

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030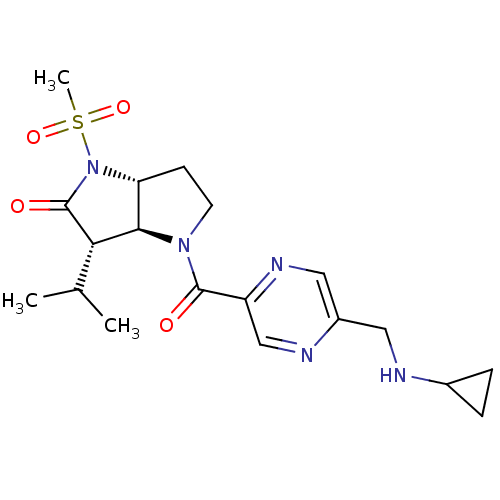 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028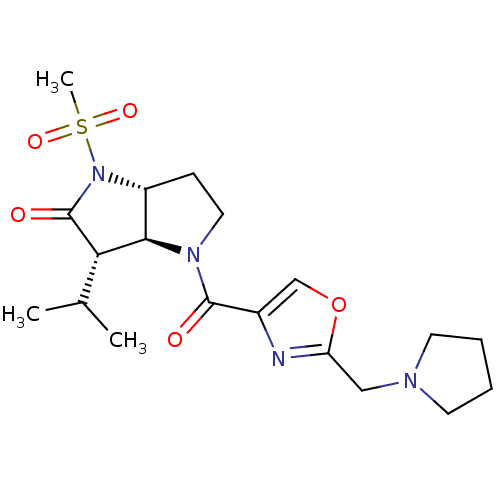 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | 4.90 | 3.05E+4 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | 0.00000207 | 6.63E+3 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027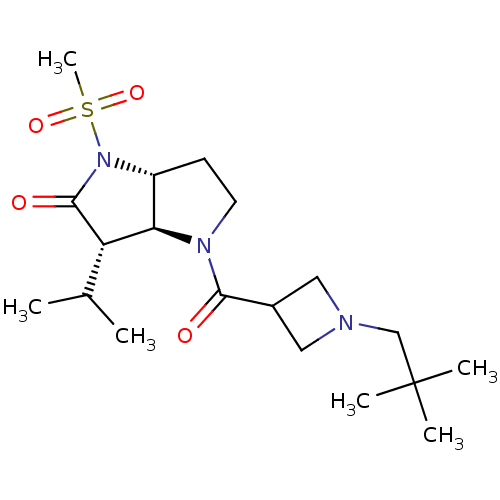 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.73 | n/a | n/a | n/a | n/a | 0.0000109 | 6.31E+3 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096484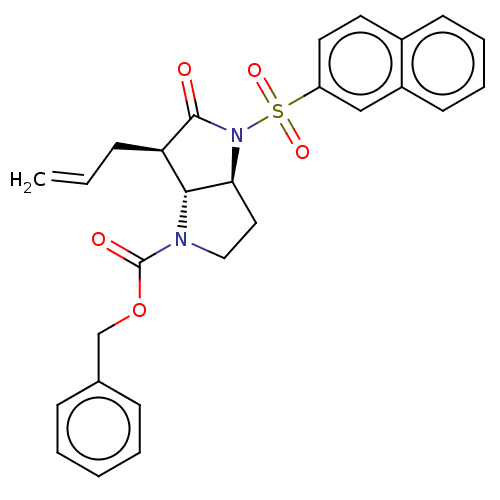 ((4S,6R)-6-Allyl-4-(naphthalene-2-sulfonyl)-5-oxo-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066997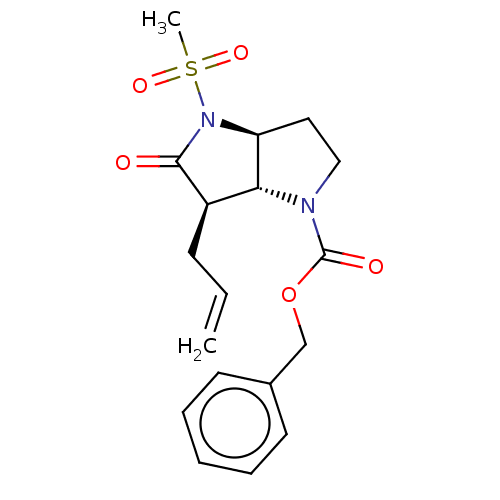 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096486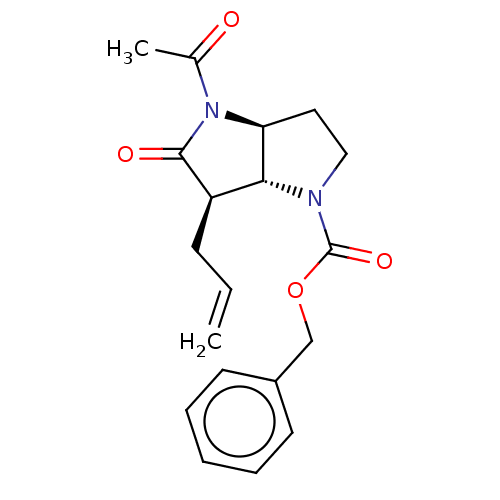 (CHEMBL2367646 | benzyl (3aS,6aR)-4-acetyl-6-allyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096488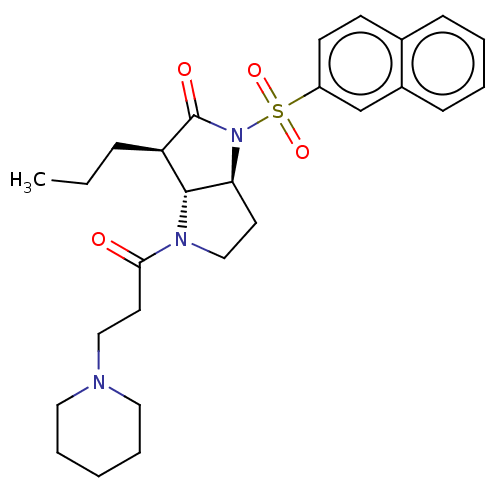 ((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Binding affinity for dopamine D4-like receptor labelled with [3H]YM-09151-2 in retina | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096488 ((1S,3R)-1-(Naphthalene-2-sulfonyl)-4-(3-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096487 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-pyrrolo[3,2-b]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096483 ((3R,6aS)-1-Methanesulfonyl-4-(3-piperidin-1-yl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase at a concentration of 10 uM | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50566686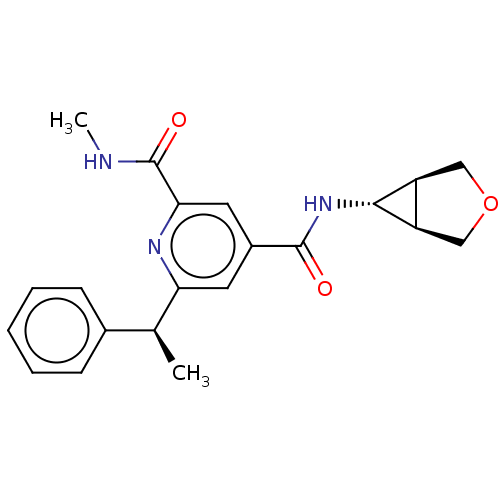 (CHEMBL4873182) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50061024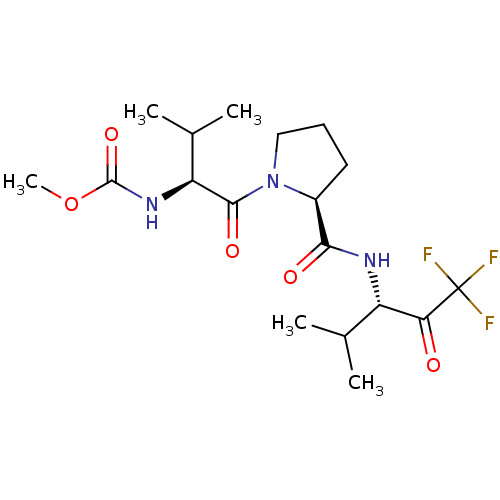 ((2-Methyl-1-{(S)-oxo-[(S)-2-((S)-3,3,3-trifluoro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity in human whole blood (HWB) elastase | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50543349 (CHEMBL4640769) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from His6-tagged BRD4 BD2 (1 to 477 residues)/BD1 Y97A mutant (unknown origin) after 30 mins by TR-FR... | ACS Med Chem Lett 11: 1581-1587 (2020) Article DOI: 10.1021/acsmedchemlett.0c00247 BindingDB Entry DOI: 10.7270/Q279487V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50566679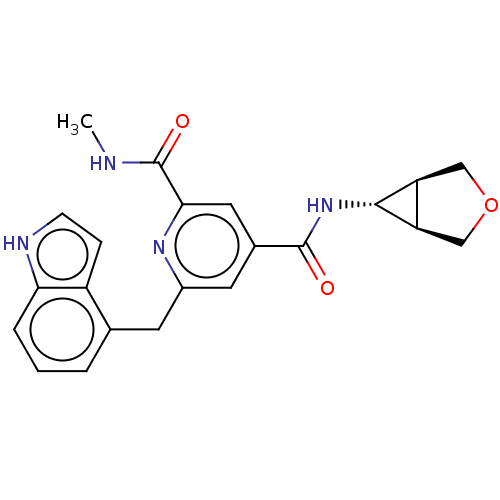 (CHEMBL4846199) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50566697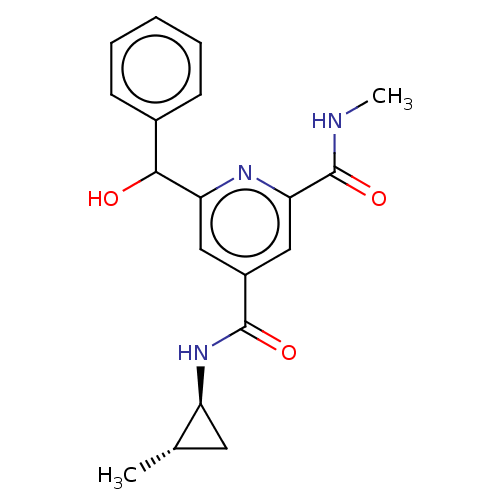 (CHEMBL4862372) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD3 BD2/BD1 Y73A mutant (1 to 435 residues) (unknown origin) measured after 30 ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50566697 (CHEMBL4862372) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50543360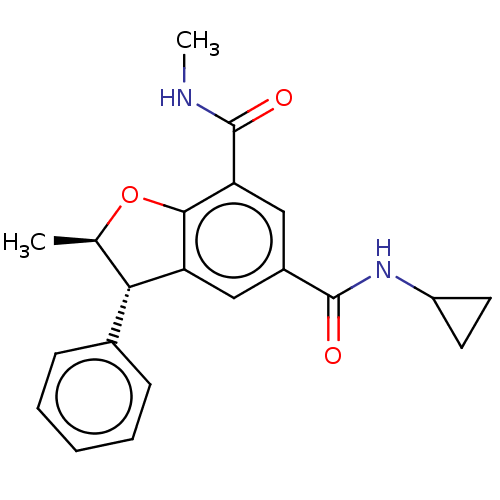 (CHEMBL4643660) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from His6-tagged BRD4 BD2 (1 