 Found 201 hits with Last Name = 'harp' and Initial = 'jm'
Found 201 hits with Last Name = 'harp' and Initial = 'jm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430877
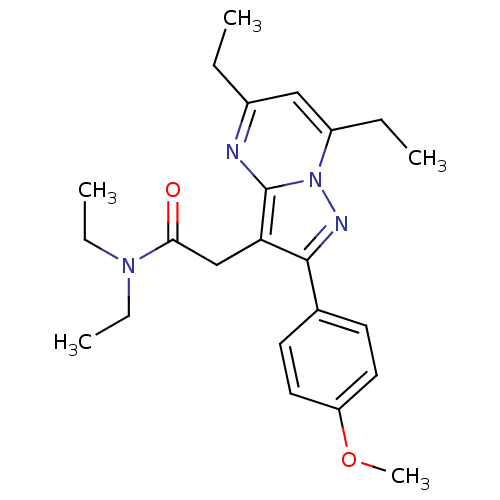
(CHEMBL2336480)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(CC)cc(CC)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C23H30N4O2/c1-6-17-14-18(7-2)27-23(24-17)20(15-21(28)26(8-3)9-4)22(25-27)16-10-12-19(29-5)13-11-16/h10-14H,6-9,15H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430878

(CHEMBL2336474)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(CC)cc(CC)nc12)-c1ccc(OCCF)cc1 Show InChI InChI=1S/C24H31FN4O2/c1-5-18-15-19(6-2)29-24(26-18)21(16-22(30)28(7-3)8-4)23(27-29)17-9-11-20(12-10-17)31-14-13-25/h9-12,15H,5-8,13-14,16H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50243007
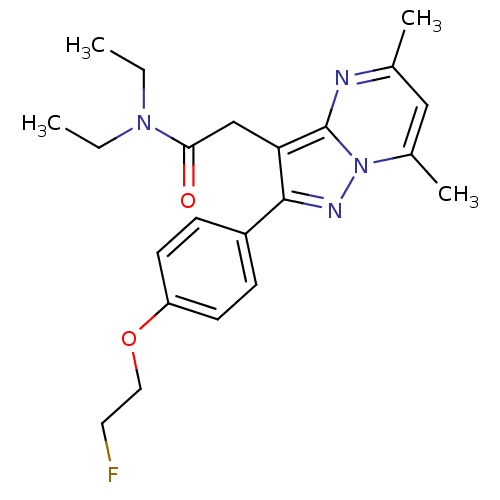
(2-(2-(4-(2-Fluoroethoxy)phenyl)-5,7-dimethylpyrazo...)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCCF)cc1 Show InChI InChI=1S/C22H27FN4O2/c1-5-26(6-2)20(28)14-19-21(17-7-9-18(10-8-17)29-12-11-23)25-27-16(4)13-15(3)24-22(19)27/h7-10,13H,5-6,11-12,14H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50327238
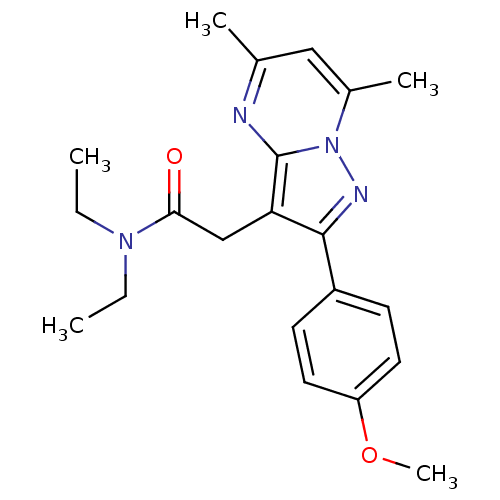
(2-[2-(4'-Methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a...)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H26N4O2/c1-6-24(7-2)19(26)13-18-20(16-8-10-17(27-5)11-9-16)23-25-15(4)12-14(3)22-21(18)25/h8-12H,6-7,13H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596239

