 Found 450 hits with Last Name = 'haydar' and Initial = 's'
Found 450 hits with Last Name = 'haydar' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308167

((3R)-N,N-Dimethyl-1-[3-(1-naphthylsulfonyl)-1H-ind...)Show SMILES CN(C)[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2S/c1-26(2)18-12-13-27(15-18)17-10-11-21-20(14-17)23(25-24-21)30(28,29)22-9-5-7-16-6-3-4-8-19(16)22/h3-11,14,18H,12-13,15H2,1-2H3,(H,24,25)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303164
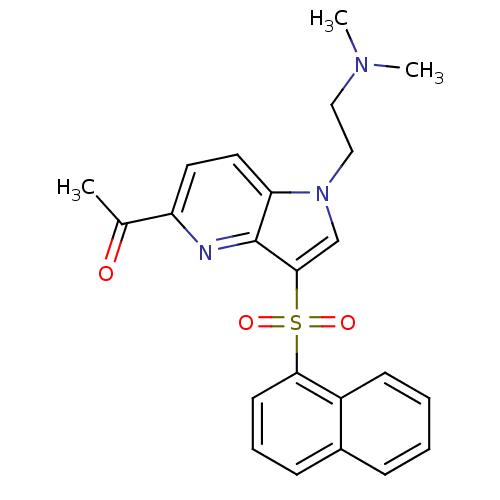
(1-(1-(2-(dimethylamino)ethyl)-3-(naphthalen-1-ylsu...)Show SMILES CN(C)CCn1cc(c2nc(ccc12)C(C)=O)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C23H23N3O3S/c1-16(27)19-11-12-20-23(24-19)22(15-26(20)14-13-25(2)3)30(28,29)21-10-6-8-17-7-4-5-9-18(17)21/h4-12,15H,13-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308181
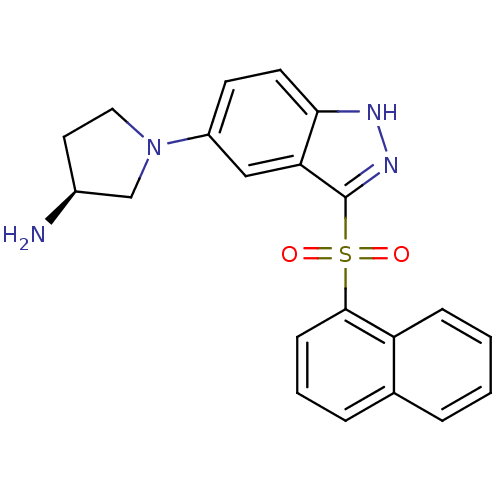
((3S)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308183
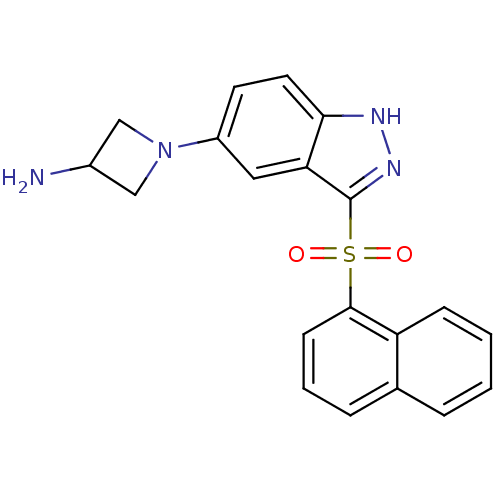
(1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]azetidin...)Show SMILES NC1CN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H18N4O2S/c21-14-11-24(12-14)15-8-9-18-17(10-15)20(23-22-18)27(25,26)19-7-3-5-13-4-1-2-6-16(13)19/h1-10,14H,11-12,21H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308177

(1-{3-[(4-Methyl-1-naphthyl)sulfonyl]-1H-indazol-5-...)Show SMILES Cc1ccc(c2ccccc12)S(=O)(=O)c1n[nH]c2ccc(cc12)N1CCC(N)CC1 Show InChI InChI=1S/C23H24N4O2S/c1-15-6-9-22(19-5-3-2-4-18(15)19)30(28,29)23-20-14-17(7-8-21(20)25-26-23)27-12-10-16(24)11-13-27/h2-9,14,16H,10-13,24H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303163

