

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM92358 (HGXPRT Inhibitor, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.650 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.6 | 37 |
Albert Einstein College of Medicine | Assay Description PfHGXPRT activity was measured using spectrophotometric assay observing the conversion of xanthine and 5-phospho-alpha-D-ribose-1-pyrophosphate to xa... | Chem Biol 19: 721-30 (2012) Article DOI: 10.1016/j.chembiol.2012.04.012 BindingDB Entry DOI: 10.7270/Q2639NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine-xanthine phosphoribosyltransferase (Plasmodium falciparum) | BDBM92357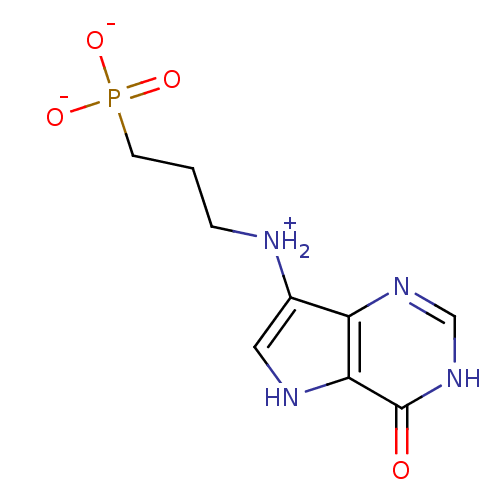 (HGXPRT Inhibitor, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10.6 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 37 |
Albert Einstein College of Medicine | Assay Description PfHGXPRT activity was measured using spectrophotometric assay observing the conversion of xanthine and 5-phospho-alpha-D-ribose-1-pyrophosphate to xa... | Chem Biol 19: 721-30 (2012) Article DOI: 10.1016/j.chembiol.2012.04.012 BindingDB Entry DOI: 10.7270/Q2639NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214779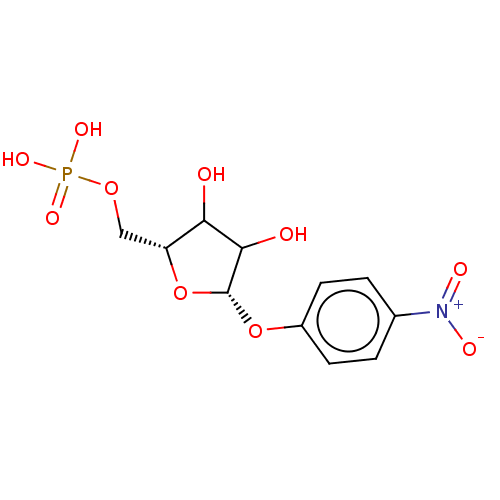 (OPRT inhibitor, 2) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214779 (OPRT inhibitor, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | -42.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214786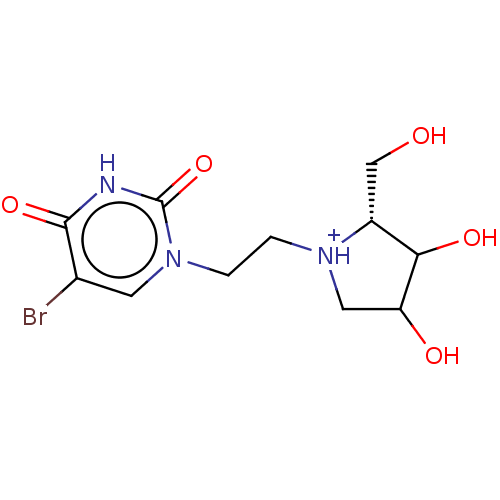 (OPRT inhibitor, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 80 | -40.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214791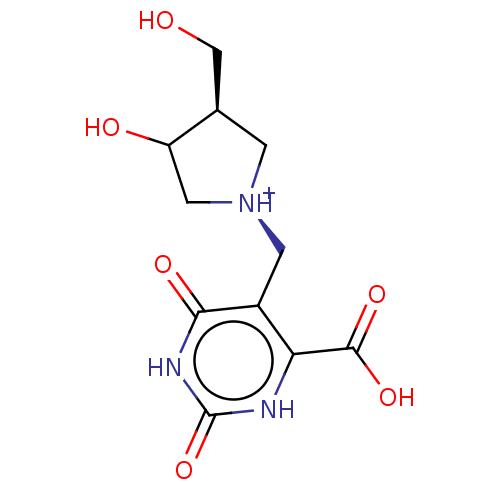 (OPRT inhibitor, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 83 | -40.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214785 (OPRT inhibitor, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | -40.