to 477 residues)/BD1 Y97A mutant (unknown origin) after 30 mins by TR-FR... | ACS Med Chem Lett 11: 1581-1587 (2020) Article DOI: 10.1021/acsmedchemlett.0c00247 BindingDB Entry DOI: 10.7270/Q279487V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50544308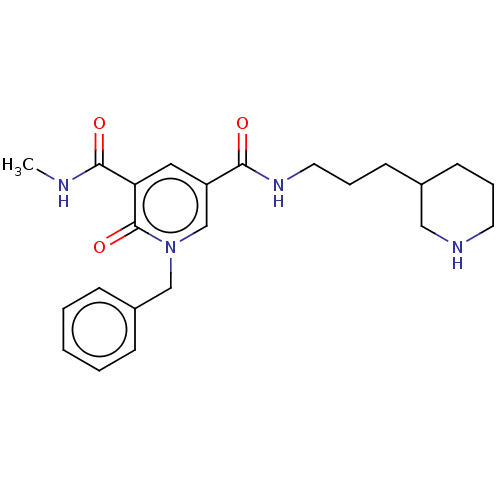 (CHEMBL4646820) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to 6His-Thr-BRD4 (1 to 477 residues) BD2 domain Y390A mutant (unknown origin) incubated for 30 mins by TR-FRET assay | J Med Chem 63: 9093-9126 (2020) Article DOI: 10.1021/acs.jmedchem.0c00796 BindingDB Entry DOI: 10.7270/Q27W6GS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50544335 (CHEMBL4648431) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to 6His-Thr-BRD4 (1 to 477 residues) BD2 domain Y390A mutant (unknown origin) incubated for 30 mins by TR-FRET assay | J Med Chem 63: 9093-9126 (2020) Article DOI: 10.1021/acs.jmedchem.0c00796 BindingDB Entry DOI: 10.7270/Q27W6GS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50566685 (CHEMBL4860220) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50544335 (CHEMBL4648431) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50543343 (CHEMBL4637298) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from His6-tagged BRD4 BD2 (1 to 477 residues)/BD1 Y97A mutant (unknown origin) after 30 mins by TR-FR... | ACS Med Chem Lett 11: 1581-1587 (2020) Article DOI: 10.1021/acsmedchemlett.0c00247 BindingDB Entry DOI: 