 Found 68 hits with Last Name = 'havale' and Initial = 'sh'
Found 68 hits with Last Name = 'havale' and Initial = 'sh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50225074

((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |TLB:1:12:15:20.18.17,THB:13:14:17:22.12.21,13:12:15.14.20:17,21:12:15:20.18.17,21:18:15:22.13.12,19:18:15:22.13.12| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11694
((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...)Show InChI InChI=1S/C13H21N3O/c14-9-11-7-4-8-16(11)13(17)12(15)10-5-2-1-3-6-10/h10-12H,1-8,15H2/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276467

((1R,3R,5R)-2-((2S,4S)-4-(3-chloro-4-cyanophenylami...)Show SMILES Clc1cc(N[C@@H]2CN[C@@H](C2)C(=O)N2[C@@H]3C[C@@H]3C[C@@H]2C#N)ccc1C#N |r| Show InChI InChI=1S/C18H18ClN5O/c19-15-5-12(2-1-10(15)7-20)23-13-6-16(22-9-13)18(25)24-14(8-21)3-11-4-17(11)24/h1-2,5,11,13-14,16-17,22-23H,3-4,6,9H2/t11-,13-,14+,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173963
((2S,4S)-1-((R)-2-amino-3-(4-methoxybenzylsulfonyl)...)Show SMILES COc1ccc(CS(=O)(=O)C(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)cc1 |r| Show InChI InChI=1S/C18H24FN3O4S/c1-18(2,16(21)17(23)22-10-13(19)8-14(22)9-20)27(24,25)11-12-4-6-15(26-3)7-5-12/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of QPP |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM11463
(CHEMBL22310 | N-{4-[(1S)-1-amino-2-[(3S)-3-fluorop...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)cc1F)[C@H](N)C(=O)N1CC[C@H](F)C1 |r,wU:4.7,19.21,1.0,wD:1.1,26.28,(1.34,.92,;-.05,.24,;-.05,1.79,;-1.38,2.56,;-2.72,1.79,;-2.72,.24,;-1.38,-.53,;-4.05,2.56,;-5.38,1.78,;-6.47,.7,;-4.29,.7,;-6.72,2.55,;-6.72,4.1,;-8.05,4.87,;-9.38,4.1,;-10.72,4.87,;-9.38,2.55,;-8.05,1.78,;-8.05,.24,;1.29,-.53,;1.29,-2.07,;2.62,.24,;2.62,1.78,;3.95,-.53,;5.2,.38,;6.44,-.53,;5.97,-1.99,;6.87,-3.24,;4.43,-1.99,)| Show InChI InChI=1S/C18H24F3N3O3S/c19-12-3-6-16(15(21)9-12)28(26,27)23-14-4-1-11(2-5-14)17(22)18(25)24-8-7-13(20)10-24/h3,6,9,11,13-14,17,23H,1-2,4-5,7-8,10,22H2/t11-,13-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221967

((2S,3S)-3-amino-2-(4'-fluoro-biphenyl-4-yl)-4-((S)...)Show SMILES CN(C)C(=O)[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H25F2N3O2/c1-26(2)21(28)19(20(25)22(29)27-12-11-18(24)13-27)16-5-3-14(4-6-16)15-7-9-17(23)10-8-15/h3-10,18-20H,11-13,25H2,1-2H3/t18-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221977

((2S,3S)-2-amino-3-(4'-fluoro-biphenyl-4-yl)-1-((S)...)Show SMILES C[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H22F2N2O/c1-13(19(23)20(25)24-11-10-18(22)12-24)14-2-4-15(5-3-14)16-6-8-17(21)9-7-16/h2-9,13,18-19H,10-12,23H2,1H3/t13-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50166318

((S)-1-[(2S,4S)-4-(3-Chloro-4-cyano-phenylamino)-py...)Show SMILES Clc1cc(N[C@@H]2CN[C@@H](C2)C(=O)N2CCC[C@H]2C#N)ccc1C#N Show InChI InChI=1S/C17H18ClN5O/c18-15-6-12(4-3-11(15)8-19)22-13-7-16(21-10-13)17(24)23-5-1-2-14(23)9-20/h3-4,6,13-14,16,21-22H,1-2,5,7,10H2/t13-,14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in rat plasma |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50166319

