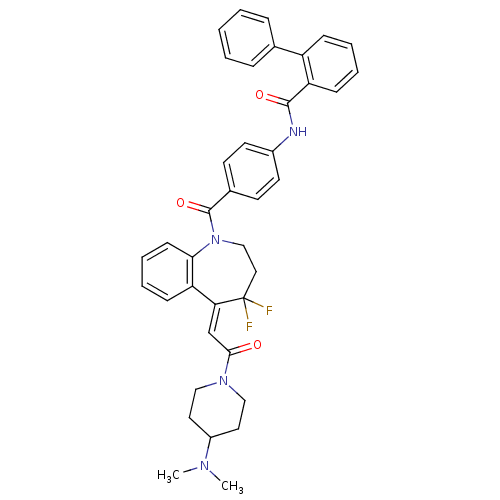
(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at Vasopressin V1a receptor, performed using [3H]-AVP on rat liver |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50295690
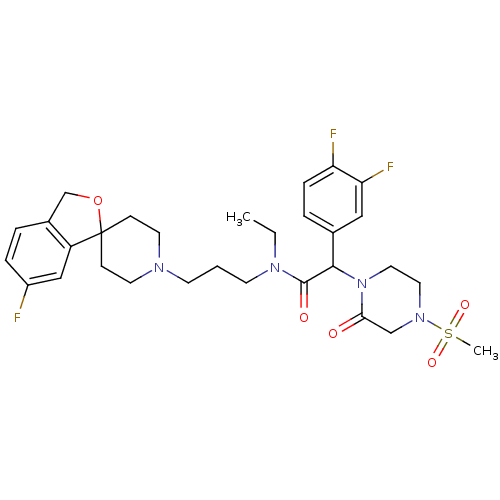
((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...)Show SMILES CCN(CCCN1CCC2(CC1)OCc1ccc(F)cc21)C(=O)C(N1CCN(CC1=O)S(C)(=O)=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C30H37F3N4O5S/c1-3-35(12-4-11-34-13-9-30(10-14-34)24-18-23(31)7-5-22(24)20-42-30)29(39)28(21-6-8-25(32)26(33)17-21)37-16-15-36(19-27(37)38)43(2,40)41/h5-8,17-18,28H,3-4,9-16,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human MCH1R expressed in CHO cells by scintillation counting per mg of protein |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85790
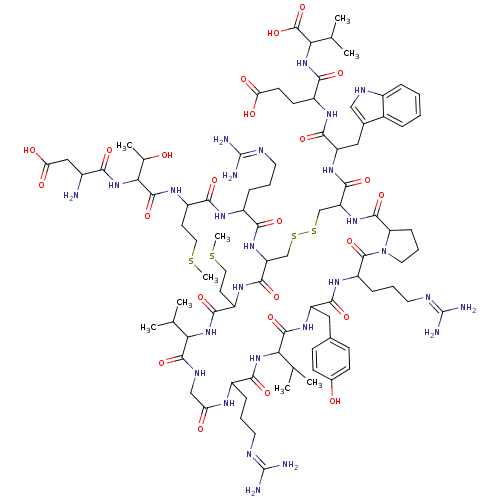
(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM35667
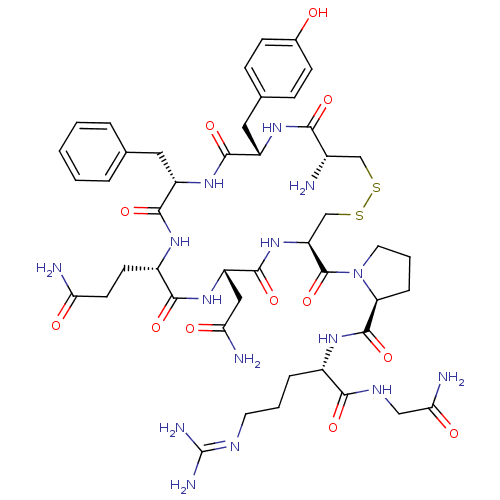
(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095
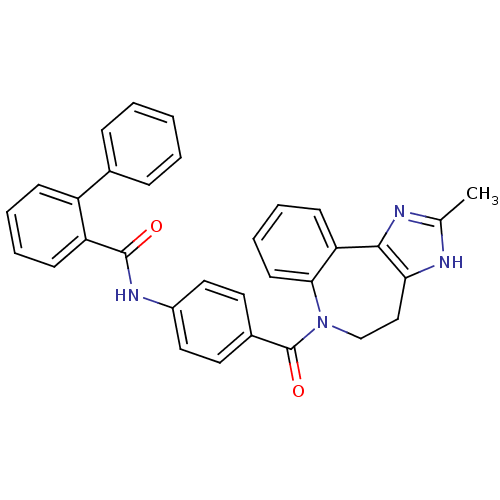
(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85789
([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85096
(CAS_105077 | NSC_105077 | d(CH2)5Tyr(Me)AVP)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H74N14O12S2/c1-78-32-16-14-31(15-17-32)25-35-46(73)63-36(24-30-10-4-2-5-11-30)47(74)61-34(18-19-40(53)67)45(72)64-37(26-41(54)68)48(75)65-38(29-79-80-52(27-43(70)60-35)20-6-3-7-21-52)50(77)66-23-9-13-39(66)49(76)62-33(12-8-22-58-51(56)57)44(71)59-28-42(55)69/h2,4-5,10-11,14-17,33-39H,3,6-9,12-13,18-29H2,1H3,(H2,53,67)(H2,54,68)(H2,55,69)(H,59,71)(H,60,70)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,56,57,58) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18869
(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to TRbeta1 |
J Med Chem 55: 5649-75 (2012)
Article DOI: 10.1021/jm2004706
BindingDB Entry DOI: 10.7270/Q2DZ09FJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50295690