(CHEMBL5189893)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430876

(CHEMBL2336473)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)c(Cl)c(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H25ClN4O2/c1-6-25(7-2)18(27)12-17-20(15-8-10-16(28-5)11-9-15)24-26-14(4)19(22)13(3)23-21(17)26/h8-11H,6-7,12H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430887
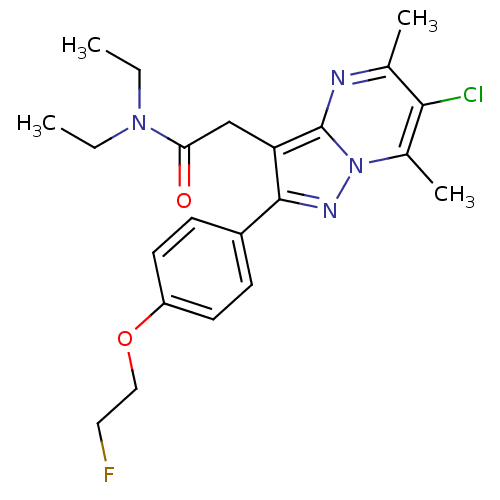
(CHEMBL2336479)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)c(Cl)c(C)nc12)-c1ccc(OCCF)cc1 Show InChI InChI=1S/C22H26ClFN4O2/c1-5-27(6-2)19(29)13-18-21(16-7-9-17(10-8-16)30-12-11-24)26-28-15(4)20(23)14(3)25-22(18)28/h7-10H,5-6,11-13H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430886

(CHEMBL2336476)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)c(C)c(C)nc12)-c1ccc(OCCF)cc1 Show InChI InChI=1S/C23H29FN4O2/c1-6-27(7-2)21(29)14-20-22(18-8-10-19(11-9-18)30-13-12-24)26-28-17(5)15(3)16(4)25-23(20)28/h8-11H,6-7,12-14H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430885
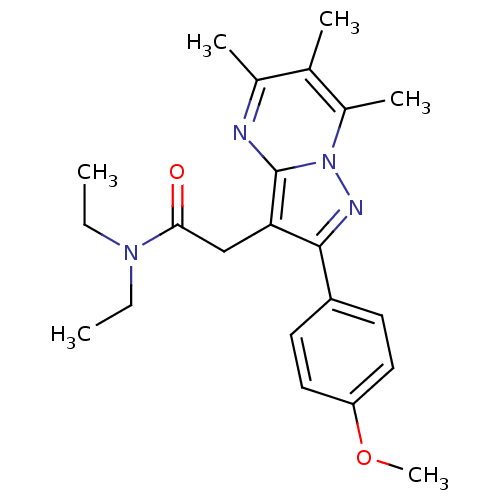
(CHEMBL2336482)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)c(C)c(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C22H28N4O2/c1-7-25(8-2)20(27)13-19-21(17-9-11-18(28-6)12-10-17)24-26-16(5)14(3)15(4)23-22(19)26/h9-12H,7-8,13H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430884
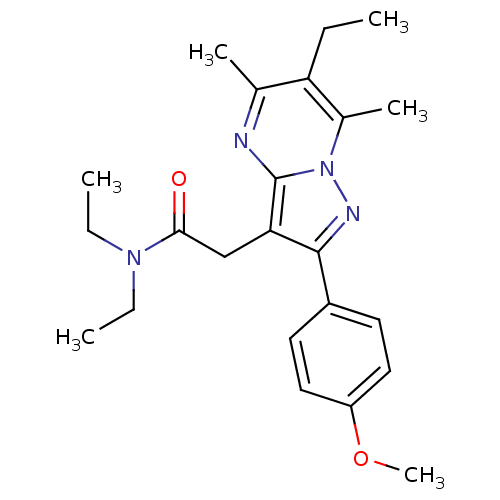
(CHEMBL2336483)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)c(CC)c(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C23H30N4O2/c1-7-19-15(4)24-23-20(14-21(28)26(8-2)9-3)22(25-27(23)16(19)5)17-10-12-18(29-6)13-11-17/h10-13H,7-9,14H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430883