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214784 (OPRT inhibitor, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | -39.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214797 (OPRT inhibitor, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214778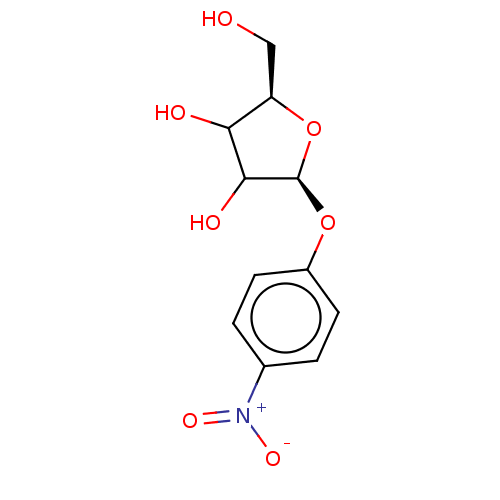 (OPRT inhibitor, 1 ) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 120 | -39.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214783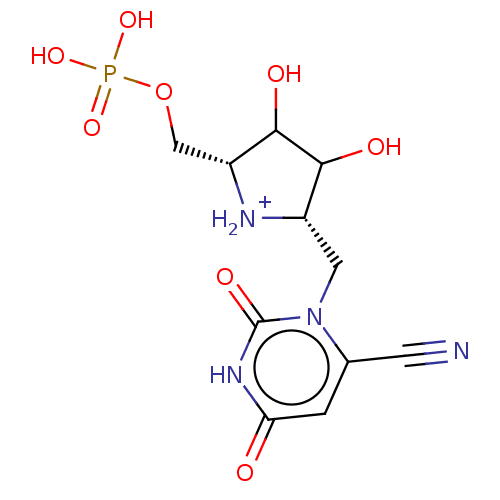 (OPRT inhibitor, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 140 | -39.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214796 (OPRT inhibitor, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | -38.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214785 (OPRT inhibitor, 8) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | -38.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214778 (OPRT inhibitor, 1 ) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 190 | -38.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214794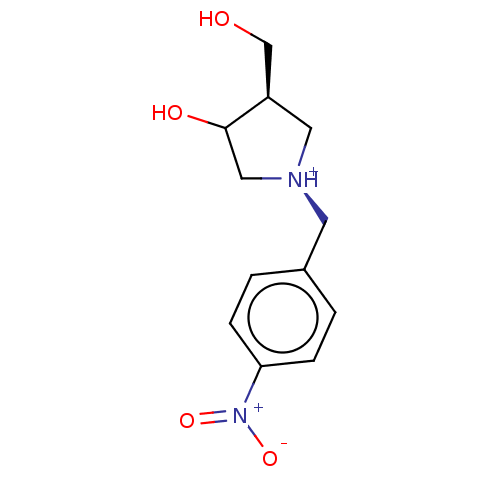 (OPRT inhibitor, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214781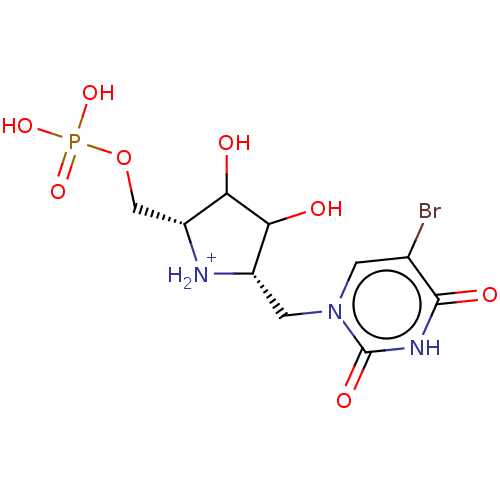 (OPRT inhibitor, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214791 (OPRT inhibitor, 14) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 220 | -38.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214793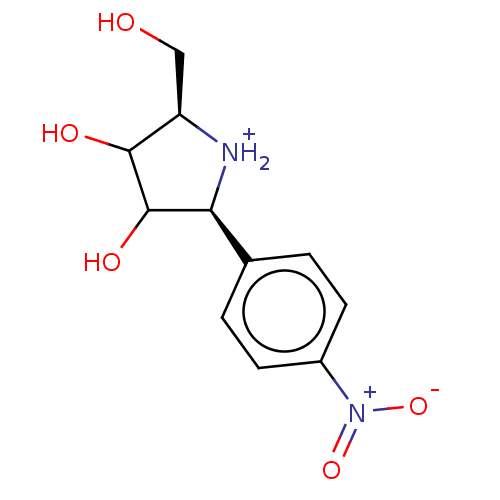 (OPRT inhibitor, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 230 | -37.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214795 (OPRT inhibitor, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 230 | -37.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214792 (OPRT inhibitor, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | -37.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214790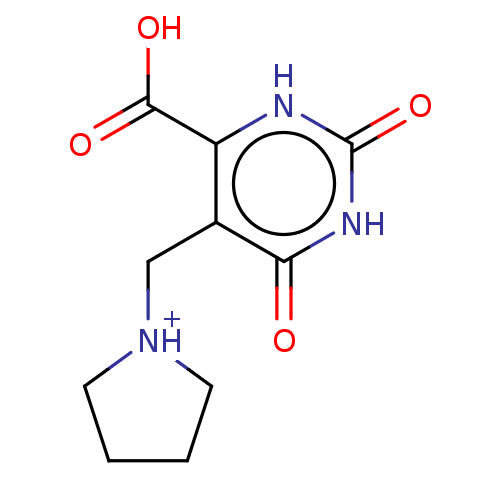 (OPRT inhibitor, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 250 | -37.