

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lanosterol synthase (Rattus norvegicus) | BDBM50055641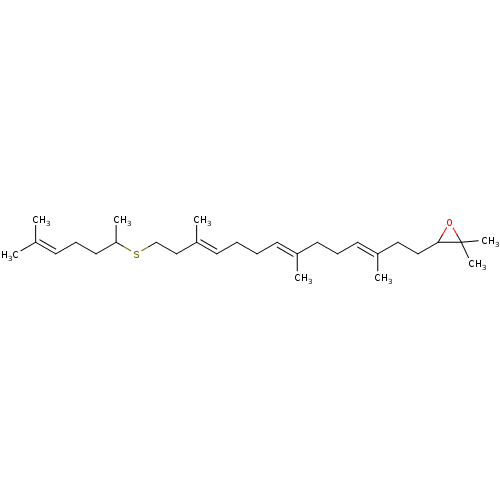 (2,2-dimethyl-3-((3E,7E,11E)-3,7,12-trimethyl-14-(6...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from rat liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055642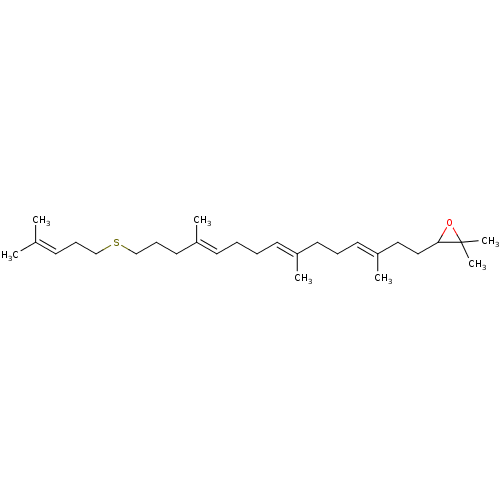 (2,2-Dimethyl-3-[(3E,7E,11E)-3,7,12-trimethyl-15-(4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from rat liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055643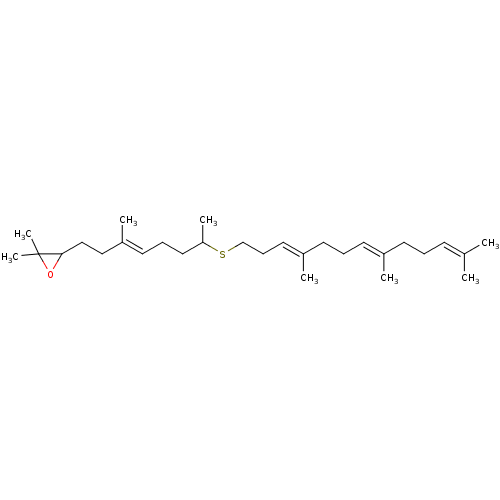 (2,2-Dimethyl-3-[(E)-3-methyl-7-((3E,7E)-4,8,12-tri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055642 (2,2-Dimethyl-3-[(3E,7E,11E)-3,7,12-trimethyl-15-(4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055641 (2,2-dimethyl-3-((3E,7E,11E)-3,7,12-trimethyl-14-(6...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055644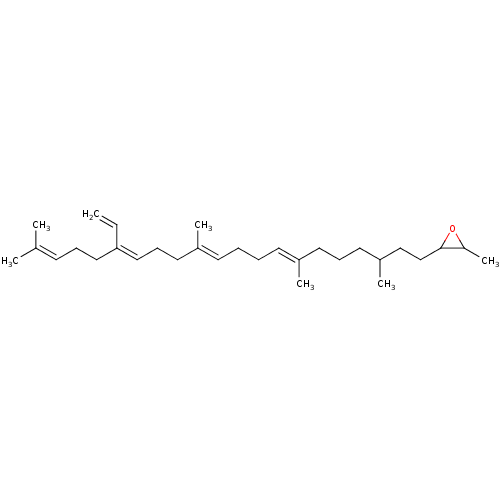 (2-Methyl-3-((7E,11E,15Z)-3,7,12,20-tetramethyl-16-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from rat liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055644 (2-Methyl-3-((7E,11E,15Z)-3,7,12,20-tetramethyl-16-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055640 (3-[(3E,7E)-11-((E)-4,8-Dimethyl-nona-3,7-dienylsul...