(4-[(3S,5S)-5-((S)-2-Cyano-pyrrolidine-1-carbonyl)-...)Show SMILES O=C([C@@H]1C[C@@H](CN1)Nc1ccc(C#N)c(c1)C#N)N1CCC[C@H]1C#N Show InChI InChI=1S/C18H18N6O/c19-8-12-3-4-14(6-13(12)9-20)23-15-7-17(22-11-15)18(25)24-5-1-2-16(24)10-21/h3-4,6,15-17,22-23H,1-2,5,7,11H2/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50166318

((S)-1-[(2S,4S)-4-(3-Chloro-4-cyano-phenylamino)-py...)Show SMILES Clc1cc(N[C@@H]2CN[C@@H](C2)C(=O)N2CCC[C@H]2C#N)ccc1C#N Show InChI InChI=1S/C17H18ClN5O/c18-15-6-12(4-3-11(15)8-19)22-13-7-16(21-10-13)17(24)23-5-1-2-14(23)9-20/h3-4,6,13-14,16,21-22H,1-2,5,7,10H2/t13-,14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50166319

(4-[(3S,5S)-5-((S)-2-Cyano-pyrrolidine-1-carbonyl)-...)Show SMILES O=C([C@@H]1C[C@@H](CN1)Nc1ccc(C#N)c(c1)C#N)N1CCC[C@H]1C#N Show InChI InChI=1S/C18H18N6O/c19-8-12-3-4-14(6-13(12)9-20)23-15-7-17(22-11-15)18(25)24-5-1-2-16(24)10-21/h3-4,6,15-17,22-23H,1-2,5,7,11H2/t15-,16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in rat plasma |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276653
((S)-2-(4-(((S)-1-((R)-3-amino-4-(2,5-difluoropheny...)Show SMILES CC(C)[C@H](Oc1ccc(CNC(=O)[C@@H]2CCCN2C(=O)C[C@H](N)Cc2cc(F)ccc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C27H33F2N3O5/c1-16(2)25(27(35)36)37-21-8-5-17(6-9-21)15-31-26(34)23-4-3-11-32(23)24(33)14-20(30)13-18-12-19(28)7-10-22(18)29/h5-10,12,16,20,23,25H,3-4,11,13-15,30H2,1-2H3,(H,31,34)(H,35,36)/t20-,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276423

((2S,4S)-1-((2S,3S)-2-amino-3-methylpentanoyl)-4-fl...)Show SMILES CC[C@H](C)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C11H18FN3O/c1-3-7(2)10(14)11(16)15-6-8(12)4-9(15)5-13/h7-10H,3-4,6,14H2,1-2H3/t7-,8-,9-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276700
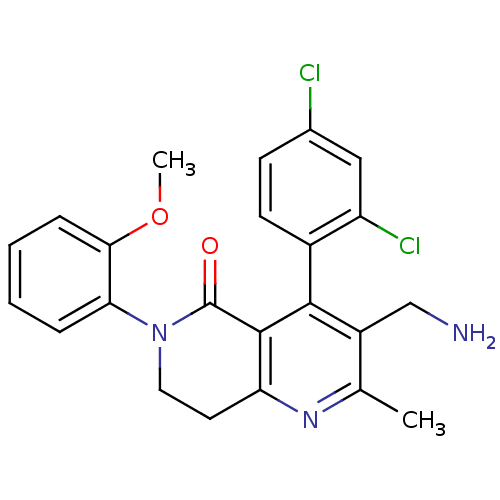
(3-(aminomethyl)-4-(2,4-dichlorophenyl)-6-(2-methox...)Show SMILES COc1ccccc1N1CCc2nc(C)c(CN)c(-c3ccc(Cl)cc3Cl)c2C1=O |(9.93,-9,;11.27,-8.23,;11.27,-6.69,;9.94,-5.92,;9.95,-4.37,;11.29,-3.61,;12.61,-4.39,;12.6,-5.93,;13.93,-6.71,;13.92,-8.24,;15.25,-9.02,;16.58,-8.26,;17.91,-9.03,;19.25,-8.26,;20.59,-9.02,;19.25,-6.7,;20.58,-5.93,;21.92,-6.69,;17.91,-5.94,;17.9,-4.4,;16.57,-3.64,;16.56,-2.1,;17.89,-1.32,;17.89,.22,;19.23,-2.09,;19.23,-3.63,;20.57,-4.4,;16.58,-6.72,;15.26,-5.95,;15.27,-4.41,)| Show InChI InChI=1S/C23H21Cl2N3O2/c1-13-16(12-26)21(15-8-7-14(24)11-17(15)25)22-18(27-13)9-10-28(23(22)29)19-5-3-4-6-20(19)30-2/h3-8,11H,9-10,12,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.28 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50276520