10.7270/Q279487V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50566690 (CHEMBL4863255) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50566687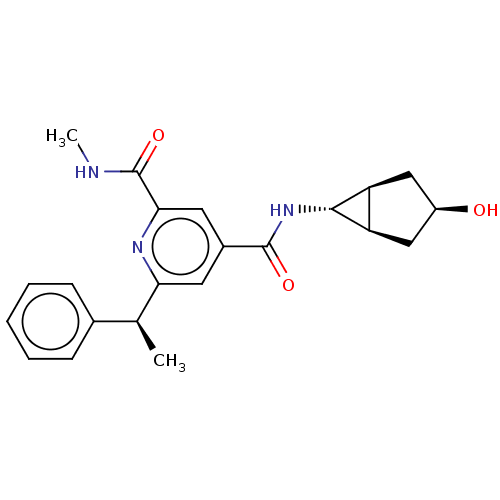 (CHEMBL4865699) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50566695 (CHEMBL4846439) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50544309 (CHEMBL4641088) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to 6His-Thr-BRD4 (1 to 477 residues) BD2 domain Y390A mutant (unknown origin) incubated for 30 mins by TR-FRET assay | J Med Chem 63: 9093-9126 (2020) Article DOI: 10.1021/acs.jmedchem.0c00796 BindingDB Entry DOI: 10.7270/Q27W6GS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50543348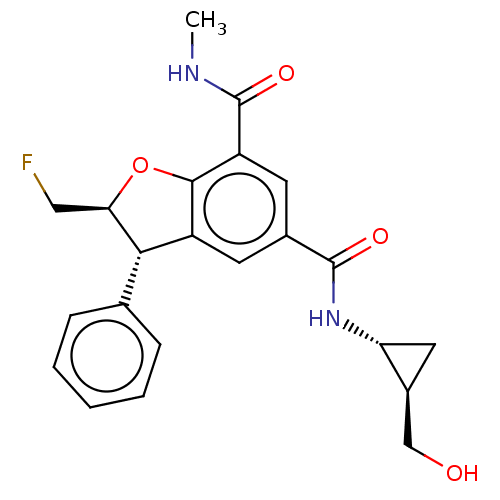 (CHEMBL4647044) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from His6-tagged BRD4 BD2 (1 to 477 residues)/BD1 Y97A mutant (unknown origin) after 30 mins by TR-FR... | ACS Med Chem Lett 11: 1581-1587 (2020) Article DOI: 10.1021/acsmedchemlett.0c00247 BindingDB Entry DOI: 10.7270/Q279487V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50603088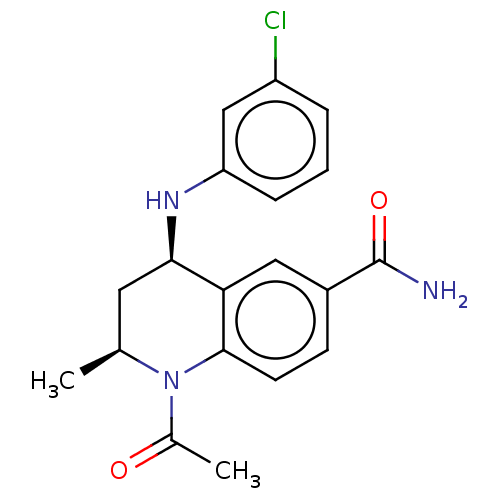 (CHEMBL5203965) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01747 BindingDB Entry DOI: 10.7270/Q23B646M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against dog neutrophil elastase using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Chymotrypsinogen using selectivity assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098095 ((3S,3aR,6aS)-3-Isopropyl-1-methanesulfonyl-5-((E)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against Elastase | Bioorg Med Chem Lett 11: 895-8 (2001) BindingDB Entry DOI: 10.7270/Q29C6XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 0 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50566693 (CHEMBL4857098) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50543350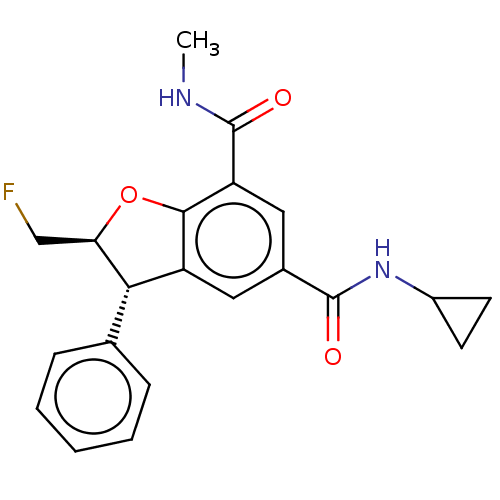 (CHEMBL4646186) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from His6-tagged BRD4 BD2 (1 to 477 residues)/BD1 Y97A mutant (unknown origin) after 30 mins by TR-FR... | ACS Med Chem Lett 11: 1581-1587 (2020) Article DOI: 10.1021/acsmedchemlett.0c00247 BindingDB Entry DOI: 10.7270/Q279487V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50576427 (CHEMBL4865767) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 6His-FLAG-Tev-BRDT (1 to 397 residues) BD2 domain Y309A or Y66A mutant (unknown origin) incubated for 30 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00365 BindingDB Entry DOI: 10.7270/Q21N84ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50566697 (CHEMBL4862372) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRDT BD2/BD1 Y66A mutant (1 to 397 residues) (unknown origin) measured after 30 ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50566691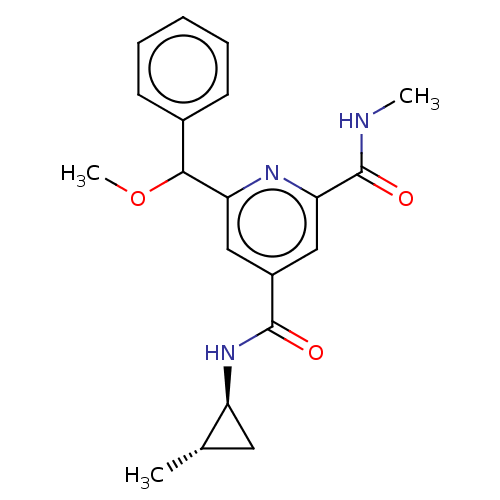 (CHEMBL4850783) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Alexa Fluor 647 labelled ligand from 6His-Thr tagged BRD4 BD2/BD1 