(1-(3-(4-bromophenylsulfonyl)-1-(2-(dimethylamino)e...)Show SMILES CN(C)CCn1cc(c2nc(ccc12)C(C)=O)S(=O)(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C19H20BrN3O3S/c1-13(24)16-8-9-17-19(21-16)18(12-23(17)11-10-22(2)3)27(25,26)15-6-4-14(20)5-7-15/h4-9,12H,10-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308175

(1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]piperidi...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c23-16-10-12-26(13-11-16)17-8-9-20-19(14-17)22(25-24-20)29(27,28)21-7-3-5-15-4-1-2-6-18(15)21/h1-9,14,16H,10-13,23H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308178
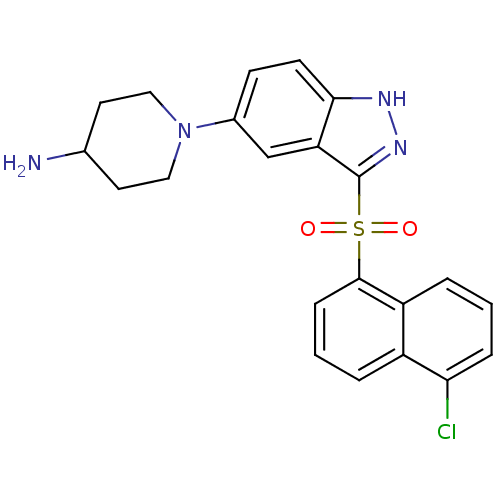
(1-{3-[(5-Chloro-1-naphthyl)sulfonyl]-1H-indazol-5-...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2c(Cl)cccc12 Show InChI InChI=1S/C22H21ClN4O2S/c23-19-5-1-4-17-16(19)3-2-6-21(17)30(28,29)22-18-13-15(7-8-20(18)25-26-22)27-11-9-14(24)10-12-27/h1-8,13-14H,9-12,24H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50300828
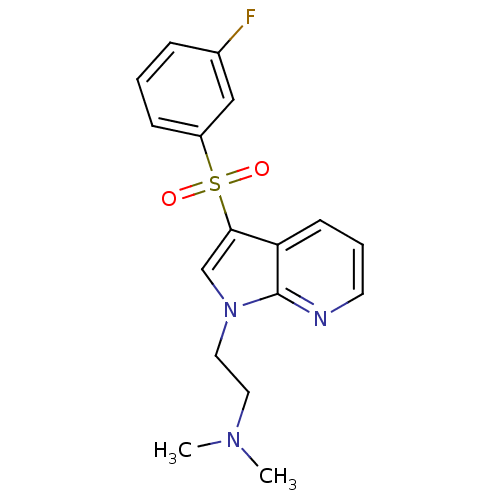
(2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...)Show InChI InChI=1S/C17H18FN3O2S/c1-20(2)9-10-21-12-16(15-7-4-8-19-17(15)21)24(22,23)14-6-3-5-13(18)11-14/h3-8,11-12H,9-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308179