((2R,4R)-4-fluoro-1-(2-(4-methyl-1-(methylsulfonyl)...)Show SMILES CC1(CCN(CC1)S(C)(=O)=O)NCC(=O)N1C[C@H](F)C[C@@H]1C#N |r| Show InChI InChI=1S/C14H23FN4O3S/c1-14(3-5-18(6-4-14)23(2,21)22)17-9-13(20)19-10-11(15)7-12(19)8-16/h11-12,17H,3-7,9-10H2,1-2H3/t11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of mouse DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50221977

((2S,3S)-2-amino-3-(4'-fluoro-biphenyl-4-yl)-1-((S)...)Show SMILES C[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H22F2N2O/c1-13(19(23)20(25)24-11-10-18(22)12-24)14-2-4-15(5-3-14)16-6-8-17(21)9-7-16/h2-9,13,18-19H,10-12,23H2,1H3/t13-,18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of QPP |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50151005
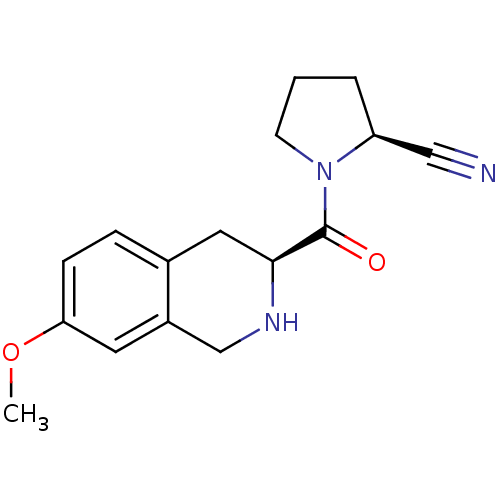
((S)-1-((S)-7-Methoxy-1,2,3,4-tetrahydro-isoquinoli...)Show SMILES COc1ccc2C[C@H](NCc2c1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H19N3O2/c1-21-14-5-4-11-8-15(18-10-12(11)7-14)16(20)19-6-2-3-13(19)9-17/h4-5,7,13,15,18H,2-3,6,8,10H2,1H3/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276598
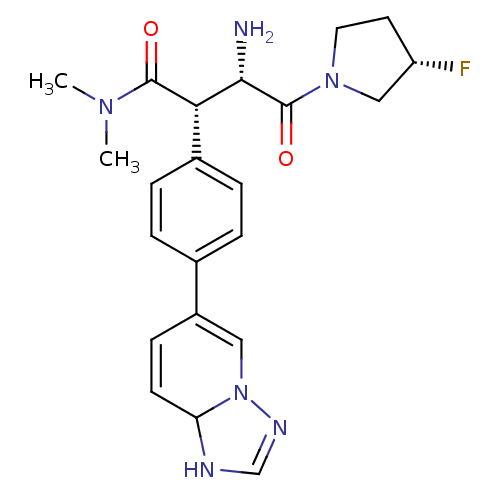
((2S,3S)-3-amino-2-(4-(1,8a-dihydro-[1,2,4]triazolo...)Show SMILES CN(C)C(=O)[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)C1=CN2N=CNC2C=C1 |r,c:27,32,t:24| Show InChI InChI=1S/C22H27FN6O2/c1-27(2)21(30)19(20(24)22(31)28-10-9-17(23)12-28)15-5-3-14(4-6-15)16-7-8-18-25-13-26-29(18)11-16/h3-8,11,13,17-20H,9-10,12,24H2,1-2H3,(H,25,26)/t17-,18?,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242513
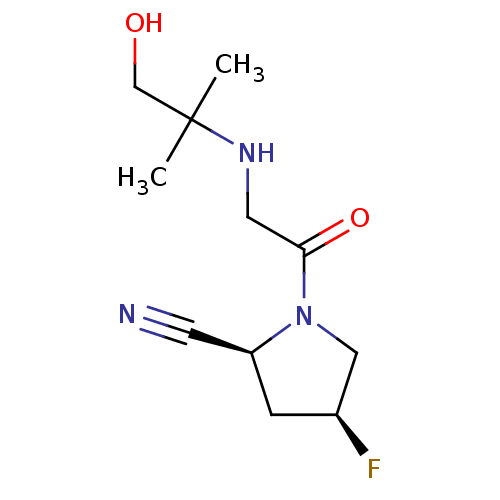
((2S,4S)-4-Fluoro-1-[N-(2-hydroxy-1,1-dimethylethyl...)Show InChI InChI=1S/C11H18FN3O2/c1-11(2,7-16)14-5-10(17)15-6-8(12)3-9(15)4-13/h8-9,14,16H,3,5-7H2,1-2H3/t8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50205136