((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...)Show SMILES CCN(CCCN1CCC2(CC1)OCc1ccc(F)cc21)C(=O)C(N1CCN(CC1=O)S(C)(=O)=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C30H37F3N4O5S/c1-3-35(12-4-11-34-13-9-30(10-14-34)24-18-23(31)7-5-22(24)20-42-30)29(39)28(21-6-8-25(32)26(33)17-21)37-16-15-36(19-27(37)38)43(2,40)41/h5-8,17-18,28H,3-4,9-16,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Mus musculus) | BDBM50295690

((+/-)-2-(3,4-difluorophenyl)-N-ethyl-N-(3-(6-fluor...)Show SMILES CCN(CCCN1CCC2(CC1)OCc1ccc(F)cc21)C(=O)C(N1CCN(CC1=O)S(C)(=O)=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C30H37F3N4O5S/c1-3-35(12-4-11-34-13-9-30(10-14-34)24-18-23(31)7-5-22(24)20-42-30)29(39)28(21-6-8-25(32)26(33)17-21)37-16-15-36(19-27(37)38)43(2,40)41/h5-8,17-18,28H,3-4,9-16,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... |
Bioorg Med Chem Lett 19: 2835-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.102
BindingDB Entry DOI: 10.7270/Q2R49QS7 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114031
((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at Vasopressin V2 receptor, performed using [3H]-AVP on rat kidney |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50249779
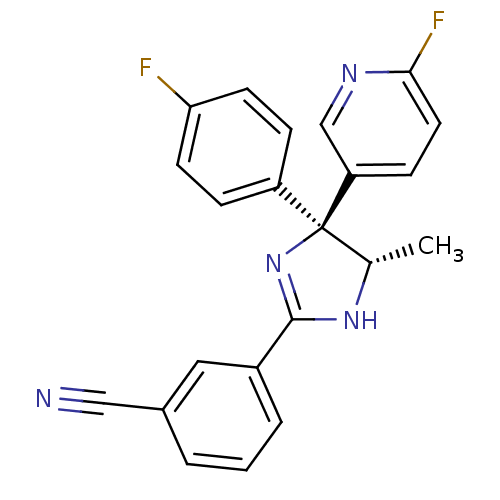
(3-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...)Show SMILES C[C@@H]1NC(=N[C@@]1(c1ccc(F)cc1)c1ccc(F)nc1)c1cccc(c1)C#N |r,c:3| Show InChI InChI=1S/C22H16F2N4/c1-14-22(17-5-8-19(23)9-6-17,18-7-10-20(24)26-13-18)28-21(27-14)16-4-2-3-15(11-16)12-25/h2-11,13-14H,1H3,(H,27,28)/t14-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells |
J Med Chem 52: 3385-96 (2009)
Article DOI: 10.1021/jm900110t
BindingDB Entry DOI: 10.7270/Q2DN450G |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85789

([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427
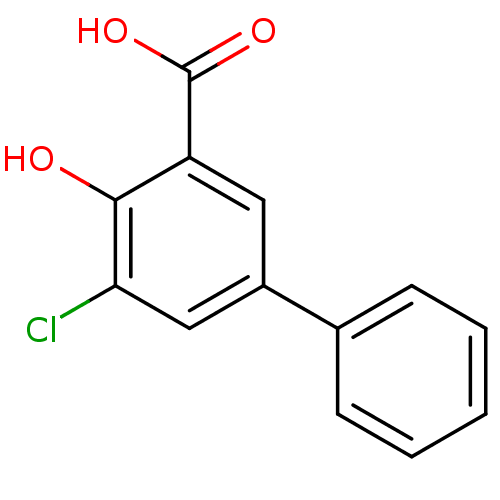
(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C1 Phe311Leu mutant by fluorescence assay |
Bioorg Med Chem Lett 21: 2564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.076