(CHEMBL2336484)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)c(CC(C)=O)c(C)nc12)-c1ccc(OC)cc1 Show InChI InChI=1S/C24H30N4O3/c1-7-27(8-2)22(30)14-21-23(18-9-11-19(31-6)12-10-18)26-28-17(5)20(13-15(3)29)16(4)25-24(21)28/h9-12H,7-8,13-14H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430882
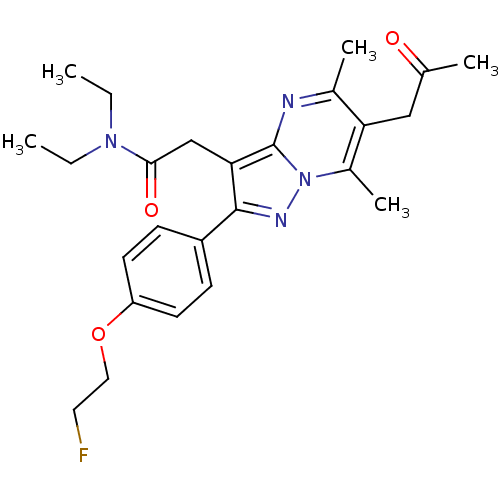
(CHEMBL2336478)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)c(CC(C)=O)c(C)nc12)-c1ccc(OCCF)cc1 Show InChI InChI=1S/C25H31FN4O3/c1-6-29(7-2)23(32)15-22-24(19-8-10-20(11-9-19)33-13-12-26)28-30-18(5)21(14-16(3)31)17(4)27-25(22)30/h8-11H,6-7,12-15H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430881

(CHEMBL2336477)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)c(CC)c(C)nc12)-c1ccc(OCCF)cc1 Show InChI InChI=1S/C24H31FN4O2/c1-6-20-16(4)26-24-21(15-22(30)28(7-2)8-3)23(27-29(24)17(20)5)18-9-11-19(12-10-18)31-14-13-25/h9-12H,6-8,13-15H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 277 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430879

(CHEMBL2336475)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(cc(nc12)C(C)C)C(C)C)-c1ccc(OCCF)cc1 Show InChI InChI=1S/C26H35FN4O2/c1-7-30(8-2)24(32)15-21-25(19-9-11-20(12-10-19)33-14-13-27)29-31-23(18(5)6)16-22(17(3)4)28-26(21)31/h9-12,16-18H,7-8,13-15H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50430880

(CHEMBL2336481)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(cc(nc12)C(C)C)C(C)C)-c1ccc(OC)cc1 Show InChI InChI=1S/C25H34N4O2/c1-8-28(9-2)23(30)14-20-24(18-10-12-19(31-7)13-11-18)27-29-22(17(5)6)15-21(16(3)4)26-25(20)29/h10-13,15-17H,8-9,14H2,1-7H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Institute of Imaging Science (VUIIS)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PK 11195 from TSPO in rat C6 cell lysates after 2 hrs by scintillation counting analysis |
J Med Chem 56: 3429-33 (2013)
Article DOI: 10.1021/jm4001874
BindingDB Entry DOI: 10.7270/Q2Z3211Z |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Displacement of [3H] substance P from recombinant human NK1 receptor expressed in CHO cells after 90 mins by scintillation counting method |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596231

(CHEMBL5182783)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596231

(CHEMBL5182783)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596229

(CHEMBL5205163)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCCCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596229

(CHEMBL5205163)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCCCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596242

(CHEMBL5185664)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NCC2CC22CCN(CC3CCCCC3)CC2)nn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596230

(CHEMBL5178468)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@H]2CC22CCN(CC3CCCCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596230

(CHEMBL5178468)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@H]2CC22CCN(CC3CCCCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596237

(CHEMBL5172967)Show SMILES Fc1cc(F)c(F)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596237

(CHEMBL5172967)Show SMILES Fc1cc(F)c(F)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596237

(CHEMBL5172967)Show SMILES Fc1cc(F)c(F)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596237

(CHEMBL5172967)Show SMILES Fc1cc(F)c(F)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596236
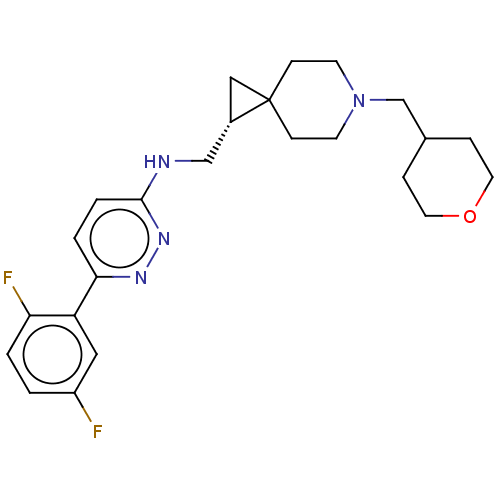
(CHEMBL5173887)Show SMILES Fc1ccc(F)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596236