((2S)-1-{[(2S,4R)-4-(3-hydroxy-2,6-dimethylphenyl)-...)Show SMILES Cc1ccc(O)c(C)c1[C@@H]1CN[C@@H](C1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H23N3O2/c1-11-5-6-16(22)12(2)17(11)13-8-15(20-10-13)18(23)21-7-3-4-14(21)9-19/h5-6,13-15,20,22H,3-4,7-8,10H2,1-2H3/t13-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276520

((2R,4R)-4-fluoro-1-(2-(4-methyl-1-(methylsulfonyl)...)Show SMILES CC1(CCN(CC1)S(C)(=O)=O)NCC(=O)N1C[C@H](F)C[C@@H]1C#N |r| Show InChI InChI=1S/C14H23FN4O3S/c1-14(3-5-18(6-4-14)23(2,21)22)17-9-13(20)19-10-11(15)7-12(19)8-16/h11-12,17H,3-7,9-10H2,1-2H3/t11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276652

(2-(adamantan-1-ylamino)-1-[(2R)-2-(dihydroxyborany...)Show SMILES OB(O)[C@@H]1CCCN1C(=O)CNC12CC3CC(CC(C3)C1)C2 |r,TLB:11:12:15.14.19:17,THB:11:12:15:19.18.17,13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12| Show InChI InChI=1S/C16H27BN2O3/c20-15(19-3-1-2-14(19)17(21)22)10-18-16-7-11-4-12(8-16)6-13(5-11)9-16/h11-14,18,21-22H,1-10H2/t11?,12?,13?,14-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276654

(3-methyl-7-(3-methylbut-2-enyl)-8-((3aS,6aR)-octah...)Show SMILES [#6]-[#7]-1-[#6]-2-[#7]=[#6](-[#6]-3-[#6]-[#6]-[#6@H]-4-[#7]-[#6]-[#6]-[#6@@H]-3-4)-[#7](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6]-2-[#6](=O)-[#7](-[#6]-[#6](=O)-c2ccccc2)-[#6]-1=O |r,t:3| Show InChI InChI=1S/C26H33N5O3/c1-16(2)12-14-30-22-24(28-23(30)19-9-10-20-18(19)11-13-27-20)29(3)26(34)31(25(22)33)15-21(32)17-7-5-4-6-8-17/h4-8,12,18-20,22,24,27H,9-11,13-15H2,1-3H3/t18-,19?,20+,22?,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11113

(6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...)Show InChI InChI=1S/C15H18N6O/c16-8-12-3-4-14(20-10-12)19-6-5-18-11-15(22)21-7-1-2-13(21)9-17/h3-4,10,13,18H,1-2,5-7,11H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276519