BindingDB Entry DOI: 10.7270/Q20G3NZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427

(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxytocin receptor
(RAT) | BDBM50013775

((oxytocin-OT) cyclo[Cys-Tyr-Ile-Gln-Asn-Cys]-Pro-L...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](N)CSSCC(NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22?,25-,26+,27+,28+,29+,30?,31?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
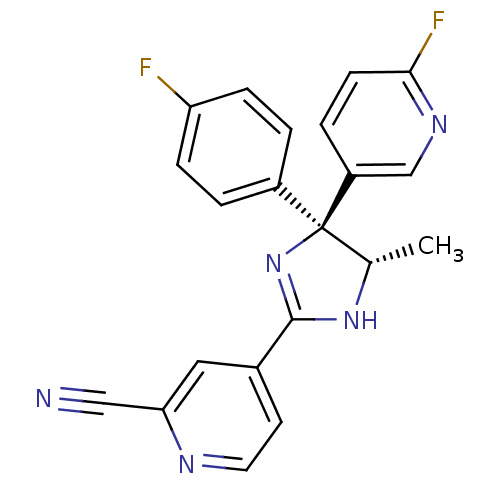
(Homo sapiens (Human)) | BDBM50249806
(4-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...)Show SMILES C[C@@H]1NC(=N[C@@]1(c1ccc(F)cc1)c1ccc(F)nc1)c1ccnc(c1)C#N |r,c:3| Show InChI InChI=1S/C21H15F2N5/c1-13-21(15-2-5-17(22)6-3-15,16-4-7-19(23)26-12-16)28-20(27-13)14-8-9-25-18(10-14)11-24/h2-10,12-13H,1H3,(H,27,28)/t13-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells |
J Med Chem 52: 3385-96 (2009)
Article DOI: 10.1021/jm900110t
BindingDB Entry DOI: 10.7270/Q2DN450G |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
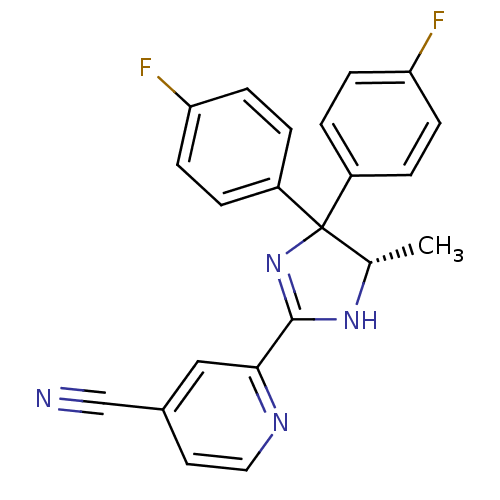
(Homo sapiens (Human)) | BDBM50249739
(2-[(5S)-4,4-Bis(4-fluorophenyl)-5-methyl-4,5-dihyd...)Show SMILES C[C@@H]1NC(=NC1(c1ccc(F)cc1)c1ccc(F)cc1)c1cc(ccn1)C#N |r,c:3| Show InChI InChI=1S/C22H16F2N4/c1-14-22(16-2-6-18(23)7-3-16,17-4-8-19(24)9-5-17)28-21(27-14)20-12-15(13-25)10-11-26-20/h2-12,14H,1H3,(H,27,28)/t14-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells |
J Med Chem 52: 3385-96 (2009)
Article DOI: 10.1021/jm900110t
BindingDB Entry DOI: 10.7270/Q2DN450G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50187381
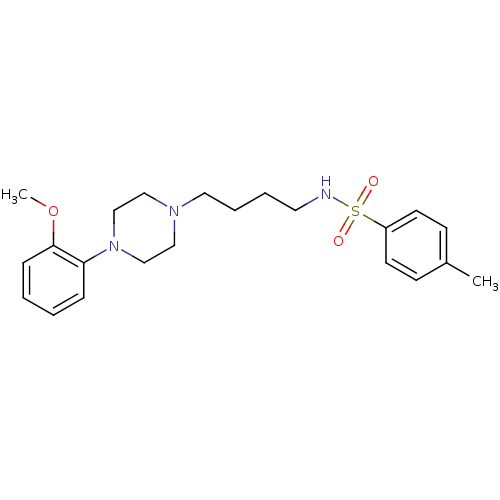
(CHEMBL209324 | N-(4-(4-(2-methoxyphenyl)piperazin-...)Show SMILES COc1ccccc1N1CCN(CCCCNS(=O)(=O)c2ccc(C)cc2)CC1 Show InChI InChI=1S/C22H31N3O3S/c1-19-9-11-20(12-10-19)29(26,27)23-13-5-6-14-24-15-17-25(18-16-24)21-7-3-4-8-22(21)28-2/h3-4,7-12,23H,5-6,13-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Predix Pharmaceuticals Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cells |
