

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50214615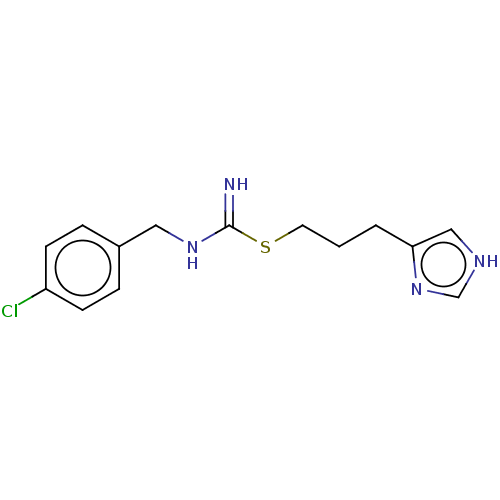 (CHEBI:64177 | Clobenpropit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494136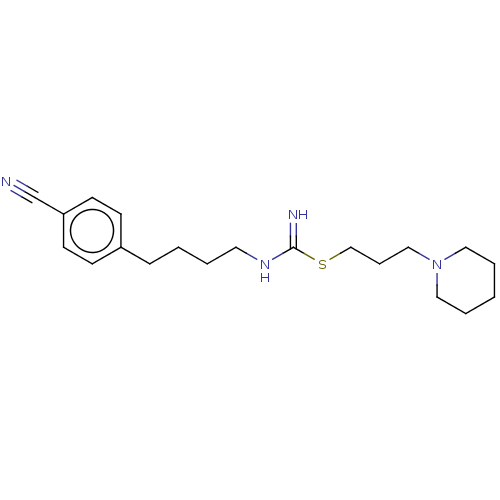 (CHEMBL2441945) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494132 (CHEMBL2441941) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494138 (CHEMBL2441946) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494137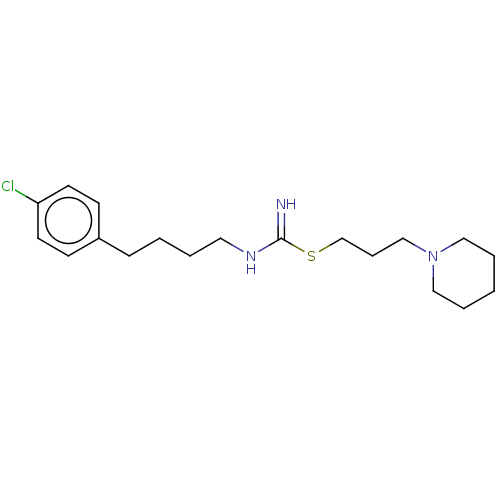 (CHEMBL2441942) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494141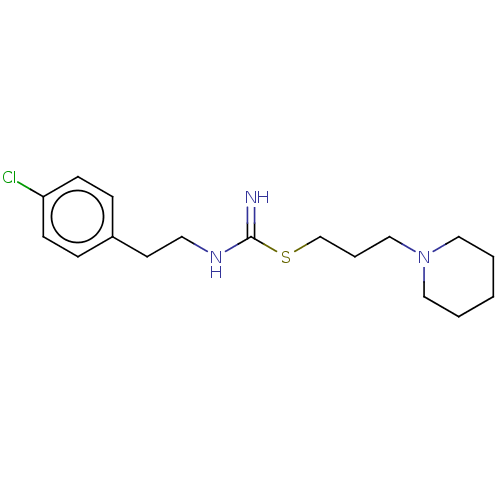 (CHEMBL2441940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494127 (CHEMBL2441944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494135 (CHEMBL2441933) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494131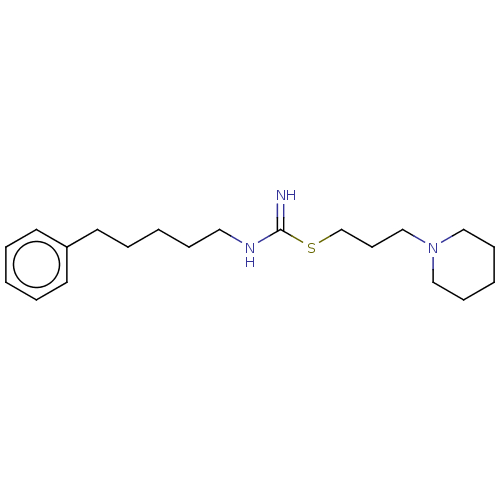 (CHEMBL2441943) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494139 (CHEMBL2441938) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494142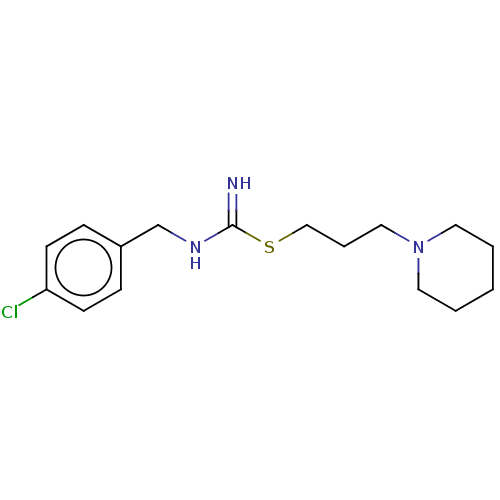 (CHEMBL2441935) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494129 (CHEMBL2441948) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494130 (CHEMBL2441947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494128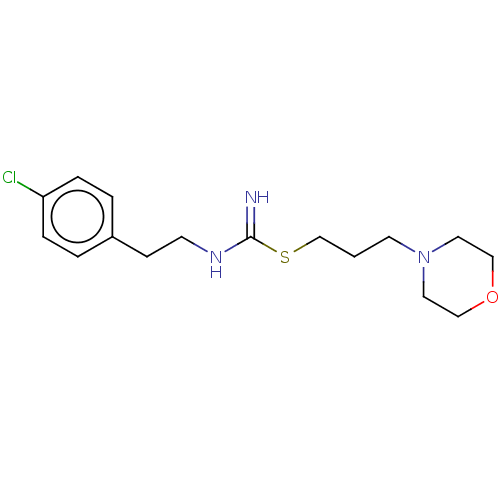 (CHEMBL2441939) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494126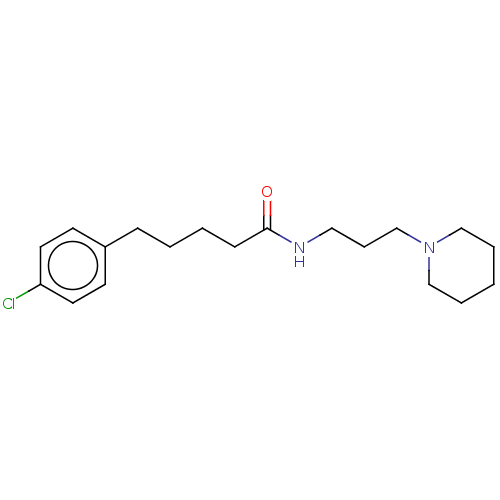 (CHEMBL2441949) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494134 (CHEMBL2441936) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494140 (CHEMBL2441934) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50360450 (CHEMBL1934618) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/Aichi/2/68 (H3N2) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50494133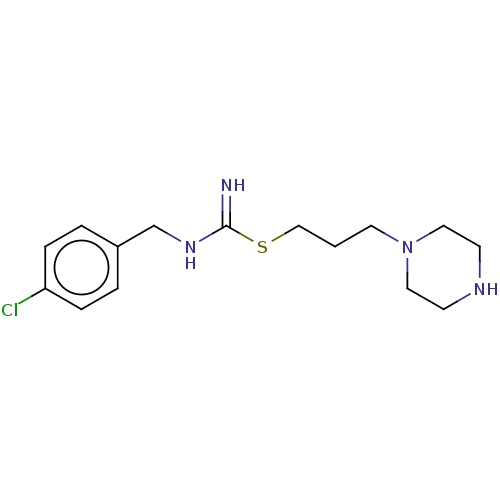 (CHEMBL2441937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... | Bioorg Med Chem Lett 23: 6415-20 (2013) Article DOI: 10.1016/j.bmcl.2013.09.052 BindingDB Entry DOI: 10.7270/Q2R78J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50360452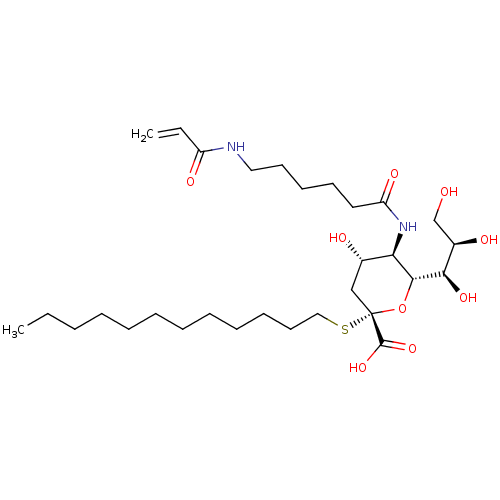 (CHEMBL1934620) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/Aichi/2/68 (H3N2) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Puerto Rico/8/1934 H1N...) | BDBM50360452 (CHEMBL1934620) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/PR/8/34 (H1N1) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Puerto Rico/8/1934 H1N...) | BDBM50360450 (CHEMBL1934618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/PR/8/34 (H1N1) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50360451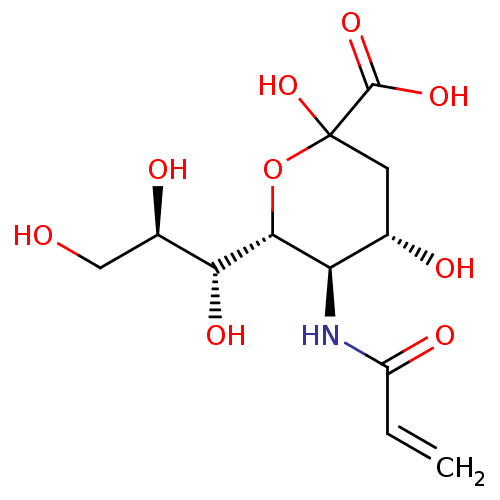 (CHEMBL1934619) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/Aichi/2/68 (H3N2) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Puerto Rico/8/1934 H1N...) | BDBM50360451 (CHEMBL1934619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/PR/8/34 (H1N1) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50295343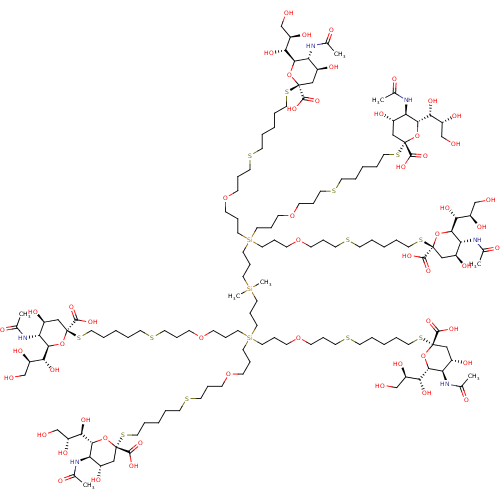 ((R,R,2S,2'S,4S,4'S,5R,5'R,6R,6'R)-2,2'-(14-(21-((2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of