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055645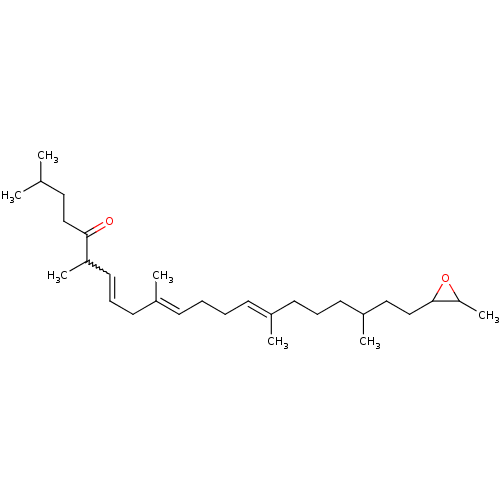 ((6E,10E,14E)-2,6,10,15,19-Pentamethyl-21-(3-methyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition constant against rat liver Oxidosqualene-lanosterol cyclase | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50055641 (2,2-dimethyl-3-((3E,7E,11E)-3,7,12-trimethyl-14-(6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for inhibition of purified Oxidosqualene-lanosterol cyclase from Candida albicans | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336375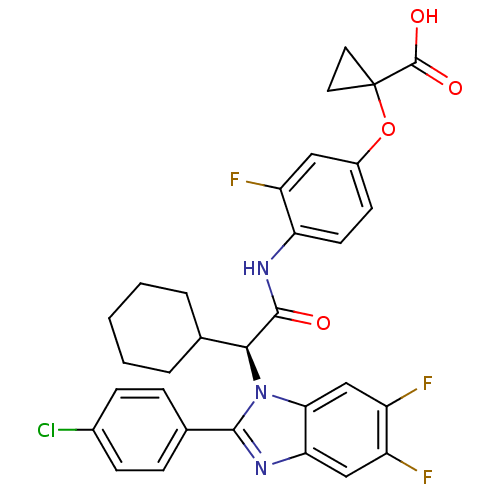 ((S)-1-(4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50192109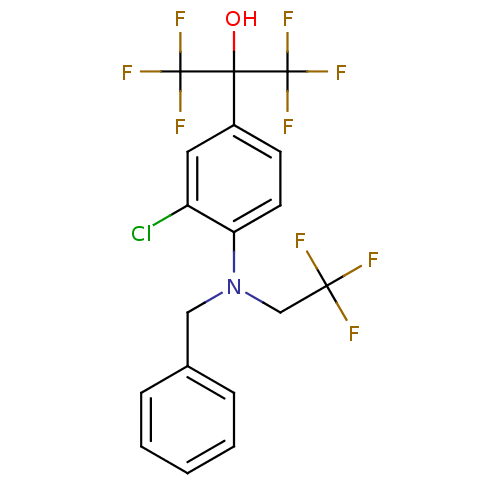 (2-(4-(benzyl(2,2,2-trifluoroethyl)amino)-3-chlorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRalpha by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192148 (2-(3-chloro-4-(ethyl(thiazol-4-ylmethyl)amino)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50192137 (2-(3-chloro-4-(((2-(3-chlorophenyl)-5-methyloxazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRalpha by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128532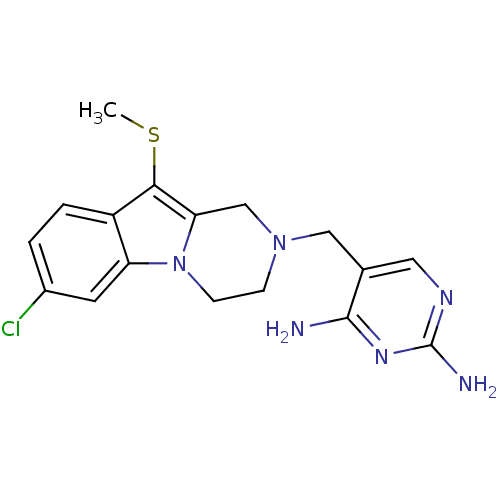 (5-(7-Chloro-10-methylsulfanyl-3,4-dihydro-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50055642 (2,2-Dimethyl-3-[(3E,7E,11E)-3,7,12-trimethyl-15-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for inhibition of purified Oxidosqualene-lanosterol cyclase from Candida albicans | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128535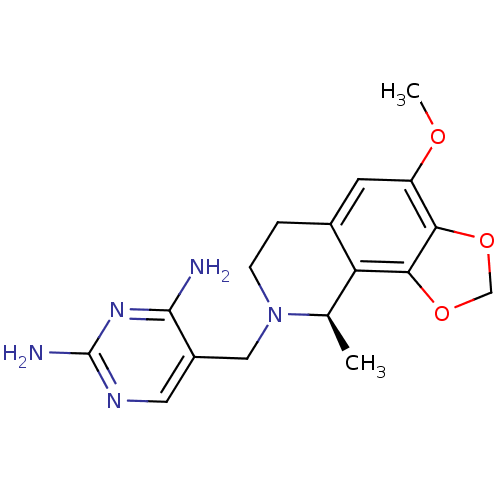 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192134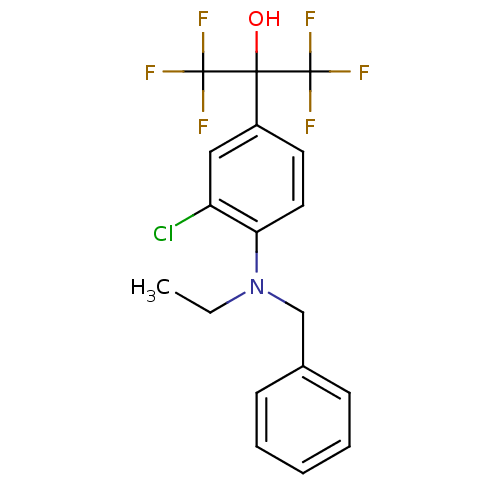 (2-(4-(benzyl(ethyl)amino)-3-chlorophenyl)-1,1,1,3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192144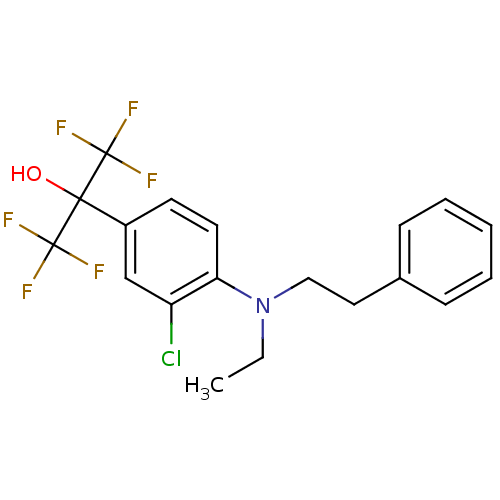 (2-(3-chloro-4-(ethyl(phenethyl)amino)phenyl)-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192109 (2-(4-(benzyl(2,2,2-trifluoroethyl)amino)-3-chlorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50192134 (2-(4-(benzyl(ethyl)amino)-3-chlorophenyl)-1,1,1,3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRalpha by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192136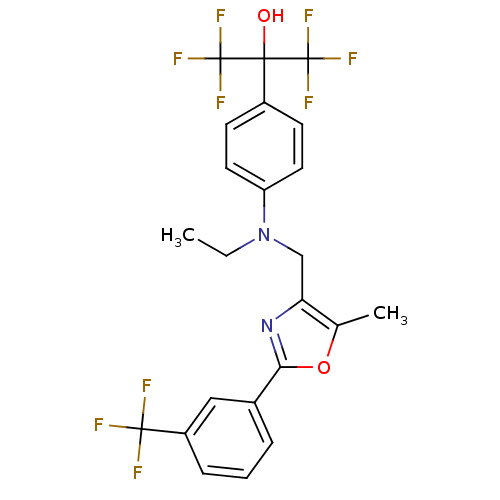 (2-(4-(ethyl((5-methyl-2-(3-(trifluoromethyl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336376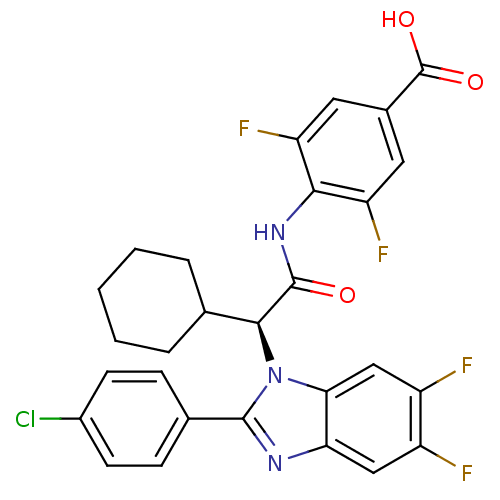 ((S)-4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192137 (2-(3-chloro-4-(((2-(3-chlorophenyl)-5-methyloxazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128540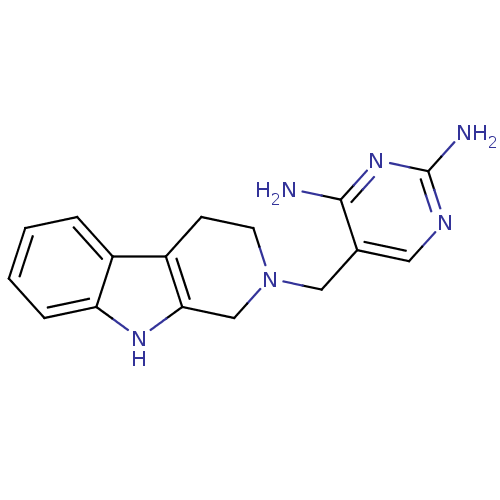 (5-(1,3,4,9-Tetrahydro-beta-carbolin-2-ylmethyl)-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128534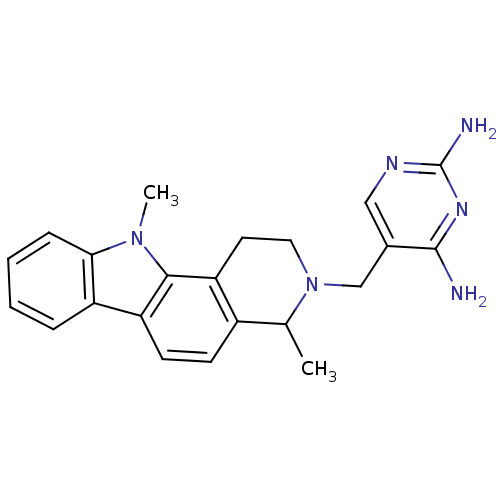 (5-(4,11-Dimethyl-1,2,4,11-tetrahydro-pyrido[4,3-a]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192117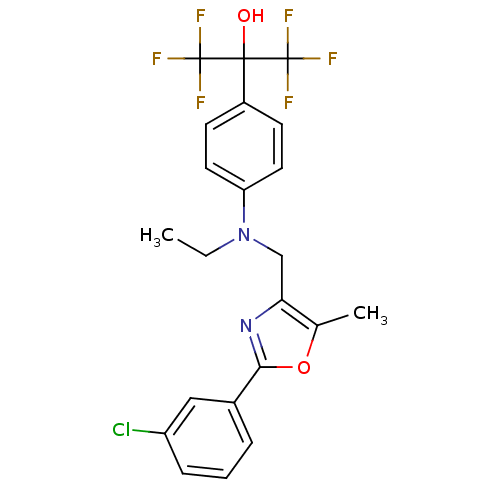 (2-(4-(((2-(3-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50192117 (2-(4-(((2-(3-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRalpha by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50192136 (2-(4-(ethyl((5-methyl-2-(3-(trifluoromethyl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRalpha by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50192113 (2-(3-chloro-4-(ethyl((5-methyl-2-(3-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRalpha by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128538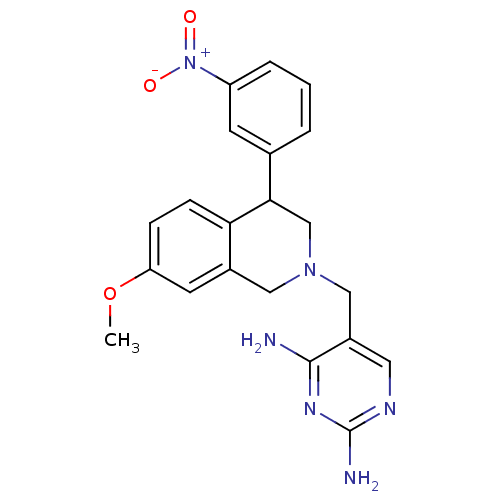 (5-[7-Methoxy-4-(3-nitro-phenyl)-3,4-dihydro-1H-iso...