J Med Chem 49: 3116-35 (2006)
Article DOI: 10.1021/jm0508641
BindingDB Entry DOI: 10.7270/Q2319VHC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114033
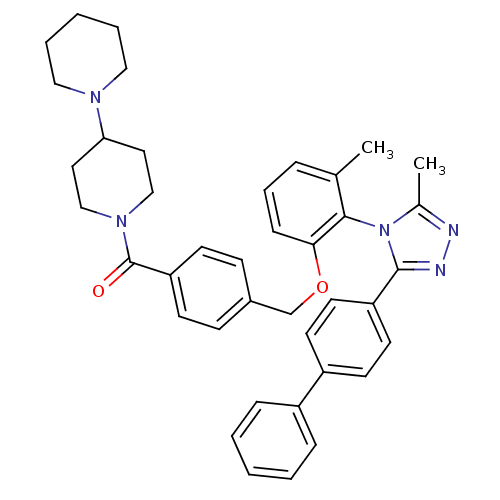
(CHEMBL86667 | {4-[2-(3-Biphenyl-4-yl-5-methyl-[1,2...)Show SMILES Cc1nnc(-c2ccc(cc2)-c2ccccc2)n1-c1c(C)cccc1OCc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCCC1 |(15.01,-.52,;13.48,-.29,;12.92,1.14,;11.39,1.05,;10.99,-.44,;9.57,-1,;9.33,-2.53,;7.9,-3.09,;6.69,-2.13,;6.92,-.61,;8.35,-.03,;5.26,-2.69,;4.05,-1.71,;2.62,-2.27,;2.38,-3.81,;3.58,-4.77,;5.02,-4.21,;12.29,-1.27,;12.45,-2.79,;11.2,-3.7,;9.8,-3.07,;11.36,-5.22,;12.77,-5.85,;14.02,-4.94,;13.86,-3.42,;15.1,-2.51,;16.53,-3.05,;16.79,-4.56,;18.23,-5.09,;18.49,-6.6,;17.32,-7.6,;15.87,-7.06,;15.59,-5.55,;17.57,-9.12,;19.02,-9.65,;16.39,-10.1,;17.53,-11.13,;17.18,-12.65,;15.71,-13.11,;14.59,-12.07,;14.92,-10.56,;15.48,-14.61,;16.67,-15.57,;16.44,-17.09,;15.01,-17.63,;13.82,-16.67,;14.05,-15.17,)| Show InChI InChI=1S/C40H43N5O2/c1-29-10-9-13-37(38(29)45-30(2)41-42-39(45)34-20-18-33(19-21-34)32-11-5-3-6-12-32)47-28-31-14-16-35(17-15-31)40(46)44-26-22-36(23-27-44)43-24-7-4-8-25-43/h3,5-6,9-21,36H,4,7-8,22-28H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114028

(1-{6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES CN1CCN(CCCCCCOc2ccccc2-n2c(C)nnc2-c2ccc(cc2)-c2ccccc2)CC1 |(27.25,-2.68,;25.78,-2.25,;24.66,-3.31,;23.19,-2.88,;22.83,-1.38,;21.58,-2.29,;20.18,-1.64,;18.92,-2.53,;17.52,-1.89,;16.26,-2.77,;14.86,-2.13,;13.62,-3.02,;13.76,-4.55,;15.14,-5.18,;15.28,-6.72,;14.02,-7.59,;12.64,-6.96,;12.51,-5.42,;11.12,-4.78,;10.9,-3.25,;11.88,-2.08,;9.38,-2.99,;8.66,-4.36,;9.75,-5.46,;9.49,-6.98,;8.05,-7.52,;7.79,-9.03,;8.98,-10,;10.41,-9.48,;10.68,-7.96,;8.71,-11.52,;7.28,-12.06,;7,-13.57,;8.19,-14.56,;9.64,-14.02,;9.91,-12.51,;23.93,-.31,;25.4,-.75,)| Show InChI InChI=1S/C32H39N5O/c1-26-33-34-32(29-18-16-28(17-19-29)27-12-6-5-7-13-27)37(26)30-14-8-9-15-31(30)38-25-11-4-3-10-20-36-23-21-35(2)22-24-36/h5-9,12-19H,3-4,10-11,20-25H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114034