((2R,4R)-1-((S)-2-amino-3,3-bis(4-fluorophenyl)prop...)Show SMILES N[C@@H](C(c1ccc(F)cc1)c1ccc(F)cc1)C(=O)N1C[C@H](F)C[C@@H]1C#N |r| Show InChI InChI=1S/C20H18F3N3O/c21-14-5-1-12(2-6-14)18(13-3-7-15(22)8-4-13)19(25)20(27)26-11-16(23)9-17(26)10-24/h1-8,16-19H,9,11,25H2/t16-,17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50221967

((2S,3S)-3-amino-2-(4'-fluoro-biphenyl-4-yl)-4-((S)...)Show SMILES CN(C)C(=O)[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H25F2N3O2/c1-26(2)21(28)19(20(25)22(29)27-12-11-18(24)13-27)16-5-3-14(4-6-16)15-7-9-17(23)10-8-15/h3-10,18-20H,11-13,25H2,1-2H3/t18-,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of QPP |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50198863

(6-((3S,5S)-5-(thiazolidine-3-carbonyl)pyrrolidin-3...)Show SMILES O=C([C@@H]1C[C@@H](CN1)Nc1ccc(cn1)C#N)N1CCSC1 |r| Show InChI InChI=1S/C14H17N5OS/c15-6-10-1-2-13(17-7-10)18-11-5-12(16-8-11)14(20)19-3-4-21-9-19/h1-2,7,11-12,16H,3-5,8-9H2,(H,17,18)/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50198863

(6-((3S,5S)-5-(thiazolidine-3-carbonyl)pyrrolidin-3...)Show SMILES O=C([C@@H]1C[C@@H](CN1)Nc1ccc(cn1)C#N)N1CCSC1 |r| Show InChI InChI=1S/C14H17N5OS/c15-6-10-1-2-13(17-7-10)18-11-5-12(16-8-11)14(20)19-3-4-21-9-19/h1-2,7,11-12,16H,3-5,8-9H2,(H,17,18)/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in rat plasma |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11695

((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1CCC[C@H]1C#N |r,TLB:9:8:6:3.2.4,4:3:10:7.6.5,4:5:10:3.2.9,THB:9:3:6:10.7.8,11:8:6:3.2.4| Show InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50276652

(2-(adamantan-1-ylamino)-1-[(2R)-2-(dihydroxyborany...)Show SMILES OB(O)[C@@H]1CCCN1C(=O)CNC12CC3CC(CC(C3)C1)C2 |r,TLB:11:12:15.14.19:17,THB:11:12:15:19.18.17,13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12| Show InChI InChI=1S/C16H27BN2O3/c20-15(19-3-1-2-14(19)17(21)22)10-18-16-7-11-4-12(8-16)6-13(5-11)9-16/h11-14,18,21-22H,1-10H2/t11?,12?,13?,14-,16?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of FAP |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11463

(CHEMBL22310 | N-{4-[(1S)-1-amino-2-[(3S)-3-fluorop...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)cc1F)[C@H](N)C(=O)N1CC[C@H](F)C1 |r,wU:4.7,19.21,1.0,wD:1.1,26.28,(1.34,.92,;-.05,.24,;-.05,1.79,;-1.38,2.56,;-2.72,1.79,;-2.72,.24,;-1.38,-.53,;-4.05,2.56,;-5.38,1.78,;-6.47,.7,;-4.29,.7,;-6.72,2.55,;-6.72,4.1,;-8.05,4.87,;-9.38,4.1,;-10.72,4.87,;-9.38,2.55,;-8.05,1.78,;-8.05,.24,;1.29,-.53,;1.29,-2.07,;2.62,.24,;2.62,1.78,;3.95,-.53,;5.2,.38,;6.44,-.53,;5.97,-1.99,;6.87,-3.24,;4.43,-1.99,)| Show InChI InChI=1S/C18H24F3N3O3S/c19-12-3-6-16(15(21)9-12)28(26,27)23-14-4-1-11(2-5-14)17(22)18(25)24-8-7-13(20)10-24/h3,6,9,11,13-14,17,23H,1-2,4-5,7-8,10,22H2/t11-,13-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50221967