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214784 (OPRT inhibitor, 7) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 270 | -37.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214786 (OPRT inhibitor, 9) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 270 | -37.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214787 (OPRT inhibitor, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | -37.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214782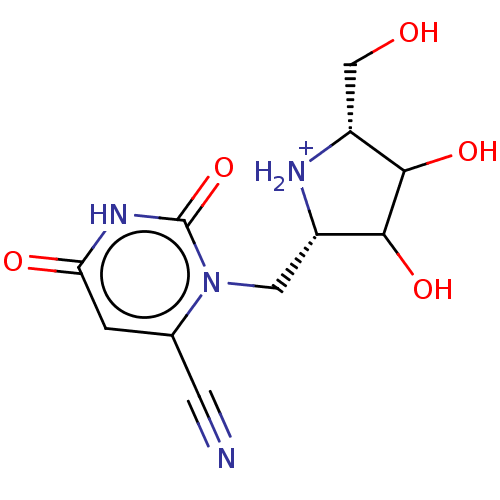 (OPRT inhibitor, 5) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 300 | -37.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214780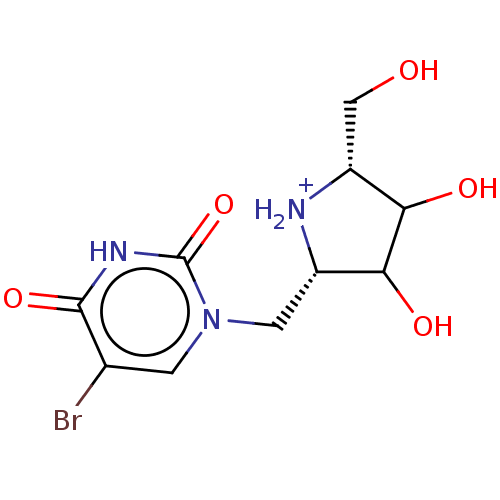 (OPRT inhibitor, 3) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 350 | -36.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214783 (OPRT inhibitor, 6) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 350 | -36.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214782 (OPRT inhibitor, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 360 | -36.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214781 (OPRT inhibitor, 4) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 370 | -36.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438479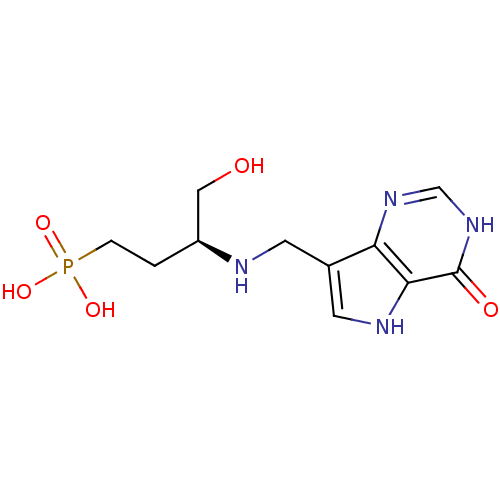 (CHEMBL2414636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Ltd Curated by ChEMBL | Assay Description Inhibition of human HGPRT using xanthine/PRPP assessed as xanthine/guanine conversion to xanthosine-5'-monophosphate/guanosine-5'-monophosphate by sp... | Bioorg Med Chem 21: 5629-46 (2013) Article DOI: 10.1016/j.bmc.2013.02.016 BindingDB Entry DOI: 10.7270/Q2N58NSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM92358 (HGXPRT Inhibitor, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 385 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 37 |