(1-(3-(3-chlorophenylsulfonyl)-1H-indazol-5-yl)pipe...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H19ClN4O2S/c19-12-2-1-3-15(10-12)26(24,25)18-16-11-14(4-5-17(16)21-22-18)23-8-6-13(20)7-9-23/h1-5,10-11,13H,6-9,20H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303162
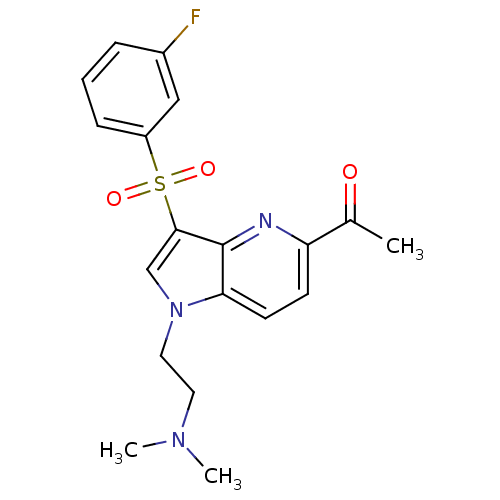
(1-(1-(2-(dimethylamino)ethyl)-3-(3-fluorophenylsul...)Show SMILES CN(C)CCn1cc(c2nc(ccc12)C(C)=O)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C19H20FN3O3S/c1-13(24)16-7-8-17-19(21-16)18(12-23(17)10-9-22(2)3)27(25,26)15-6-4-5-14(20)11-15/h4-8,11-12H,9-10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308180
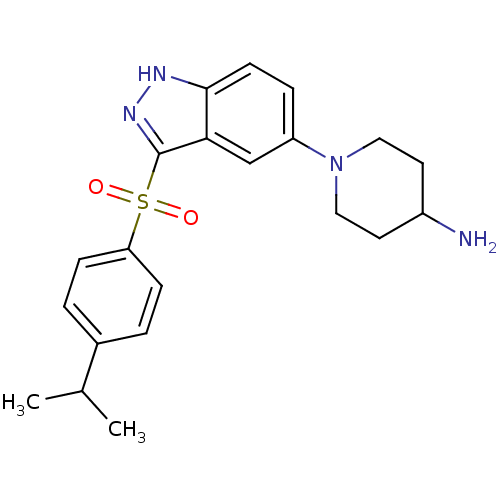
(1-{3-[(4-Isopropylphenyl)sulfonyl]-1H-indazol-5-yl...)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(cc12)N1CCC(N)CC1 Show InChI InChI=1S/C21H26N4O2S/c1-14(2)15-3-6-18(7-4-15)28(26,27)21-19-13-17(5-8-20(19)23-24-21)25-11-9-16(22)10-12-25/h3-8,13-14,16H,9-12,22H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308170
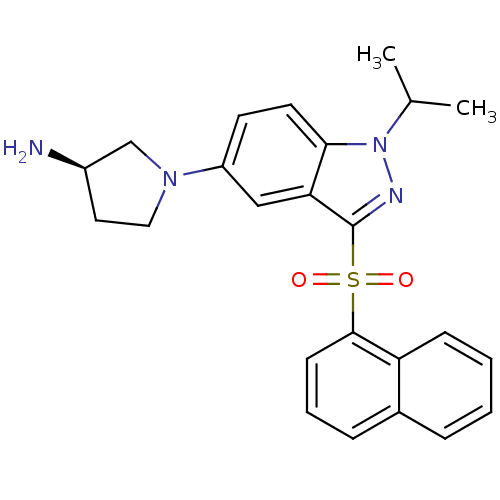
((3R)-1-[1-(1-Methylethyl)-3-(naphthalen-1-ylsulfon...)Show SMILES CC(C)n1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N4O2S/c1-16(2)28-22-11-10-19(27-13-12-18(25)15-27)14-21(22)24(26-28)31(29,30)23-9-5-7-17-6-3-4-8-20(17)23/h3-11,14,16,18H,12-13,15,25H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308176
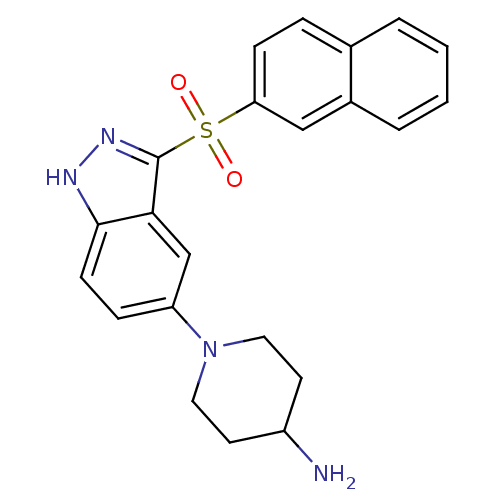
(1-[3-(2-Naphthylsulfonyl)-1H-indazol-5-yl]piperidi...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H22N4O2S/c23-17-9-11-26(12-10-17)18-6-8-21-20(14-18)22(25-24-21)29(27,28)19-7-5-15-3-1-2-4-16(15)13-19/h1-8,13-14,17H,9-12,23H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308181