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50192144 (2-(3-chloro-4-(ethyl(phenethyl)amino)phenyl)-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRalpha by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128533 (5-[4-(2,4-Dichloro-phenyl)-6,7-dimethoxy-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192115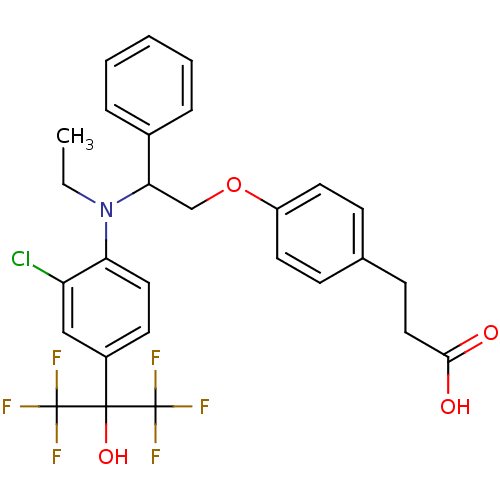 (3-(4-(2-((2-chloro-4-(1,1,1,3,3,3-hexafluoro-2-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192113 (2-(3-chloro-4-(ethyl((5-methyl-2-(3-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336377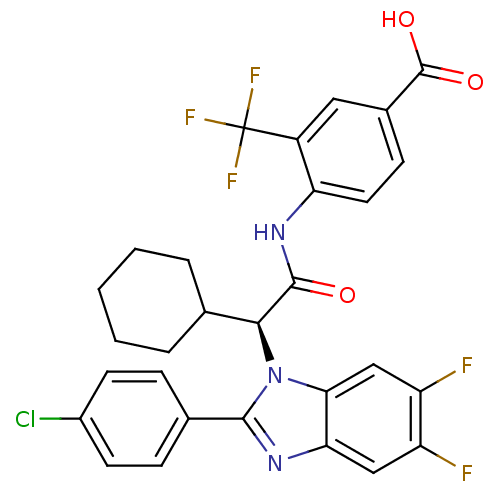 ((S)-4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336378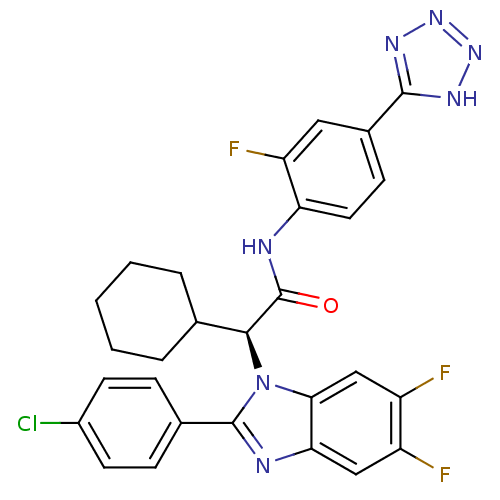 ((S)-2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo[d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192138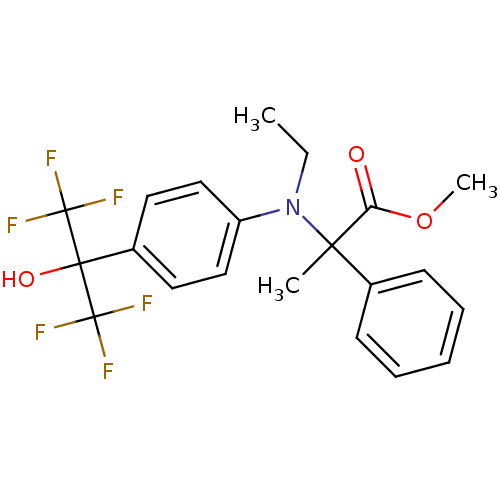 (CHEMBL215904 | methyl 2-(ethyl(4-(1,1,1,3,3,3-hexa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRbeta by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336379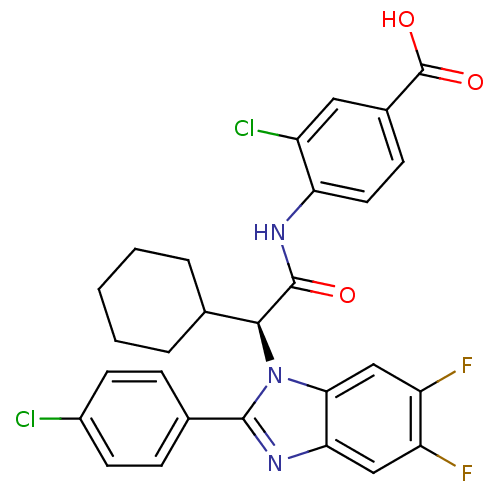 ((S)-3-chloro-4-(2-(2-(4-chlorophenyl)-5,6-difluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128535 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336380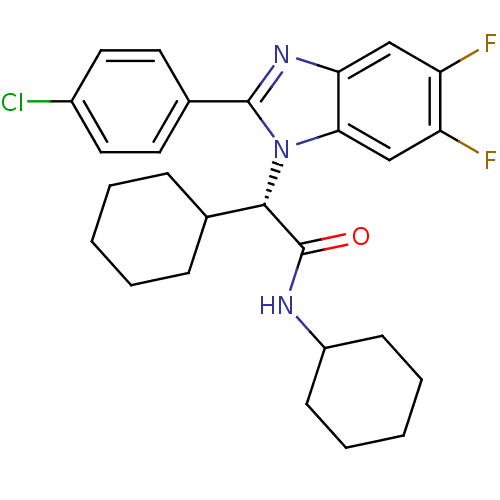 ((S)-2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo[d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336381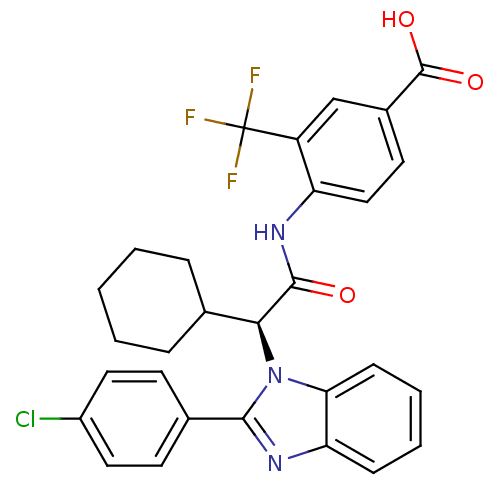 ((S)-4-(2-(2-(4-chlorophenyl)-1H-benzo[d]imidazol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128536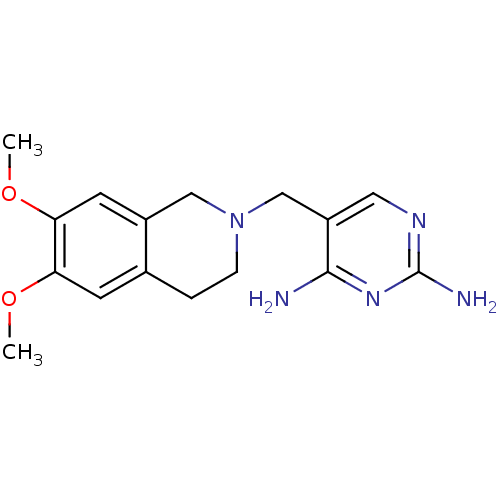 (5-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128534 (5-(4,11-Dimethyl-1,2,4,11-tetrahydro-pyrido[4,3-a]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50128537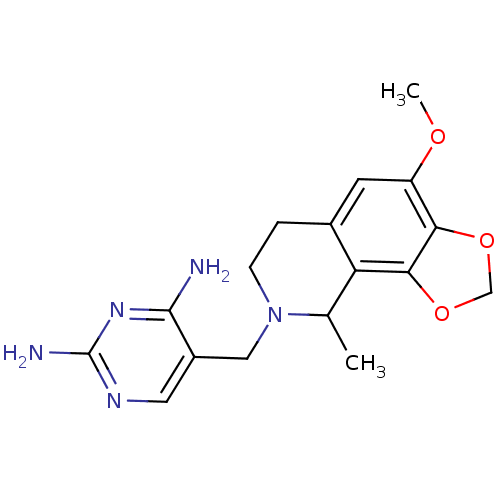 (5-(4-Methoxy-9-methyl-6,9-dihydro-7H-[1,3]dioxolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antibacterial activity of the compound against TMP-susceptible Dihydrofolate reductase from Staphylococcus pneumoniae ATCC 49619 | J Med Chem 46: 2304-12 (2003) Article DOI: 10.1021/jm020495y BindingDB Entry DOI: 10.7270/Q2J38RXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50334234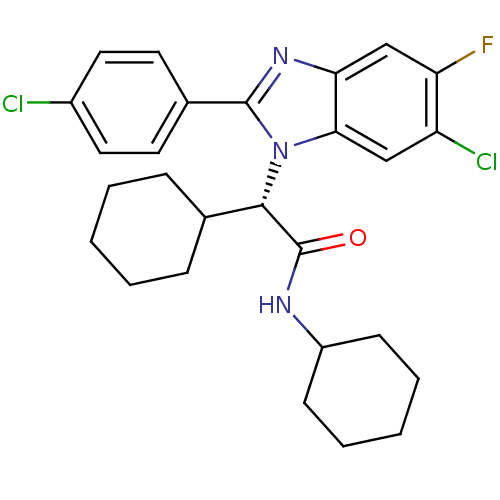 ((S)-2-(6-chloro-2-(4-chlorophenyl)-5-fluoro-1H-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche AG Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 191-4 (2010) Article DOI: 10.1016/j.bmcl.2010.11.039 BindingDB Entry