(CHEMBL5173887)Show SMILES Fc1ccc(F)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596234

(CHEMBL5203077)Show SMILES Fc1cccc(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596234

(CHEMBL5203077)Show SMILES Fc1cccc(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596239

(CHEMBL5189893)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596239

(CHEMBL5189893)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596233
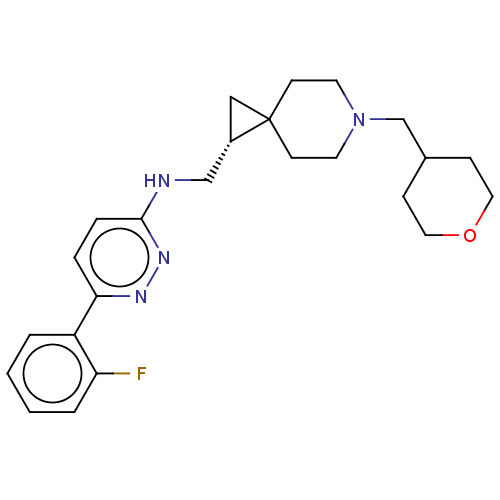
(CHEMBL5207446)Show SMILES Fc1ccccc1-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596233

(CHEMBL5207446)Show SMILES Fc1ccccc1-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50596229

(CHEMBL5205163)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCCCC3)CC2)nn1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50596229

(CHEMBL5205163)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCCCC3)CC2)nn1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596232

(CHEMBL5185022)Show SMILES C(Nc1ccc(nn1)-c1ccccc1)[C@@H]1CC11CCN(CC2CCOCC2)CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596232

(CHEMBL5185022)Show SMILES C(Nc1ccc(nn1)-c1ccccc1)[C@@H]1CC11CCN(CC2CCOCC2)CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596238
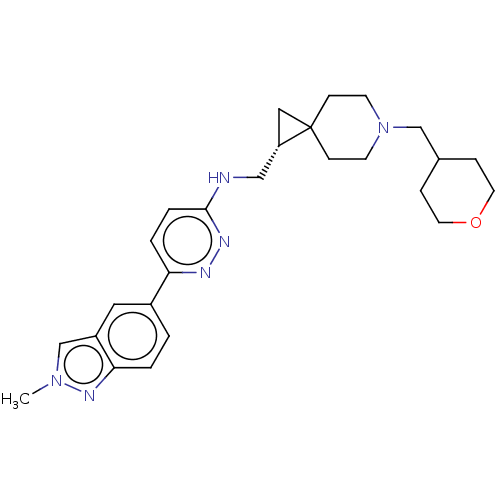
(CHEMBL5198423)Show SMILES Cn1cc2cc(ccc2n1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596238

(CHEMBL5198423)Show SMILES Cn1cc2cc(ccc2n1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50596242

(CHEMBL5185664)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NCC2CC22CCN(CC3CCCCC3)CC2)nn1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596235
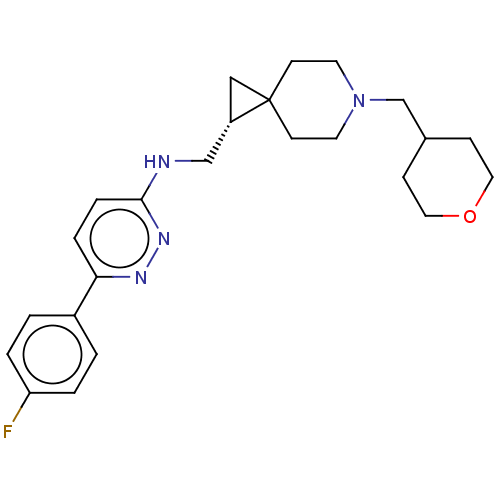
(CHEMBL5204478)Show SMILES Fc1ccc(cc1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50596235