((3S)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308169

((3R)-1-[1-Methyl-3-(naphthalen-1-ylsulfonyl)-1H-in...)Show SMILES Cn1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C22H22N4O2S/c1-25-20-10-9-17(26-12-11-16(23)14-26)13-19(20)22(24-25)29(27,28)21-8-4-6-15-5-2-3-7-18(15)21/h2-10,13,16H,11-12,14,23H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50300832
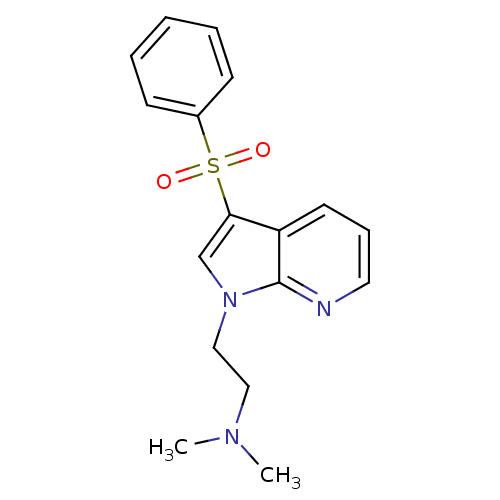
(CHEMBL578826 | N,N-Dimethyl-N-{2-[3-(phenylsulfony...)Show InChI InChI=1S/C17H19N3O2S/c1-19(2)11-12-20-13-16(15-9-6-10-18-17(15)20)23(21,22)14-7-4-3-5-8-14/h3-10,13H,11-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308168
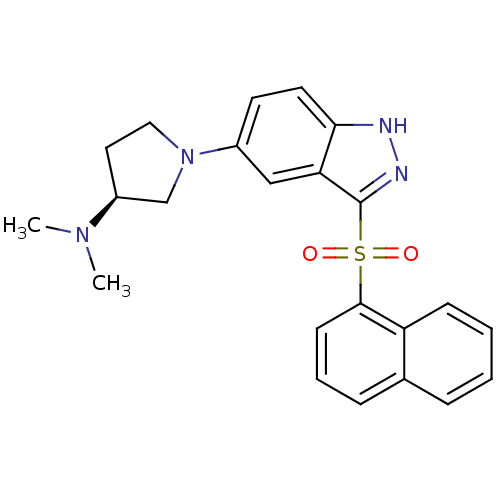
((3S)-N,N-Dimethyl-1-[3-(1-naphthylsulfonyl)-1H-ind...)Show SMILES CN(C)[C@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2S/c1-26(2)18-12-13-27(15-18)17-10-11-21-20(14-17)23(25-24-21)30(28,29)22-9-5-7-16-6-3-4-8-19(16)22/h3-11,14,18H,12-13,15H2,1-2H3,(H,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303166

(2-(3-(3-chlorophenylsulfonyl)-1H-pyrrolo[3,2-b]pyr...)Show InChI InChI=1S/C15H14ClN3O2S/c16-11-3-1-4-12(9-11)22(20,21)14-10-19(8-6-17)13-5-2-7-18-15(13)14/h1-5,7,9-10H,6,8,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308171

((3R)-1-[1-(2-Methylpropyl)-3-(naphthalen-1-ylsulfo...)Show SMILES CC(C)Cn1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C25H28N4O2S/c1-17(2)15-29-23-11-10-20(28-13-12-19(26)16-28)14-22(23)25(27-29)32(30,31)24-9-5-7-18-6-3-4-8-21(18)24/h3-11,14,17,19H,12-13,15-16,26H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303168
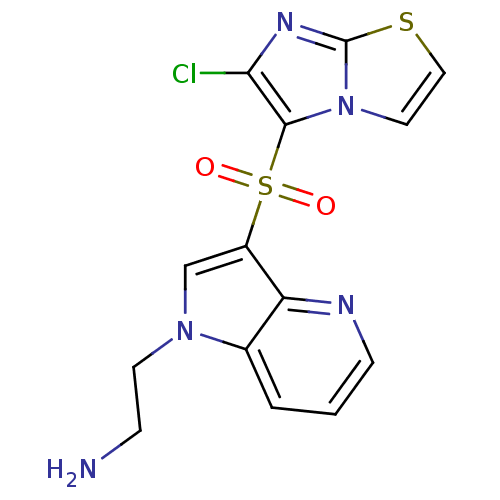
(2-(3-(6-chloroimidazo[2,1-b]thiazol-5-ylsulfonyl)-...)Show SMILES NCCn1cc(c2ncccc12)S(=O)(=O)c1c(Cl)nc2sccn12 Show InChI InChI=1S/C14H12ClN5O2S2/c15-12-13(20-6-7-23-14(20)18-12)24(21,22)10-8-19(5-3-16)9-2-1-4-17-11(9)10/h1-2,4,6-8H,3,5,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50319673
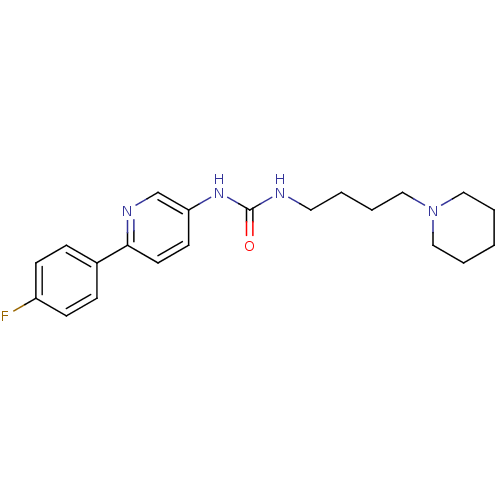
(1-[6-(4-Fluorophenyl)pyridin-3-yl]-3-(4-piperidin-...)Show InChI InChI=1S/C21H27FN4O/c22-18-8-6-17(7-9-18)20-11-10-19(16-24-20)25-21(27)23-12-2-5-15-26-13-3-1-4-14-26/h6-11,16H,1-5,12-15H2,(H2,23,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siena Biotech SpA
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha7 nAChR expressed in rat GH4C1 cells |
J Med Chem 53: 4379-89 (2010)
Article DOI: 10.1021/jm901692q
BindingDB Entry DOI: 10.7270/Q2S46S4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303167