((2S,3S)-3-amino-2-(4'-fluoro-biphenyl-4-yl)-4-((S)...)Show SMILES CN(C)C(=O)[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H25F2N3O2/c1-26(2)21(28)19(20(25)22(29)27-12-11-18(24)13-27)16-5-3-14(4-6-16)15-7-9-17(23)10-8-15/h3-10,18-20H,11-13,25H2,1-2H3/t18-,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50221977

((2S,3S)-2-amino-3-(4'-fluoro-biphenyl-4-yl)-1-((S)...)Show SMILES C[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H22F2N2O/c1-13(19(23)20(25)24-11-10-18(22)12-24)14-2-4-15(5-3-14)16-6-8-17(21)9-7-16/h2-9,13,18-19H,10-12,23H2,1H3/t13-,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50137251

(CHEMBL264738 | N-(4-((S)-1-amino-2-oxo-2-(pyrrolid...)Show SMILES N[C@@H](C1CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)cc1F)C(=O)N1CCCC1 |r,wU:1.0,wD:5.8,(27.95,-4.24,;28,-2.7,;26.69,-1.89,;25.33,-2.62,;24.02,-1.8,;24.08,-.26,;25.44,.46,;26.74,-.35,;22.77,.55,;21.41,-.17,;20.67,1.18,;22.15,-1.52,;20.07,-.93,;18.74,-.13,;17.4,-.89,;17.38,-2.43,;16.04,-3.19,;18.72,-3.22,;20.05,-2.46,;21.38,-3.24,;29.35,-1.97,;29.4,-.43,;30.66,-2.78,;31.13,-4.25,;32.67,-4.25,;33.15,-2.78,;31.9,-1.87,)| Show InChI InChI=1S/C18H25F2N3O3S/c19-13-5-8-16(15(20)11-13)27(25,26)22-14-6-3-12(4-7-14)17(21)18(24)23-9-1-2-10-23/h5,8,11-12,14,17,22H,1-4,6-7,9-10,21H2/t12?,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50221977

((2S,3S)-2-amino-3-(4'-fluoro-biphenyl-4-yl)-1-((S)...)Show SMILES C[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H22F2N2O/c1-13(19(23)20(25)24-11-10-18(22)12-24)14-2-4-15(5-3-14)16-6-8-17(21)9-7-16/h2-9,13,18-19H,10-12,23H2,1H3/t13-,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50221967

((2S,3S)-3-amino-2-(4'-fluoro-biphenyl-4-yl)-4-((S)...)Show SMILES CN(C)C(=O)[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H25F2N3O2/c1-26(2)21(28)19(20(25)22(29)27-12-11-18(24)13-27)16-5-3-14(4-6-16)15-7-9-17(23)10-8-15/h3-10,18-20H,11-13,25H2,1-2H3/t18-,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50276520

((2R,4R)-4-fluoro-1-(2-(4-methyl-1-(methylsulfonyl)...)Show SMILES CC1(CCN(CC1)S(C)(=O)=O)NCC(=O)N1C[C@H](F)C[C@@H]1C#N |r| Show InChI InChI=1S/C14H23FN4O3S/c1-14(3-5-18(6-4-14)23(2,21)22)17-9-13(20)19-10-11(15)7-12(19)8-16/h11-12,17H,3-7,9-10H2,1-2H3/t11-,12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50151843