Y97A mutant (1 to 477 residue) (unknown origin) measured after 30 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02155 BindingDB Entry DOI: 10.7270/Q2JH3QXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50543344 (CHEMBL4635048) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from His6-tagged BRD4 BD2 (1 to 477 residues)/BD1 Y97A mutant (unknown origin) after 30 mins by TR-FR... | ACS Med Chem Lett 11: 1581-1587 (2020) Article DOI: 10.1021/acsmedchemlett.0c00247 BindingDB Entry DOI: 10.7270/Q279487V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50098094 ((3R,6aR)-3-Isopropyl-1-methanesulfonyl-5-((E)-4-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against Elastase | Bioorg Med Chem Lett 11: 895-8 (2001) BindingDB Entry DOI: 10.7270/Q29C6XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096490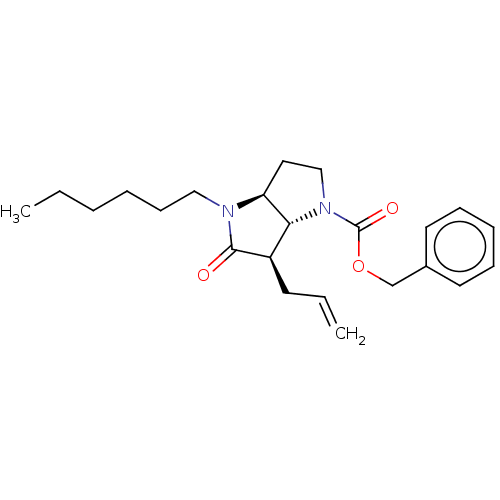 ((3aS,6R)-6-Allyl-4-hexyl-5-oxo-hexahydro-pyrrolo[3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096485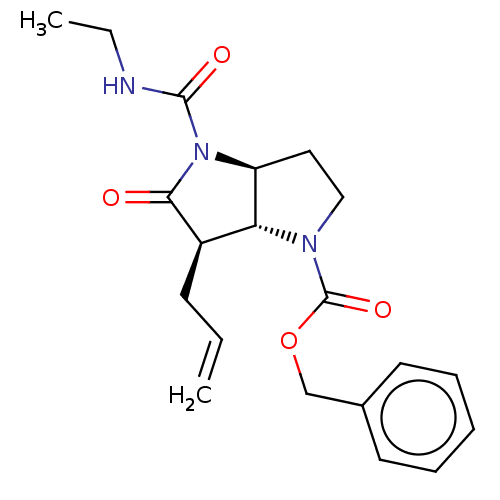 ((3aS,6R)-6-Allyl-4-ethylcarbamoyl-5-oxo-hexahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50575644 (CHEMBL4856853) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 6His-Thr-BRD3 (1 to 435 residues) BD2 domain Y348A or Y73A mutant (unknown origin) incubated for 30 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02156 BindingDB Entry DOI: 10.7270/Q2F193JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 488 total ) | Next | Last >> |