(2-(3-(m-tolylsulfonyl)-1H-pyrrolo[3,2-b]pyridin-1-...)Show InChI InChI=1S/C16H17N3O2S/c1-12-4-2-5-13(10-12)22(20,21)15-11-19(9-7-17)14-6-3-8-18-16(14)15/h2-6,8,10-11H,7,9,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308173

((3R)-1-[2-(1-Methylethyl)-3-(naphthalen-1-ylsulfon...)Show SMILES CC(C)n1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C24H26N4O2S/c1-16(2)28-24(31(29,30)23-9-5-7-17-6-3-4-8-20(17)23)21-14-19(10-11-22(21)26-28)27-13-12-18(25)15-27/h3-11,14,16,18H,12-13,15,25H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308172
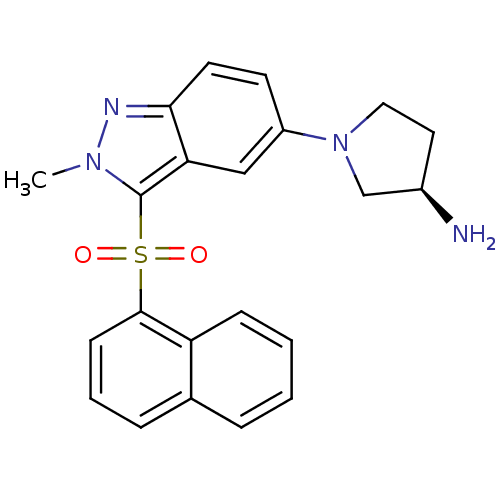
((3R)-1-[2-Methyl-3-(naphthalen-1-ylsulfonyl)-2H-in...)Show SMILES Cn1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C22H22N4O2S/c1-25-22(29(27,28)21-8-4-6-15-5-2-3-7-18(15)21)19-13-17(9-10-20(19)24-25)26-12-11-16(23)14-26/h2-10,13,16H,11-12,14,23H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303161