((R)-3-Amino-1-((R)-2-benzyl-piperazin-1-yl)-4-(2-f...)Show SMILES N[C@@H](CC(=O)N1CCNC[C@H]1Cc1ccccc1)Cc1ccccc1F |r| Show InChI InChI=1S/C21H26FN3O/c22-20-9-5-4-8-17(20)13-18(23)14-21(26)25-11-10-24-15-19(25)12-16-6-2-1-3-7-16/h1-9,18-19,24H,10-15,23H2/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50150854
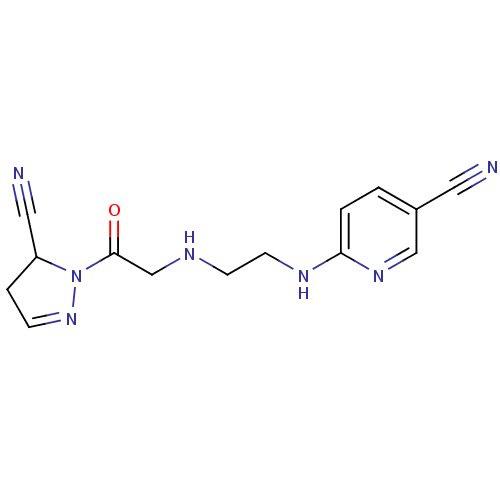
(6-(2-(2-(5-cyano-4,5-dihydro-1H-pyrazol-1-yl)-2-ox...)Show InChI InChI=1S/C14H15N7O/c15-7-11-1-2-13(19-9-11)18-6-5-17-10-14(22)21-12(8-16)3-4-20-21/h1-2,4,9,12,17H,3,5-6,10H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276465

((3R)-3-amino-4-((S)-2-cyanopyrrolidin-1-yl)-N-(2,2...)Show SMILES CC(C)(C)C(NC(=O)C[C@@H](N)C(=O)N1CCC[C@H]1C#N)c1ccccc1 |r| Show InChI InChI=1S/C20H28N4O2/c1-20(2,3)18(14-8-5-4-6-9-14)23-17(25)12-16(22)19(26)24-11-7-10-15(24)13-21/h4-6,8-9,15-16,18H,7,10-12,22H2,1-3H3,(H,23,25)/t15-,16+,18?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12191
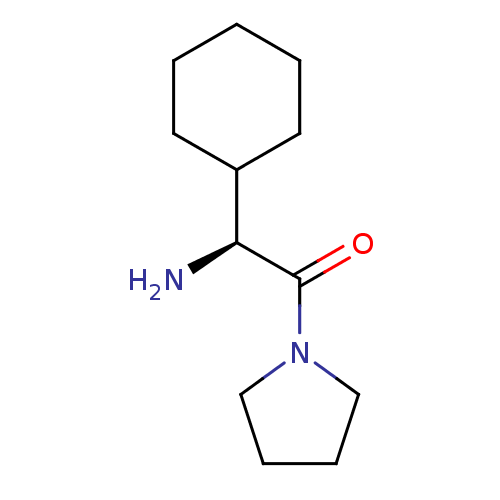
((2S)-2-amino-2-cyclohexyl-1-(pyrrolidin-1-yl)ethan...)Show InChI InChI=1S/C12H22N2O/c13-11(10-6-2-1-3-7-10)12(15)14-8-4-5-9-14/h10-11H,1-9,13H2/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50237207
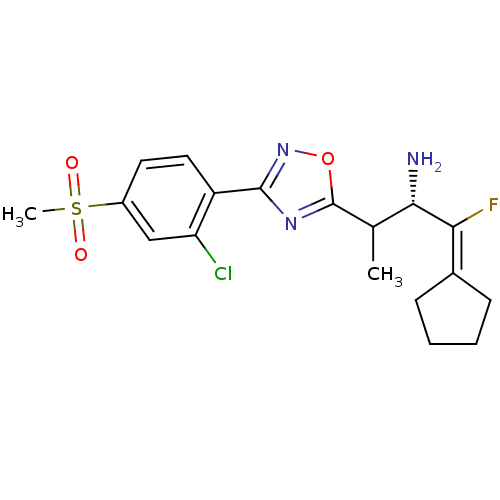
((2S)-3-(3-(2-chloro-4-(methylsulfonyl)phenyl)-1,2,...)Show SMILES [#6]-[#6](-[#6@H](-[#7])-[#6](\F)=[#6]-1/[#6]-[#6]-[#6]-[#6]-1)-c1nc(no1)-c1ccc(cc1Cl)S([#6])(=O)=O |r| Show InChI InChI=1S/C18H21ClFN3O3S/c1-10(16(21)15(20)11-5-3-4-6-11)18-22-17(23-26-18)13-8-7-12(9-14(13)19)27(2,24)25/h7-10,16H,3-6,21H2,1-2H3/t10?,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of QPP |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50276652