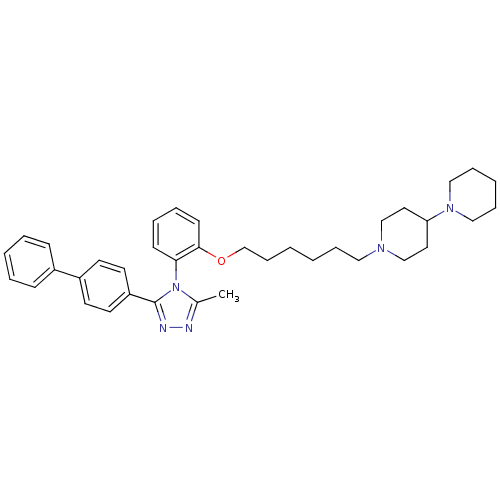
(1'-{6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-...)Show SMILES Cc1nnc(-c2ccc(cc2)-c2ccccc2)n1-c1ccccc1OCCCCCCN1CCC(CC1)N1CCCCC1 |(6.93,-1.75,;5.55,-2.43,;4.25,-1.61,;3.06,-2.57,;3.62,-4.01,;2.78,-5.3,;1.24,-5.23,;.4,-6.52,;1.1,-7.87,;2.64,-7.96,;3.48,-6.68,;.26,-9.17,;.96,-10.54,;.12,-11.84,;-1.42,-11.74,;-2.11,-10.37,;-1.28,-9.08,;5.16,-3.93,;6.17,-5.07,;5.68,-6.54,;6.69,-7.68,;8.2,-7.38,;8.69,-5.91,;7.66,-4.77,;8.15,-3.3,;9.52,-2.62,;10.8,-3.48,;12.18,-2.78,;13.46,-3.65,;14.84,-2.95,;16.13,-3.81,;17.5,-3.13,;19.04,-3.27,;19.93,-2.04,;19.3,-.63,;17.76,-.47,;16.87,-1.73,;20.09,.67,;19.39,2.01,;20.21,3.3,;21.73,3.24,;22.45,1.91,;21.63,.61,)| Show InChI InChI=1S/C37H47N5O/c1-30-38-39-37(33-20-18-32(19-21-33)31-14-6-4-7-15-31)42(30)35-16-8-9-17-36(35)43-29-13-3-2-10-24-40-27-22-34(23-28-40)41-25-11-5-12-26-41/h4,6-9,14-21,34H,2-3,5,10-13,22-29H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50151448
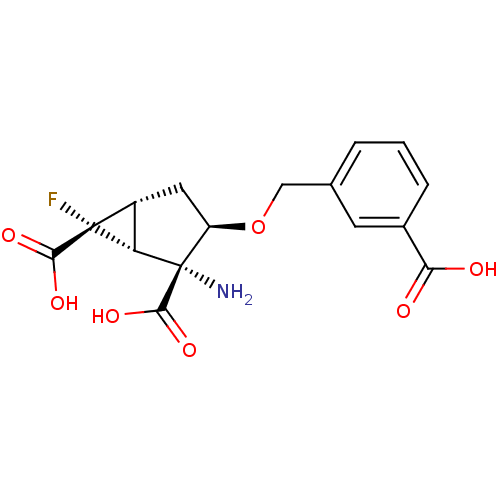
((1R,2R,3R,5R,6R)-2-Amino-3-(3-carboxy-benzyloxy)-6...)Show SMILES N[C@@]1([C@H]2[C@@H](C[C@H]1OCc1cccc(c1)C(O)=O)[C@]2(F)C(O)=O)C(O)=O Show InChI InChI=1S/C16H16FNO7/c17-15(13(21)22)9-5-10(16(18,11(9)15)14(23)24)25-6-7-2-1-3-8(4-7)12(19)20/h1-4,9-11H,5-6,18H2,(H,19,20)(H,21,22)(H,23,24)/t9-,10-,11+,15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards metabotropic glutamate receptor 2 of rat expressed in CHO cells was determined by using [3H]-MGS0008 |
J Med Chem 47: 4570-87 (2004)
Article DOI: 10.1021/jm0400294
BindingDB Entry DOI: 10.7270/Q2GH9HFK |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50249777
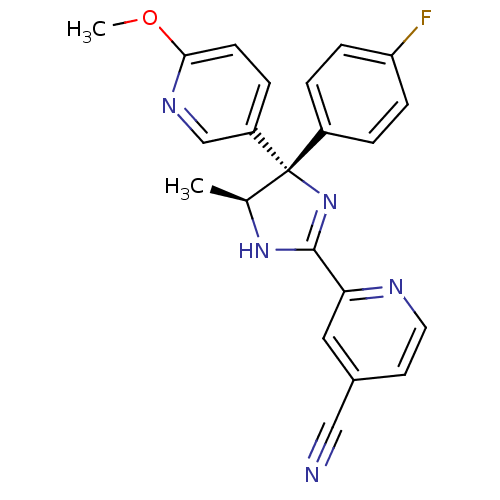
(2-{(4S,5S)-4-(4-Fluorophenyl)-5-methyl-4-[6-(methy...)Show SMILES COc1ccc(cn1)[C@@]1(N=C(N[C@H]1C)c1cc(ccn1)C#N)c1ccc(F)cc1 |r,c:10| Show InChI InChI=1S/C22H18FN5O/c1-14-22(16-3-6-18(23)7-4-16,17-5-8-20(29-2)26-13-17)28-21(27-14)19-11-15(12-24)9-10-25-19/h3-11,13-14H,1-2H3,(H,27,28)/t14-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells |
J Med Chem 52: 3385-96 (2009)
Article DOI: 10.1021/jm900110t
BindingDB Entry DOI: 10.7270/Q2DN450G |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V2 receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427

(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C1 Leu308Ala mutant by fluorescence assay |
Bioorg Med Chem Lett 21: 2564-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.076
BindingDB Entry DOI: 10.7270/Q20G3NZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V2) receptor in rat kidney membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50249758
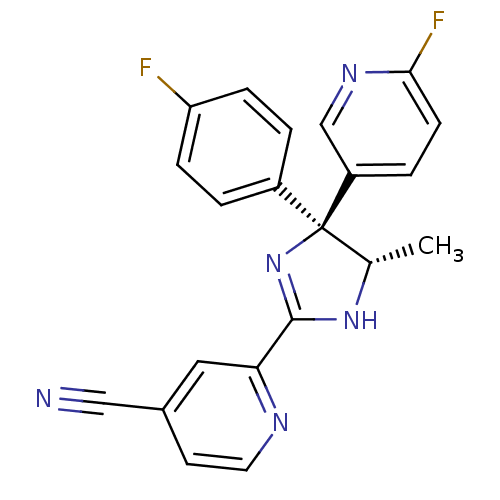
(2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...)Show SMILES C[C@@H]1NC(=N[C@@]1(c1ccc(F)cc1)c1ccc(F)nc1)c1cc(ccn1)C#N |r,c:3| Show InChI InChI=1S/C21H15F2N5/c1-13-21(15-2-5-17(22)6-3-15,16-4-7-19(23)26-12-16)28-20(27-13)18-10-14(11-24)8-9-25-18/h2-10,12-13H,1H3,(H,27,28)/t13-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells |
J Med Chem 52: 3385-96 (2009)
Article DOI: 10.1021/jm900110t
BindingDB Entry DOI: 10.7270/Q2DN450G |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330426
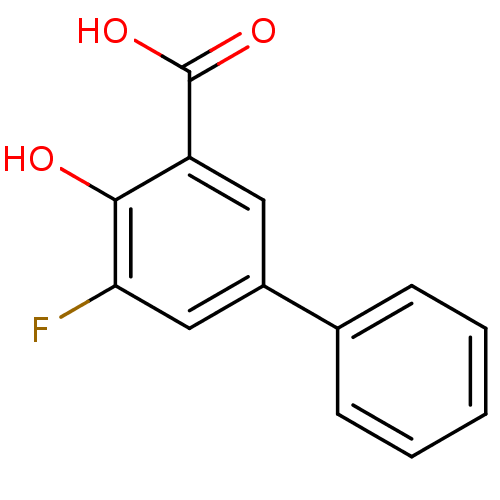
(5-Fluoro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9FO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330428
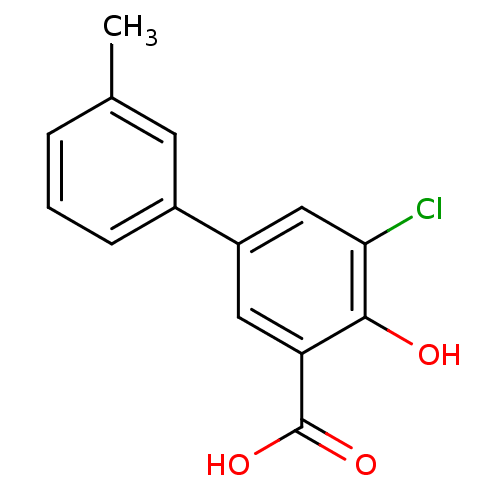
(5-Chloro-4-hydroxy-3'-methylbiphenyl-3-carboxylic ...)Show InChI InChI=1S/C14H11ClO3/c1-8-3-2-4-9(5-8)10-6-11(14(17)18)13(16)12(15)7-10/h2-7,16H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50442489

(CHEMBL2440417)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H20N2O4/c1-29-20-11-8-18(9-12-20)26-24-21(13-17-7-10-19(27)14-22(17)30-24)23(28)25-15-16-5-3-2-4-6-16/h2-14,27H,15H2,1H3,(H,25,28)/b26-24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of wild-type human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli using geraniol as substrate by dou... |
