

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain | Bioorg Med Chem 17: 5782-90 (2009) Article DOI: 10.1016/j.bmc.2009.07.024 BindingDB Entry DOI: 10.7270/Q2VQ32RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304074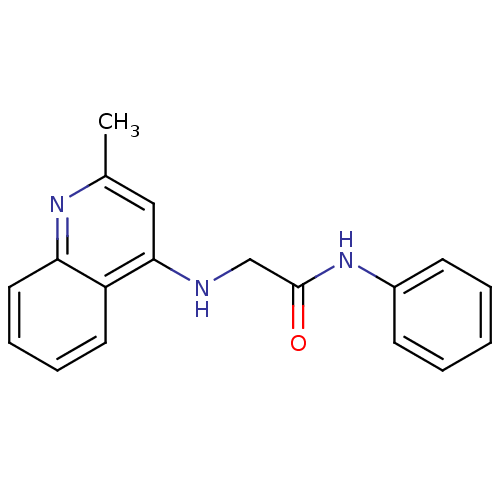 (2-(2-methylquinolin-4-ylamino)-N-phenylacetamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Displacement of [3H]dynorphin from human kappa opioid receptor expressed in C6 giloma cells | Bioorg Med Chem 17: 5782-90 (2009) Article DOI: 10.1016/j.bmc.2009.07.024 BindingDB Entry DOI: 10.7270/Q2VQ32RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304074 (2-(2-methylquinolin-4-ylamino)-N-phenylacetamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain | Bioorg Med Chem 17: 5782-90 (2009) Article DOI: 10.1016/j.bmc.2009.07.024 BindingDB Entry DOI: 10.7270/Q2VQ32RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Biology Curated by ChEMBL | Assay Description Displacement of [3H]dynorphin from human kappa opioid receptor expressed in C6 giloma cells | Bioorg Med Chem 17: 5782-90 (2009) Article DOI: 10.1016/j.bmc.2009.07.024 BindingDB Entry DOI: 10.7270/Q2VQ32RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263562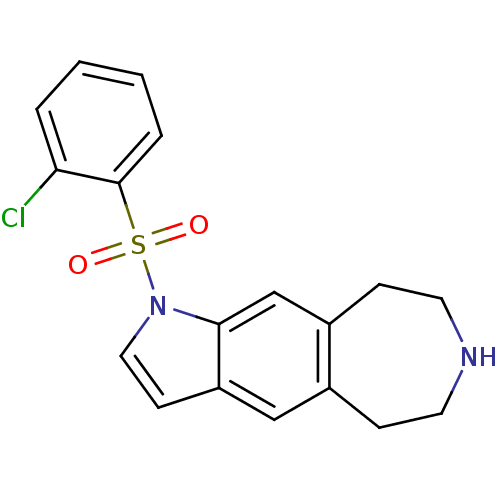 (1-(2-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263563 (1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263562 (1-(2-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263563 (1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263617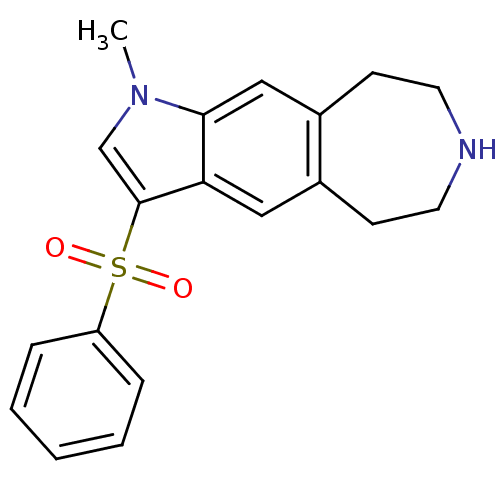 (1-methyl-3-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263566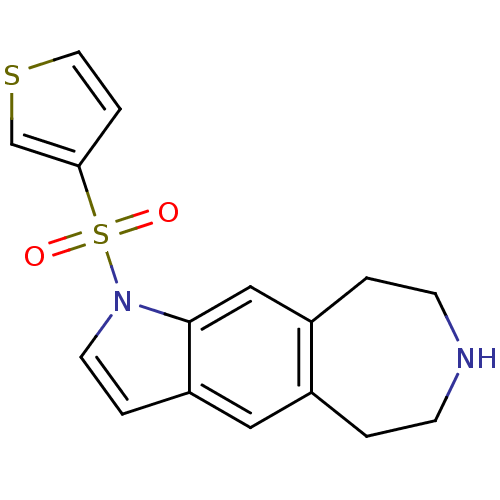 (1-(thiophen-3-ylsulfonyl)-1,5,6,7,8,9-hexahydroaze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263615 (3-chloro-1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263614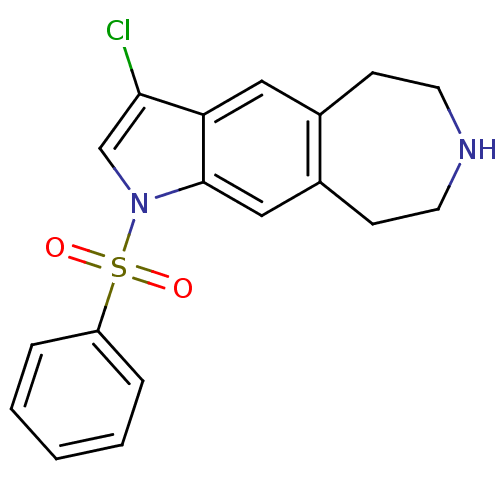 (3-chloro-1-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263518 (1-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroazepino[4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263617 (1-methyl-3-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263616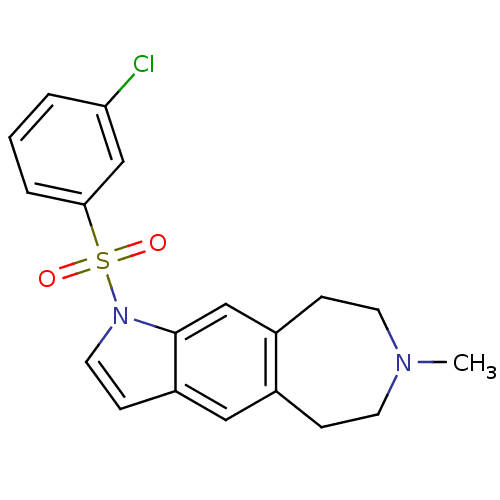 (1-(3-chlorophenylsulfonyl)-7-methyl-1,5,6,7,8,9-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263564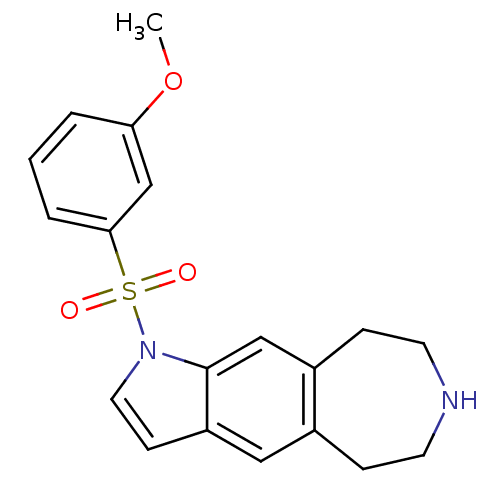 (1-(3-methoxyphenylsulfonyl)-1,5,6,7,8,9-hexahydroa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263614 (3-chloro-1-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263615 (3-chloro-1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263518 (1-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroazepino[4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263566 (1-(thiophen-3-ylsulfonyl)-1,5,6,7,8,9-hexahydroaze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263564 (1-(3-methoxyphenylsulfonyl)-1,5,6,7,8,9-hexahydroa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263565 (1-(pyridin-2-ylsulfonyl)-1,5,6,7,8,9-hexahydroazep...