(2-(adamantan-1-ylamino)-1-[(2R)-2-(dihydroxyborany...)Show SMILES OB(O)[C@@H]1CCCN1C(=O)CNC12CC3CC(CC(C3)C1)C2 |r,TLB:11:12:15.14.19:17,THB:11:12:15:19.18.17,13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12| Show InChI InChI=1S/C16H27BN2O3/c20-15(19-3-1-2-14(19)17(21)22)10-18-16-7-11-4-12(8-16)6-13(5-11)9-16/h11-14,18,21-22H,1-10H2/t11?,12?,13?,14-,16?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP7 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11464
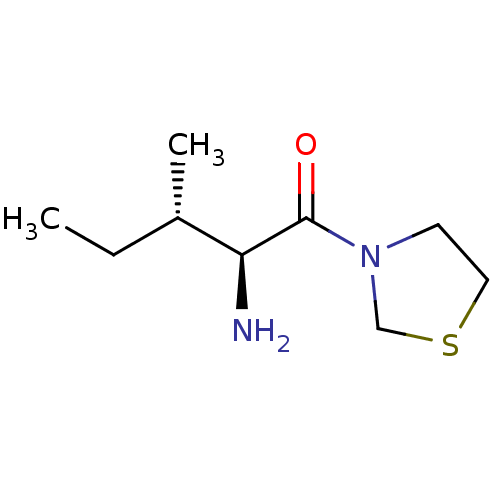
((2S,3S)-2-amino-3-methyl-1-(1,3-thiazolidin-3-yl)p...)Show InChI InChI=1S/C9H18N2OS/c1-3-7(2)8(10)9(12)11-4-5-13-6-11/h7-8H,3-6,10H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50150854

(6-(2-(2-(5-cyano-4,5-dihydro-1H-pyrazol-1-yl)-2-ox...)Show InChI InChI=1S/C14H15N7O/c15-7-11-1-2-13(19-9-11)18-6-5-17-10-14(22)21-12(8-16)3-4-20-21/h1-2,4,9,12,17H,3,5-6,10H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney DPP4 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50150854

(6-(2-(2-(5-cyano-4,5-dihydro-1H-pyrazol-1-yl)-2-ox...)Show InChI InChI=1S/C14H15N7O/c15-7-11-1-2-13(19-9-11)18-6-5-17-10-14(22)21-12(8-16)3-4-20-21/h1-2,4,9,12,17H,3,5-6,10H2,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in rat plasma |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM11463

(CHEMBL22310 | N-{4-[(1S)-1-amino-2-[(3S)-3-fluorop...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)cc1F)[C@H](N)C(=O)N1CC[C@H](F)C1 |r,wU:4.7,19.21,1.0,wD:1.1,26.28,(1.34,.92,;-.05,.24,;-.05,1.79,;-1.38,2.56,;-2.72,1.79,;-2.72,.24,;-1.38,-.53,;-4.05,2.56,;-5.38,1.78,;-6.47,.7,;-4.29,.7,;-6.72,2.55,;-6.72,4.1,;-8.05,4.87,;-9.38,4.1,;-10.72,4.87,;-9.38,2.55,;-8.05,1.78,;-8.05,.24,;1.29,-.53,;1.29,-2.07,;2.62,.24,;2.62,1.78,;3.95,-.53,;5.2,.38,;6.44,-.53,;5.97,-1.99,;6.87,-3.24,;4.43,-1.99,)| Show InChI InChI=1S/C18H24F3N3O3S/c19-12-3-6-16(15(21)9-12)28(26,27)23-14-4-1-11(2-5-14)17(22)18(25)24-8-7-13(20)10-24/h3,6,9,11,13-14,17,23H,1-2,4-5,7-8,10,22H2/t11-,13-,14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 993 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50221977

((2S,3S)-2-amino-3-(4'-fluoro-biphenyl-4-yl)-1-((S)...)Show SMILES C[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H22F2N2O/c1-13(19(23)20(25)24-11-10-18(22)12-24)14-2-4-15(5-3-14)16-6-8-17(21)9-7-16/h2-9,13,18-19H,10-12,23H2,1H3/t13-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data