Bioorg Med Chem 21: 6378-84 (2013)
Article DOI: 10.1016/j.bmc.2013.08.059
BindingDB Entry DOI: 10.7270/Q25T3MX4 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50249758

(2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...)Show SMILES C[C@@H]1NC(=N[C@@]1(c1ccc(F)cc1)c1ccc(F)nc1)c1cc(ccn1)C#N |r,c:3| Show InChI InChI=1S/C21H15F2N5/c1-13-21(15-2-5-17(22)6-3-15,16-4-7-19(23)26-12-16)28-20(27-13)18-10-14(11-24)8-9-25-18/h2-10,12-13H,1H3,(H,27,28)/t13-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human NPYY5 receptor |
Bioorg Med Chem 17: 6106-22 (2009)
Article DOI: 10.1016/j.bmc.2009.05.069
BindingDB Entry DOI: 10.7270/Q2513ZHZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50187394

(CHEMBL207220 | N-(3-{4-[4-(cyclohexylmethanesulfon...)Show SMILES CN(CCCCN1CCN(CC1)c1cccc(NC(C)=O)c1)S(=O)(=O)CC1CCCCC1 Show InChI InChI=1S/C24H40N4O3S/c1-21(29)25-23-11-8-12-24(19-23)28-17-15-27(16-18-28)14-7-6-13-26(2)32(30,31)20-22-9-4-3-5-10-22/h8,11-12,19,22H,3-7,9-10,13-18,20H2,1-2H3,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Predix Pharmaceuticals Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor in HEK293 cells |
J Med Chem 49: 3116-35 (2006)
Article DOI: 10.1021/jm0508641
BindingDB Entry DOI: 10.7270/Q2319VHC |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50249833

(6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...)Show SMILES C[C@@H]1NC(=N[C@@]1(c1ccc(F)cc1)c1ccc(F)nc1)c1cccc(=O)[nH]1 |r,c:3| Show InChI InChI=1S/C20H16F2N4O/c1-12-20(13-5-8-15(21)9-6-13,14-7-10-17(22)23-11-14)26-19(24-12)16-3-2-4-18(27)25-16/h2-12H,1H3,(H,24,26)(H,25,27)/t12-,20-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells |
Bioorg Med Chem 17: 6106-22 (2009)
Article DOI: 10.1016/j.bmc.2009.05.069
BindingDB Entry DOI: 10.7270/Q2513ZHZ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50249833