Albert Einstein College of Medicine | Assay Description PfHGXPRT activity was measured using spectrophotometric assay observing the conversion of xanthine and 5-phospho-alpha-D-ribose-1-pyrophosphate to xa... | Chem Biol 19: 721-30 (2012) Article DOI: 10.1016/j.chembiol.2012.04.012 BindingDB Entry DOI: 10.7270/Q2639NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438477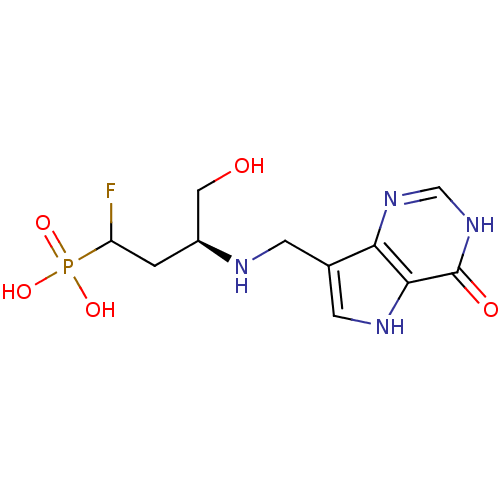 (CHEMBL2414638) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Ltd Curated by ChEMBL | Assay Description Inhibition of human HGPRT using xanthine/PRPP assessed as xanthine/guanine conversion to xanthosine-5'-monophosphate/guanosine-5'-monophosphate by sp... | Bioorg Med Chem 21: 5629-46 (2013) Article DOI: 10.1016/j.bmc.2013.02.016 BindingDB Entry DOI: 10.7270/Q2N58NSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214790 (OPRT inhibitor, 13) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 470 | -36.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214793 (OPRT inhibitor, 16) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 480 | -36.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214792 (OPRT inhibitor, 15) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | -36.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214796 (OPRT inhibitor, 19) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | -36.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214797 (OPRT inhibitor, 20) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 510 | -35.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214788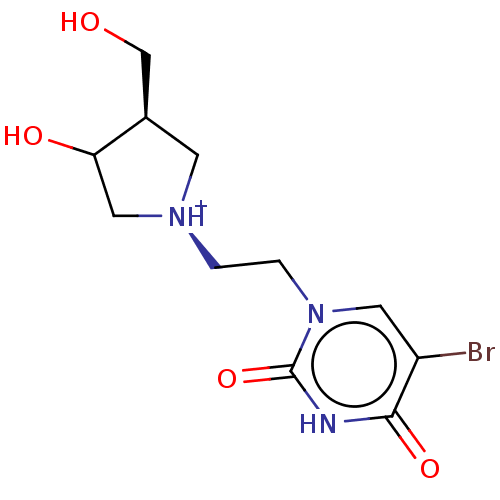 (OPRT inhibitor, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 520 | -35.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214780 (OPRT inhibitor, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 530 | -35.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214795 (OPRT inhibitor, 18) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 570 | -35.