human influenza A/Memphis/1/71(H3N2) sialidase by fluorescence spectrophotometry | Bioorg Med Chem 17: 5451-64 (2009) Article DOI: 10.1016/j.bmc.2009.06.036 BindingDB Entry DOI: 10.7270/Q26973MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50295347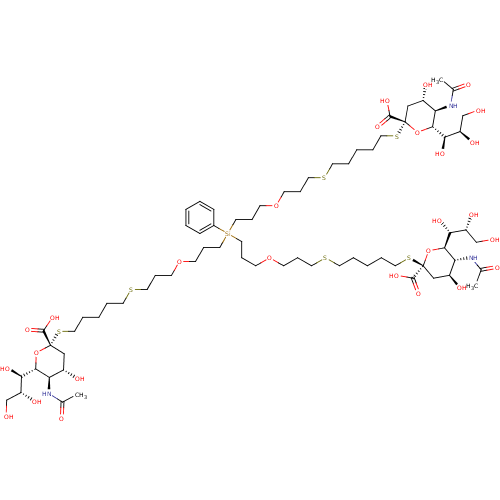 ((R,R,2S,2'S,4S,4'S,5R,5'R,6R,6'R)-2,2'-(14-(3-(3-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of human influenza A/Memphis/1/71(H3N2) sialidase by fluorescence spectrophotometry | Bioorg Med Chem 17: 5451-64 (2009) Article DOI: 10.1016/j.bmc.2009.06.036 BindingDB Entry DOI: 10.7270/Q26973MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50295345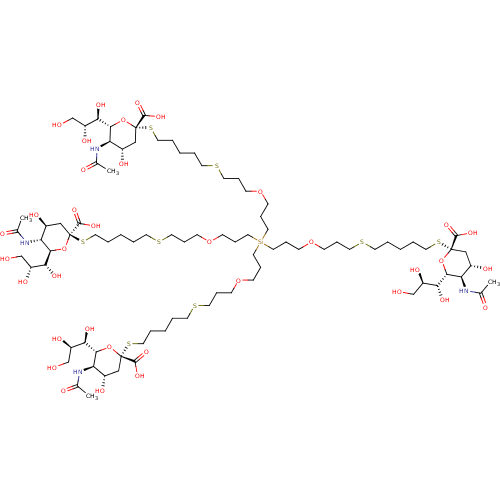 ((R,R,2S,2'S,4S,4'S,5R,5'R,6R,6'R)-2,2'-(14,14-bis(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of human influenza A/Memphis/1/71(H3N2) sialidase by fluorescence spectrophotometry | Bioorg Med Chem 17: 5451-64 (2009) Article DOI: 10.1016/j.bmc.2009.06.036 BindingDB Entry DOI: 10.7270/Q26973MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50295350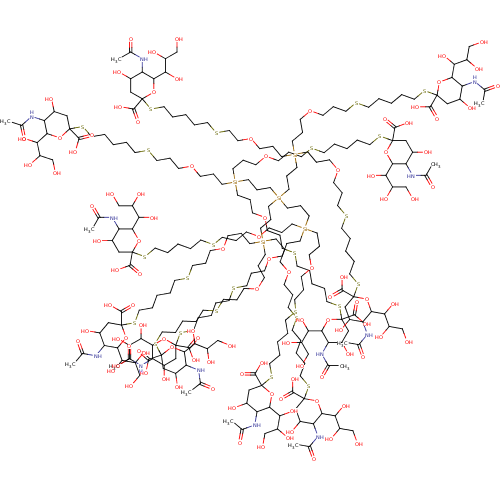 (Ball(1)12-ether-S-Neu5Ac12 | CHEMBL559353) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of human influenza A/Memphis/1/71(H3N2) sialidase by fluorescence spectrophotometry | Bioorg Med Chem 17: 5451-64 (2009) Article DOI: 10.1016/j.bmc.2009.06.036 BindingDB Entry DOI: 10.7270/Q26973MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50295348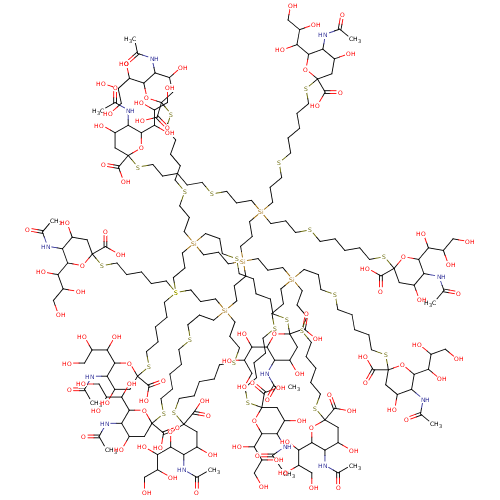 ((R,R,2S,2'S,4S,4'S,5R,5'R,6R,6'R)-2,2'-(10,10,18,1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of