(6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...)Show SMILES C[C@@H]1NC(=N[C@@]1(c1ccc(F)cc1)c1ccc(F)nc1)c1cccc(=O)[nH]1 |r,c:3| Show InChI InChI=1S/C20H16F2N4O/c1-12-20(13-5-8-15(21)9-6-13,14-7-10-17(22)23-11-14)26-19(24-12)16-3-2-4-18(27)25-16/h2-12H,1H3,(H,24,26)(H,25,27)/t12-,20-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells |
J Med Chem 52: 3385-96 (2009)
Article DOI: 10.1021/jm900110t
BindingDB Entry DOI: 10.7270/Q2DN450G |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50114031

((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at Vasopressin V1a receptor, performed using [3H]-AVP on rat liver |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50151486
((1R,2R,3R,5R,6R)-2-Amino-3-[(R)-1-(3,4-dichloro-ph...)Show SMILES C[C@@H](O[C@@H]1C[C@@H]2[C@@H]([C@@]2(F)C(O)=O)[C@]1(N)C(O)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C16H16Cl2FNO5/c1-6(7-2-3-9(17)10(18)4-7)25-11-5-8-12(15(8,19)13(21)22)16(11,20)14(23)24/h2-4,6,8,11-12H,5,20H2,1H3,(H,21,22)(H,23,24)/t6-,8-,11-,12+,15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards metabotropic glutamate receptor 2 of rat expressed in CHO cells was determined by using [3H]-MGS0008 |
J Med Chem 47: 4570-87 (2004)
Article DOI: 10.1021/jm0400294
BindingDB Entry DOI: 10.7270/Q2GH9HFK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50151439

((1R,2R,3R,5R,6R)-2-Amino-3-[(R)-1-(3,4-dichloro-ph...)Show SMILES CCC[C@@H](O[C@@H]1C[C@@H]2[C@@H]([C@@]2(F)C(O)=O)[C@]1(N)C(O)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C18H20Cl2FNO5/c1-2-3-12(8-4-5-10(19)11(20)6-8)27-13-7-9-14(17(9,21)15(23)24)18(13,22)16(25)26/h4-6,9,12-14H,2-3,7,22H2,1H3,(H,23,24)(H,25,26)/t9-,12-,13-,14+,17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards metabotropic glutamate receptor 2 of rat expressed in CHO cells was determined by using [3H]-MGS0008 |
J Med Chem 47: 4570-87 (2004)
Article DOI: 10.1021/jm0400294
BindingDB Entry DOI: 10.7270/Q2GH9HFK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data























