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214787 (OPRT inhibitor, 10) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 690 | -35.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214794 (OPRT inhibitor, 17) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 750 | -35.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotate phosphoribosyltransferase (Plasmodium falciparum) | BDBM214788 (OPRT inhibitor, 11) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | -33.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM214789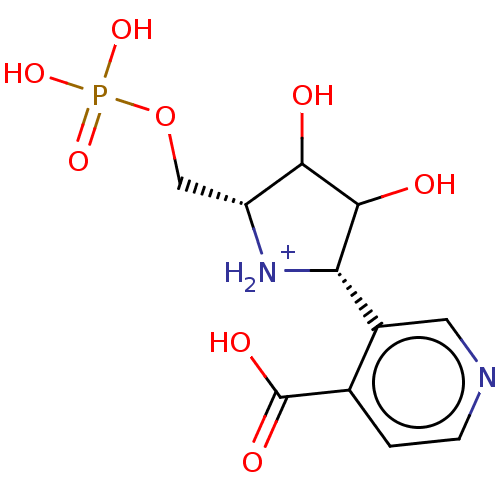 (OPRT inhibitor, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Albert Einstein College of Medicine | Assay Description Inhibition constants (Ki, equivalent to Kd for competitive inhibitors) were measured at 25 °C by adding purified OPRT enzymes (25 nM for PfOPRT a... | J Biol Chem 288: 34746-54 (2013) Article DOI: 10.1074/jbc.M113.521955 BindingDB Entry DOI: 10.7270/Q2T152GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438487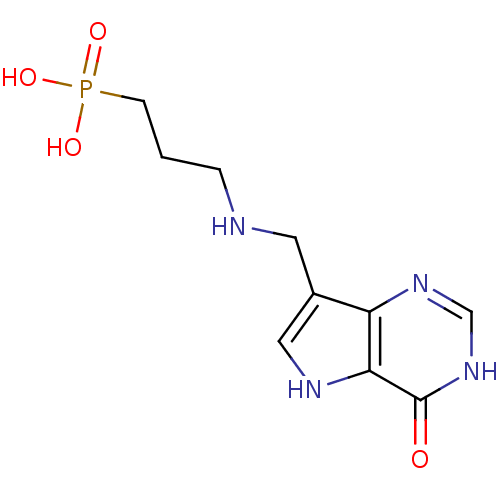 (CHEMBL2414703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Ltd Curated by ChEMBL | Assay Description Inhibition of human HGPRT using xanthine/PRPP assessed as xanthine/guanine conversion to xanthosine-5'-monophosphate/guanosine-5'-monophosphate by sp... | Bioorg Med Chem 21: 5629-46 (2013) Article DOI: 10.1016/j.bmc.2013.02.016 BindingDB Entry DOI: 10.7270/Q2N58NSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM92357 (HGXPRT Inhibitor, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.94E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 7.6 | 37 |
Albert Einstein College of Medicine | Assay Description PfHGXPRT activity was measured using spectrophotometric assay observing the conversion of xanthine and 5-phospho-alpha-D-ribose-1-pyrophosphate to xa... | Chem Biol 19: 721-30 (2012) Article DOI: 10.1016/j.chembiol.2012.04.012 BindingDB Entry DOI: 