human influenza A/Memphis/1/71(H3N2) sialidase by fluorescence spectrophotometry | Bioorg Med Chem 17: 5451-64 (2009) Article DOI: 10.1016/j.bmc.2009.06.036 BindingDB Entry DOI: 10.7270/Q26973MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50295346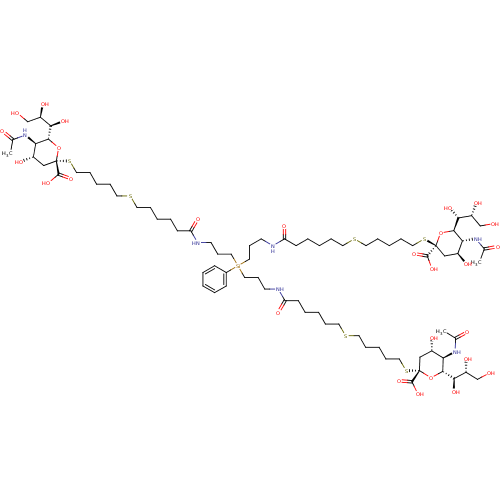 ((R,R,2S,2'S,4S,4'S,5R,5'R,6R,6'R)-2,2'-(17-(3-(6-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of human influenza A/Memphis/1/71(H3N2) sialidase by fluorescence spectrophotometry | Bioorg Med Chem 17: 5451-64 (2009) Article DOI: 10.1016/j.bmc.2009.06.036 BindingDB Entry DOI: 10.7270/Q26973MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50295349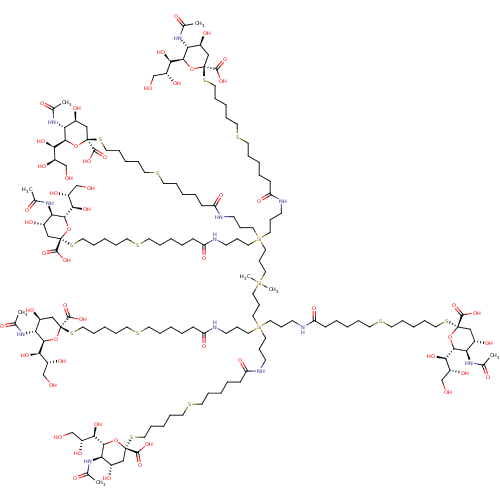 ((R,R,2S,2'S,4S,4'S,5R,5'R,6R,6'R)-2,2'-(17-(24-((2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of human influenza A/Memphis/1/71(H3N2) sialidase by fluorescence spectrophotometry | Bioorg Med Chem 17: 5451-64 (2009) Article DOI: 10.1016/j.bmc.2009.06.036 BindingDB Entry DOI: 10.7270/Q26973MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50295344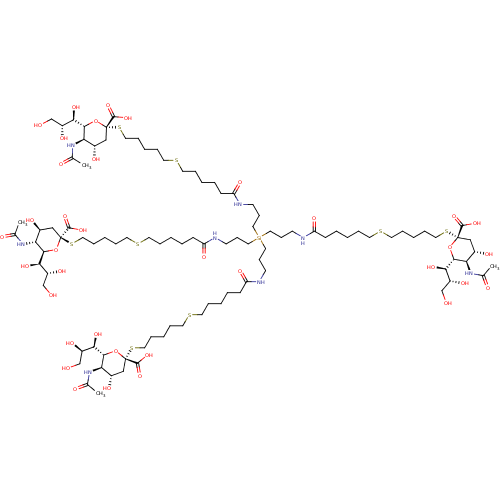 ((R,R,2S,2'S,4S,4'S,5R,5'R,6R,6'R)-2,2'-(17,17-bis(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of human influenza A/Memphis/1/71(H3N2) sialidase by fluorescence spectrophotometry | Bioorg Med Chem 17: 5451-64 (2009) Article DOI: 10.1016/j.bmc.2009.06.036 BindingDB Entry DOI: 10.7270/Q26973MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50360451 (CHEMBL1934619) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.08E+6 | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/Aichi/2/68 (H3N2) neuraminidase using 4-MU-Neu5Ac as substrate after 30 mins by fluorimetry | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50360450 (CHEMBL1934618) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.28E+5 | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/Aichi/2/68 (H3N2) neuraminidase using 4-MU-Neu5Ac as substrate after 30 mins by fluorimetry | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50360452 (CHEMBL1934620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.43E+5 | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/PR/8/34 (H1N1) neuraminidase using 4-MU-Neu5Ac as substrate after 30 mins by fluorimetry | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50360451 (CHEMBL1934619) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.48E+6 | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/PR/8/34 (H1N1) neuraminidase using 4-MU-Neu5Ac as substrate after 30 mins by fluorimetry | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50360450 (CHEMBL1934618) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.35E+6 | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/PR/8/34 (H1N1) neuraminidase using 4-MU-Neu5Ac as substrate after 30 mins by fluorimetry | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50360452 (CHEMBL1934620) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.40E+5 | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/Aichi/2/68 (H3N2) neuraminidase using 4-MU-Neu5Ac as substrate after 30 mins by fluorimetry | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||