(1-(1-(2-(dimethylamino)ethyl)-3-(phenylsulfonyl)-1...)Show SMILES CN(C)CCn1cc(c2nc(ccc12)C(C)=O)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H21N3O3S/c1-14(23)16-9-10-17-19(20-16)18(13-22(17)12-11-21(2)3)26(24,25)15-7-5-4-6-8-15/h4-10,13H,11-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308174
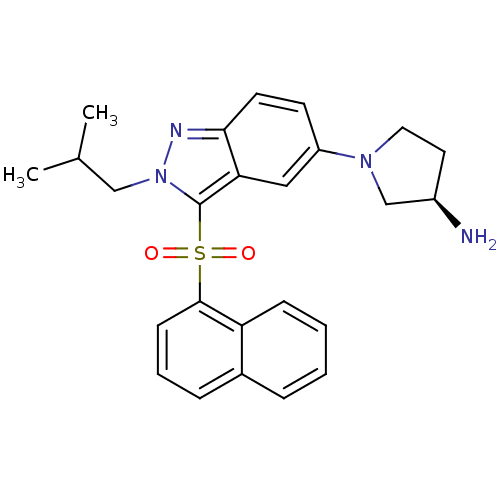
((3R)-1-[2-(2-Methylpropyl)-3-(naphthalen-1-ylsulfo...)Show SMILES CC(C)Cn1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C25H28N4O2S/c1-17(2)15-29-25(32(30,31)24-9-5-7-18-6-3-4-8-21(18)24)22-14-20(10-11-23(22)27-29)28-13-12-19(26)16-28/h3-11,14,17,19H,12-13,15-16,26H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303165

(2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[3,2-b]pyr...)Show InChI InChI=1S/C15H14FN3O2S/c16-11-3-1-4-12(9-11)22(20,21)14-10-19(8-6-17)13-5-2-7-18-15(13)14/h1-5,7,9-10H,6,8,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308178

(1-{3-[(5-Chloro-1-naphthyl)sulfonyl]-1H-indazol-5-...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2c(Cl)cccc12 Show InChI InChI=1S/C22H21ClN4O2S/c23-19-5-1-4-17-16(19)3-2-6-21(17)30(28,29)22-18-13-15(7-8-20(18)25-26-22)27-11-9-14(24)10-12-27/h1-8,13-14H,9-12,24H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308183

(1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]azetidin...)Show SMILES NC1CN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H18N4O2S/c21-14-11-24(12-14)15-8-9-18-17(10-15)20(23-22-18)27(25,26)19-7-3-5-13-4-1-2-6-16(13)19/h1-10,14H,11-12,21H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308175

(1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]piperidi...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c23-16-10-12-26(13-11-16)17-8-9-20-19(14-17)22(25-24-20)29(27,28)21-7-3-5-15-4-1-2-6-18(15)21/h1-9,14,16H,10-13,23H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308169

((3R)-1-[1-Methyl-3-(naphthalen-1-ylsulfonyl)-1H-in...)Show SMILES Cn1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C22H22N4O2S/c1-25-20-10-9-17(26-12-11-16(23)14-26)13-19(20)22(24-25)29(27,28)21-8-4-6-15-5-2-3-7-18(15)21/h2-10,13,16H,11-12,14,23H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308170

((3R)-1-[1-(1-Methylethyl)-3-(naphthalen-1-ylsulfon...)Show SMILES CC(C)n1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N4O2S/c1-16(2)28-22-11-10-19(27-13-12-18(25)15-27)14-21(22)24(26-28)31(29,30)23-9-5-7-17-6-3-4-8-20(17)23/h3-11,14,16,18H,12-13,15,25H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303159
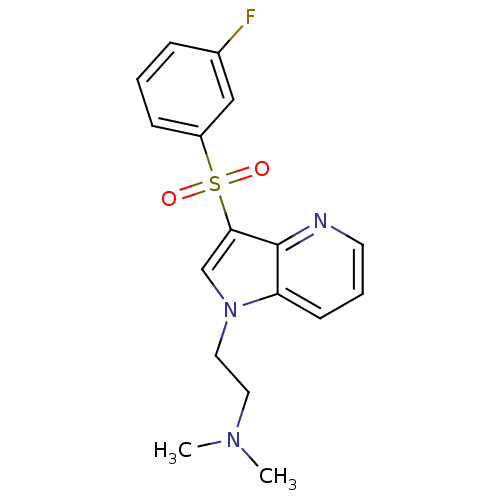
(2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[3,2-b]pyr...)Show InChI InChI=1S/C17H18FN3O2S/c1-20(2)9-10-21-12-16(17-15(21)7-4-8-19-17)24(22,23)14-6-3-5-13(18)11-14/h3-8,11-12H,9-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303160

(2-(5-chloro-3-(phenylsulfonyl)-1H-pyrrolo[3,2-b]py...)Show SMILES CN(C)CCn1cc(c2nc(Cl)ccc12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C17H18ClN3O2S/c1-20(2)10-11-21-12-15(17-14(21)8-9-16(18)19-17)24(22,23)13-6-4-3-5-7-13/h3-9,12H,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308174