(CHEMBL5204478)Show SMILES Fc1ccc(cc1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50283479
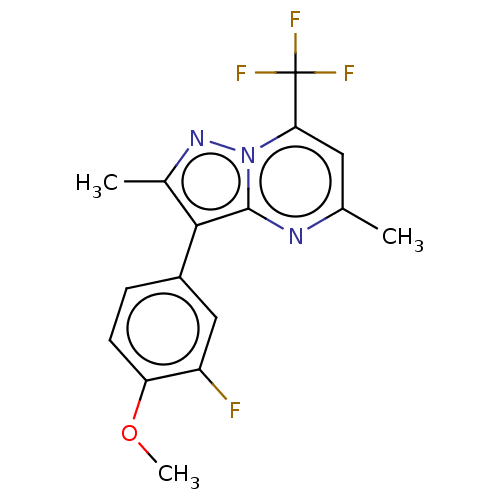
(CHEMBL4165248)Show SMILES COc1ccc(cc1F)-c1c(C)nn2c(cc(C)nc12)C(F)(F)F Show InChI InChI=1S/C16H13F4N3O/c1-8-6-13(16(18,19)20)23-15(21-8)14(9(2)22-23)10-4-5-12(24-3)11(17)7-10/h4-7H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50596230

(CHEMBL5178468)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@H]2CC22CCN(CC3CCCCC3)CC2)nn1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 525 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(RAT) | BDBM50596230

(CHEMBL5178468)Show SMILES Fc1ccc(Cl)c(c1)-c1ccc(NC[C@H]2CC22CCN(CC3CCCCC3)CC2)nn1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50596237

(CHEMBL5172967)Show SMILES Fc1cc(F)c(F)c(c1)-c1ccc(NC[C@@H]2CC22CCN(CC3CCOCC3)CC2)nn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128479
BindingDB Entry DOI: 10.7270/Q2F193SZ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280374
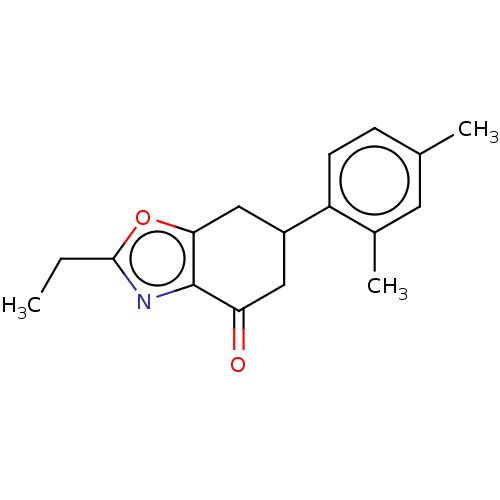
(CHEMBL4174742)Show InChI InChI=1S/C17H19NO2/c1-4-16-18-17-14(19)8-12(9-15(17)20-16)13-6-5-10(2)7-11(13)3/h5-7,12H,4,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGlu7 receptor expressed in HEK cells co-expressing Galphai5 assessed as decrease in glutamate-induced thallium... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50283483
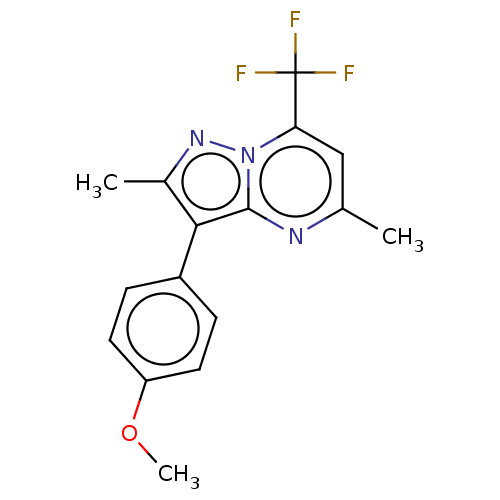
(CHEMBL2361777)Show InChI InChI=1S/C16H14F3N3O/c1-9-8-13(16(17,18)19)22-15(20-9)14(10(2)21-22)11-4-6-12(23-3)7-5-11/h4-8H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data