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50270510 (CHEMBL3589199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of recombinant human full length MMP9 preincubated for 1 hr followed by gelatin addition measured after 18 hrs by SDS-PAGE analysis | J Nat Prod 80: 1347-1353 (2017) Article DOI: 10.1021/acs.jnatprod.6b00957 BindingDB Entry DOI: 10.7270/Q2HH6NJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263616 (1-(3-chlorophenylsulfonyl)-7-methyl-1,5,6,7,8,9-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50263565 (1-(pyridin-2-ylsulfonyl)-1,5,6,7,8,9-hexahydroazep...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate | Bioorg Med Chem Lett 18: 5698-700 (2008) Article DOI: 10.1016/j.bmcl.2008.08.010 BindingDB Entry DOI: 10.7270/Q28W3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239990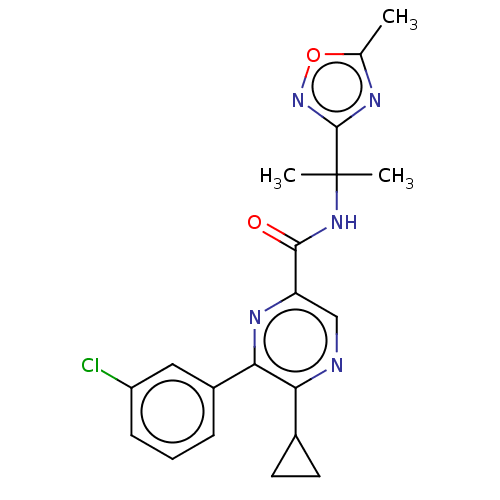 (US9403808, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 88.1 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239991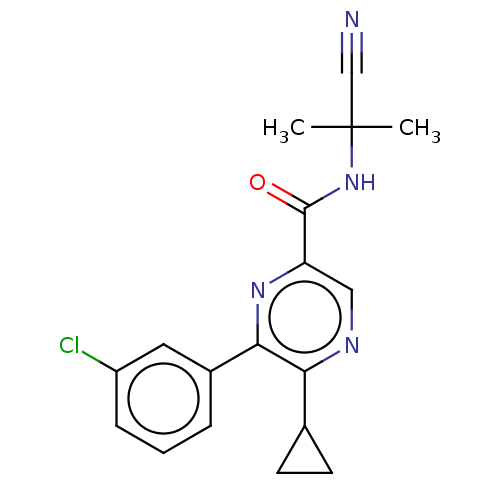 (US9403808, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 104 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239992 (US9403808, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 156 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239993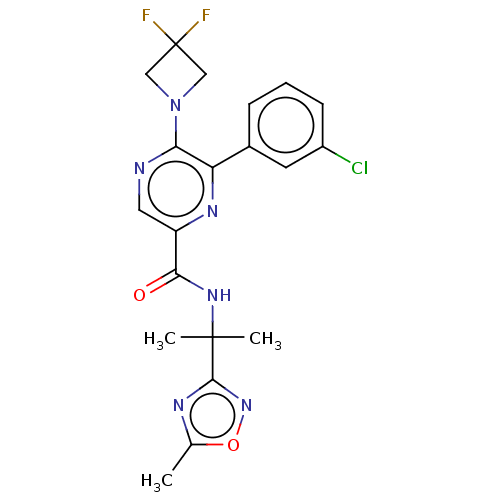 (US9403808, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 108 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239994 (US9403808, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239995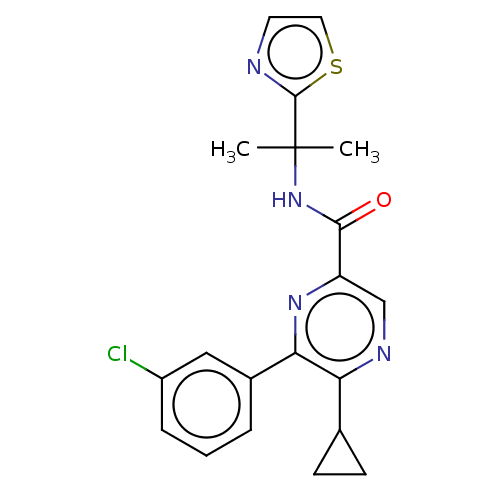 (US9403808, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239996 (US9403808, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 255 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239997 (US9403808, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 18.5 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239998 (US9403808, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 87.9 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239999 (US9403808, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 257 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240000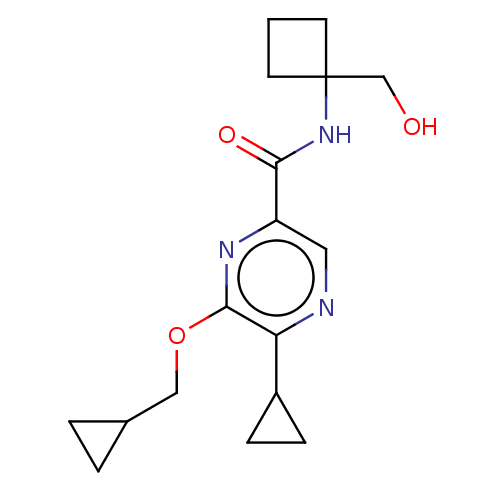 (US9403808, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 124 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240001 (US9403808, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 85.2 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240002 (US9403808, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 63.4 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240003 (US9403808, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.80 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240004 (US9403808, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240005 (US9403808, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 36.9 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240006 (US9403808, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 198 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240007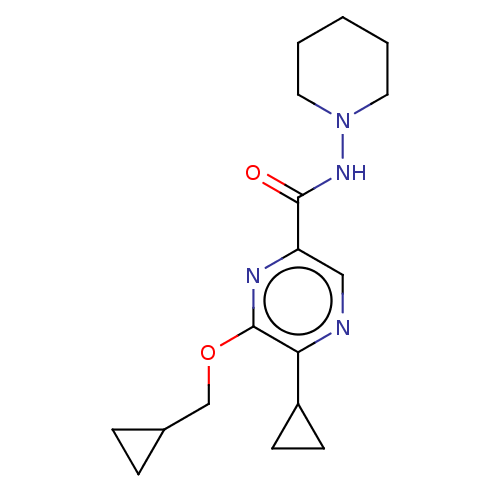 (US9403808, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 327 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240008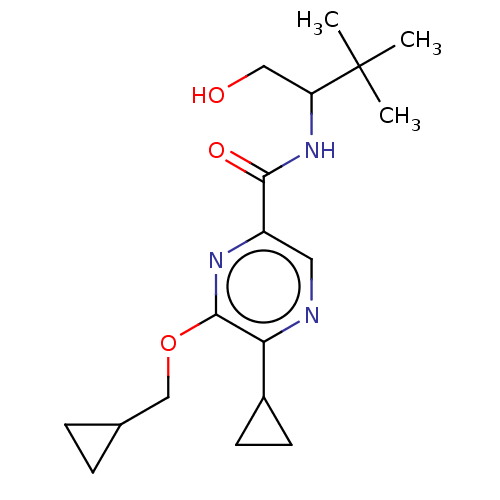 (US9403808, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 53.5 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240009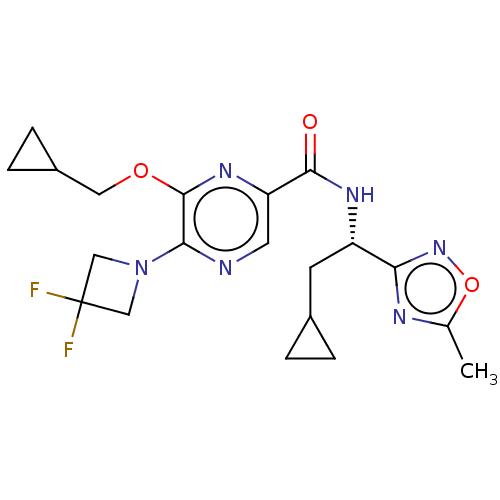 (US9403808, 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 28.4 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240010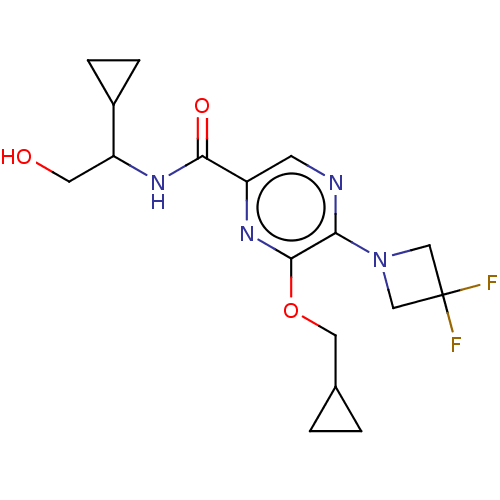 (US9403808, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 61.3 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240011 (US9403808, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240012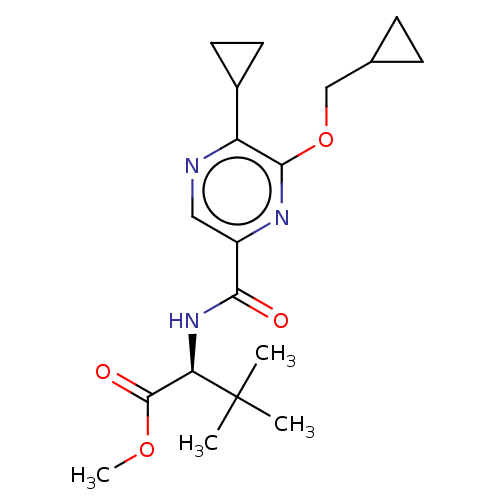 (US9403808, 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240013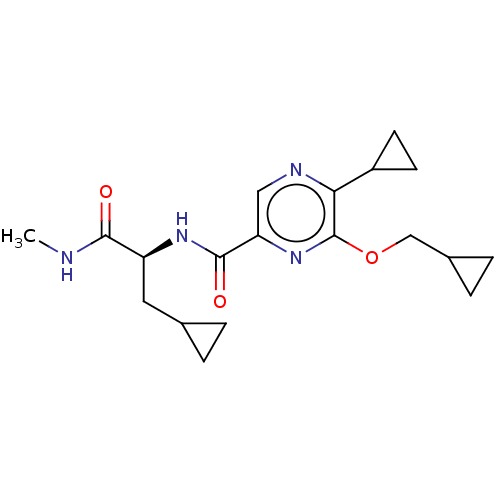 (US9403808, 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 199 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM240014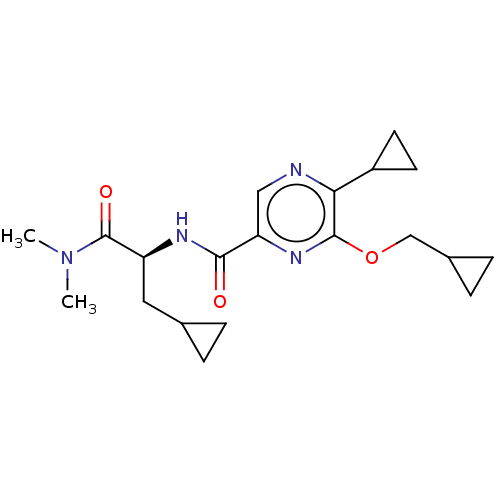 (US9403808, 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 14.6 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... | US Patent US9403808 (2016) BindingDB Entry DOI: 10.7270/Q2V69HHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 221 total ) | Next | Last >> |