((3R)-1-[2-(2-Methylpropyl)-3-(naphthalen-1-ylsulfo...)Show SMILES CC(C)Cn1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C25H28N4O2S/c1-17(2)15-29-25(32(30,31)24-9-5-7-18-6-3-4-8-21(18)24)22-14-20(10-11-23(22)27-29)28-13-12-19(26)16-28/h3-11,14,17,19H,12-13,15-16,26H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50300802
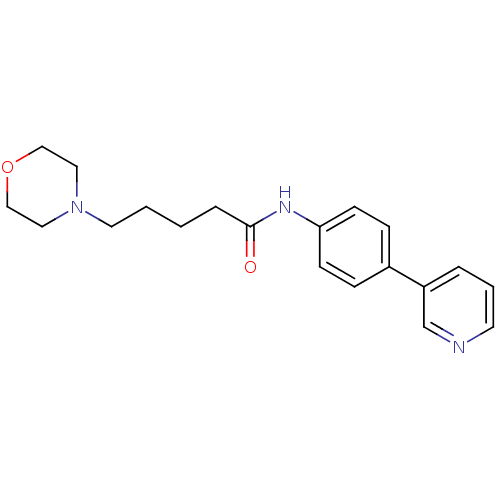
(5-Morpholin-4-ylpentanoic acid(4-pyridin-3-ylpheny...)Show InChI InChI=1S/C20H25N3O2/c24-20(5-1-2-11-23-12-14-25-15-13-23)22-19-8-6-17(7-9-19)18-4-3-10-21-16-18/h3-4,6-10,16H,1-2,5,11-15H2,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat rat alpha7 nAChR expressed in rat GH4C1 cells |
Bioorg Med Chem 17: 5247-58 (2009)
Article DOI: 10.1016/j.bmc.2009.05.040
BindingDB Entry DOI: 10.7270/Q29K4B8H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 274 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50303158
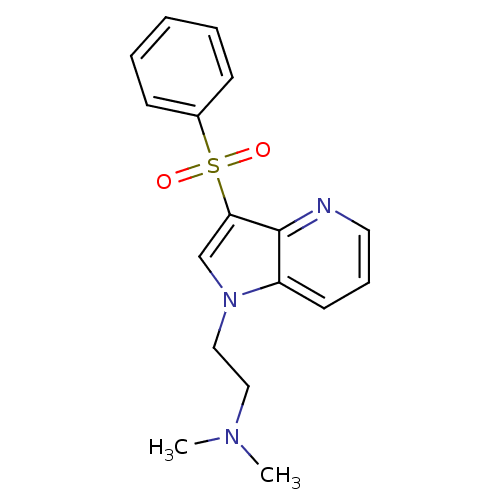
(CHEMBL576312 | N,N-dimethyl-2-(3-(phenylsulfonyl)-...)Show InChI InChI=1S/C17H19N3O2S/c1-19(2)11-12-20-13-16(17-15(20)9-6-10-18-17)23(21,22)14-7-4-3-5-8-14/h3-10,13H,11-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 368 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem Lett 19: 6935-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.067
BindingDB Entry DOI: 10.7270/Q20K28PH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50399292
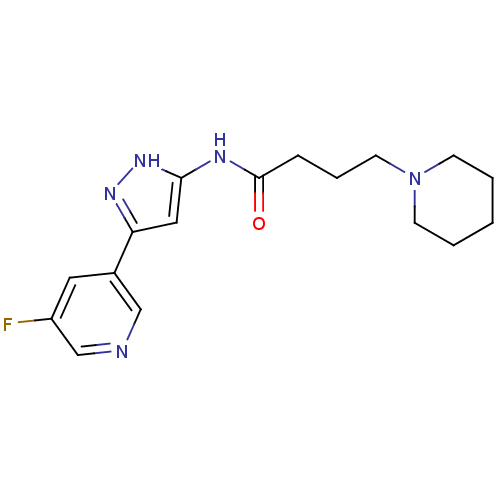
(CHEMBL2180842)Show InChI InChI=1S/C17H22FN5O/c18-14-9-13(11-19-12-14)15-10-16(22-21-15)20-17(24)5-4-8-23-6-2-1-3-7-23/h9-12H,1-8H2,(H2,20,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siena Biotech SpA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-epibatidine from rat alpha7 nAchR expressed in rat GH4C1 cell membranes |
J Med Chem 55: 10277-81 (2012)
Article DOI: 10.1021/jm3013568
BindingDB Entry DOI: 10.7270/Q2DJ5GRD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308167