DOI: 10.7270/Q2XP757R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50192126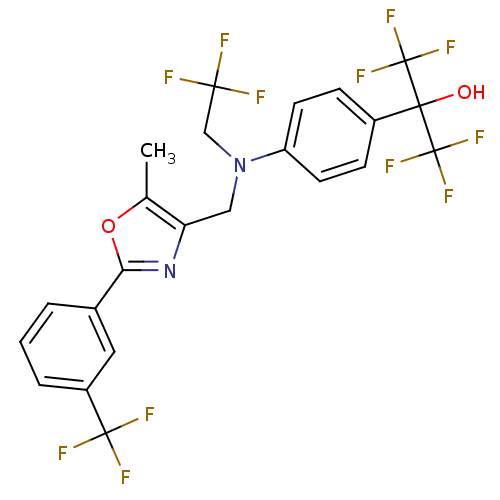 (1,1,1,3,3,3-hexafluoro-2-(4-(((5-methyl-2-(3-(trif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to LXRalpha by radioligand displacement assay | Bioorg Med Chem Lett 16: 5231-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.081 BindingDB Entry DOI: 10.7270/Q2HD7V80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50334234 ((S)-2-(6-chloro-2-(4-chlorophenyl)-5-fluoro-1H-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336382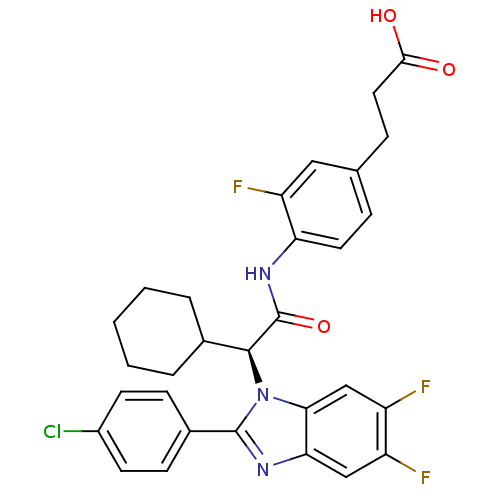 ((S)-3-(4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM28154 (5-[(S)-(benzenesulfonyl)[(2R)-7-chloro-1H,2H,3H,4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | 120 | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd | Assay Description LXR receptor binding assays were performed in 96-well plates. For each well reaction, GST-LXR-LBD fusion proteins were bound to SPA beads, and radiol... | Bioorg Med Chem Lett 19: 1654-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.109 BindingDB Entry DOI: 10.7270/Q2BK19PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 454 total ) | Next | Last >> |