10.7270/Q2639NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438481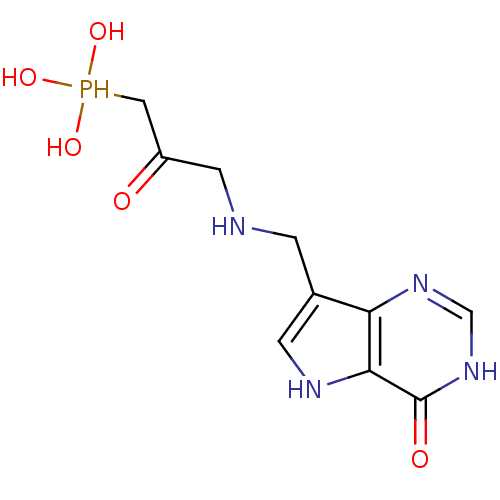 (CHEMBL2414634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Ltd Curated by ChEMBL | Assay Description Inhibition of human HGPRT using xanthine/PRPP assessed as xanthine/guanine conversion to xanthosine-5'-monophosphate/guanosine-5'-monophosphate by sp... | Bioorg Med Chem 21: 5629-46 (2013) Article DOI: 10.1016/j.bmc.2013.02.016 BindingDB Entry DOI: 10.7270/Q2N58NSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438480 (CHEMBL2414635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Ltd Curated by ChEMBL | Assay Description Inhibition of human HGPRT using xanthine/PRPP assessed as xanthine/guanine conversion to xanthosine-5'-monophosphate/guanosine-5'-monophosphate by sp... | Bioorg Med Chem 21: 5629-46 (2013) Article DOI: 10.1016/j.bmc.2013.02.016 BindingDB Entry DOI: 10.7270/Q2N58NSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438476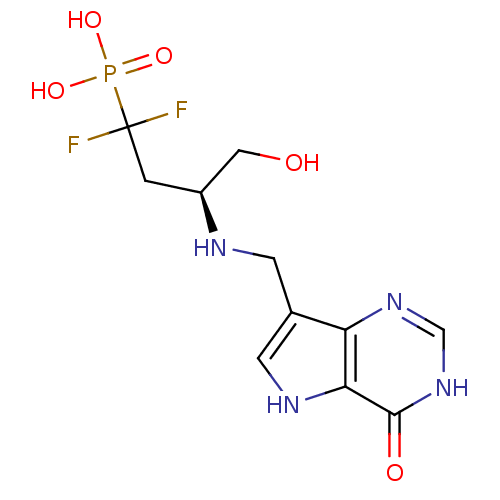 (CHEMBL2414639) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Ltd Curated by ChEMBL | Assay Description Inhibition of human HGPRT using xanthine/PRPP assessed as xanthine/guanine conversion to xanthosine-5'-monophosphate/guanosine-5'-monophosphate by sp... | Bioorg Med Chem 21: 5629-46 (2013) Article DOI: 10.1016/j.bmc.2013.02.016 BindingDB Entry DOI: 10.7270/Q2N58NSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438475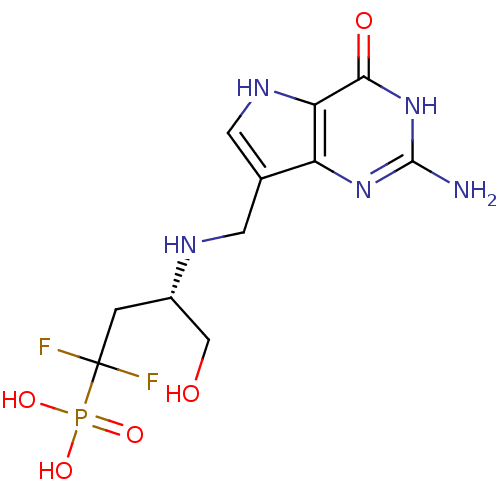 (CHEMBL2414640) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Ltd Curated by ChEMBL | Assay Description Inhibition of human HGPRT using xanthine/PRPP assessed as xanthine/guanine conversion to xanthosine-5'-monophosphate/guanosine-5'-monophosphate by sp... | Bioorg Med Chem 21: 5629-46 (2013) Article DOI: 10.1016/j.bmc.2013.02.016 BindingDB Entry DOI: 10.7270/Q2N58NSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 67 total ) | Next | Last >> |