((3R)-N,N-Dimethyl-1-[3-(1-naphthylsulfonyl)-1H-ind...)Show SMILES CN(C)[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2S/c1-26(2)18-12-13-27(15-18)17-10-11-21-20(14-17)23(25-24-21)30(28,29)22-9-5-7-16-6-3-4-8-19(16)22/h3-11,14,18H,12-13,15H2,1-2H3,(H,24,25)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 423 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308179

(1-(3-(3-chlorophenylsulfonyl)-1H-indazol-5-yl)pipe...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H19ClN4O2S/c19-12-2-1-3-15(10-12)26(24,25)18-16-11-14(4-5-17(16)21-22-18)23-8-6-13(20)7-9-23/h1-5,10-11,13H,6-9,20H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 442 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308172

((3R)-1-[2-Methyl-3-(naphthalen-1-ylsulfonyl)-2H-in...)Show SMILES Cn1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C22H22N4O2S/c1-25-22(29(27,28)21-8-4-6-15-5-2-3-7-18(15)21)19-13-17(9-10-20(19)24-25)26-12-11-16(23)14-26/h2-10,13,16H,11-12,14,23H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308177

(1-{3-[(4-Methyl-1-naphthyl)sulfonyl]-1H-indazol-5-...)Show SMILES Cc1ccc(c2ccccc12)S(=O)(=O)c1n[nH]c2ccc(cc12)N1CCC(N)CC1 Show InChI InChI=1S/C23H24N4O2S/c1-15-6-9-22(19-5-3-2-4-18(15)19)30(28,29)23-20-14-17(7-8-21(20)25-26-23)27-12-10-16(24)11-13-27/h2-9,14,16H,10-13,24H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 493 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308168

((3S)-N,N-Dimethyl-1-[3-(1-naphthylsulfonyl)-1H-ind...)Show SMILES CN(C)[C@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2S/c1-26(2)18-12-13-27(15-18)17-10-11-21-20(14-17)23(25-24-21)30(28,29)22-9-5-7-16-6-3-4-8-19(16)22/h3-11,14,18H,12-13,15H2,1-2H3,(H,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50399293
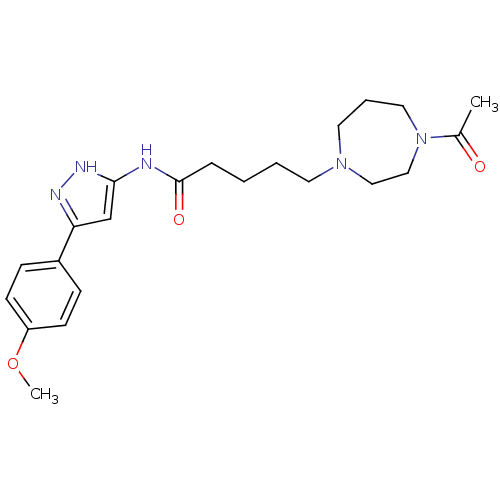
(CHEMBL2086582)Show SMILES COc1ccc(cc1)-c1cc(NC(=O)CCCCN2CCCN(CC2)C(C)=O)[nH]n1 Show InChI InChI=1S/C22H31N5O3/c1-17(28)27-13-5-12-26(14-15-27)11-4-3-6-22(29)23-21-16-20(24-25-21)18-7-9-19(30-2)10-8-18/h7-10,16H,3-6,11-15H2,1-2H3,(H2,23,24,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siena Biotech SpA
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha7 nAChR expressed in GH4C1 cells after 1 hr by TopCount method |
J Med Chem 55: 4806-23 (2012)
Article DOI: 10.1021/jm300247y
BindingDB Entry DOI: 10.7270/Q2VM4DJ7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308176

(1-[3-(2-Naphthylsulfonyl)-1H-indazol-5-yl]piperidi...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H22N4O2S/c23-17-9-11-26(12-10-17)18-6-8-21-20(14-18)22(25-24-21)29(27,28)19-7-5-15-3-1-2-4-16(15)13-19/h1-8,13-14,17H,9-12,23H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1D receptor |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data