 Found 1108 hits with Last Name = 'heerding' and Initial = 'd'
Found 1108 hits with Last Name = 'heerding' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278693
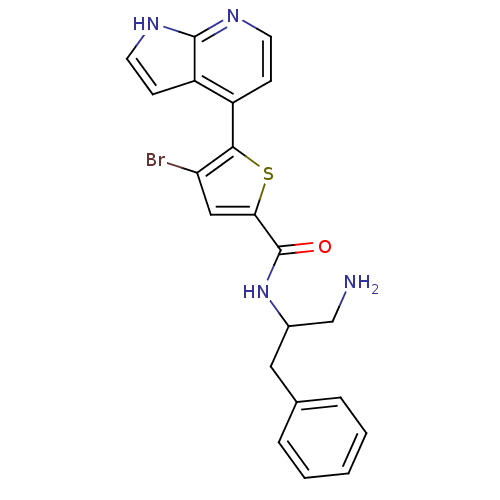
((+/-)-N-(1-amino-3-phenylpropan-2-yl)-4-bromo-5-(1...)Show SMILES NCC(Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098
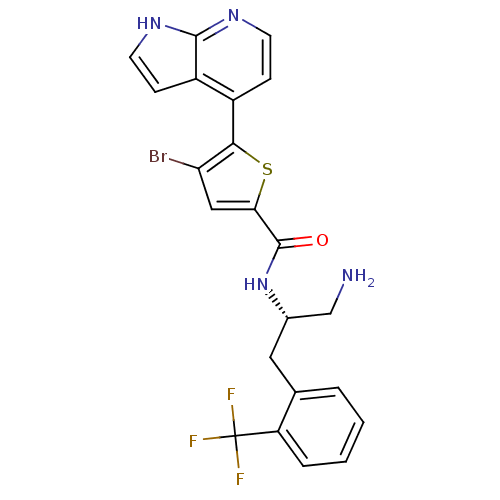
(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278837
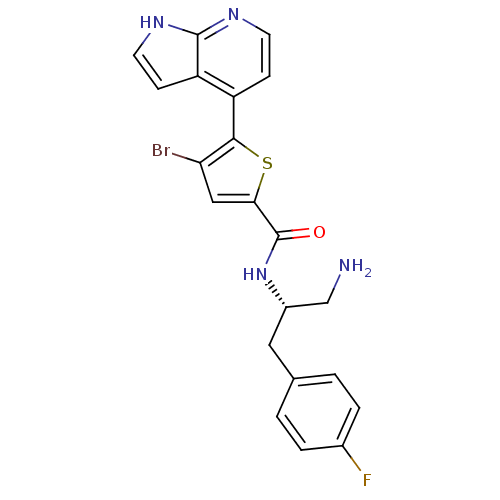
(CHEMBL496690 | N-((S)-1-amino-3-(4-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1ccc(F)cc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-5-7-25-20-16(15)6-8-26-20)21(28)27-14(11-24)9-12-1-3-13(23)4-2-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099
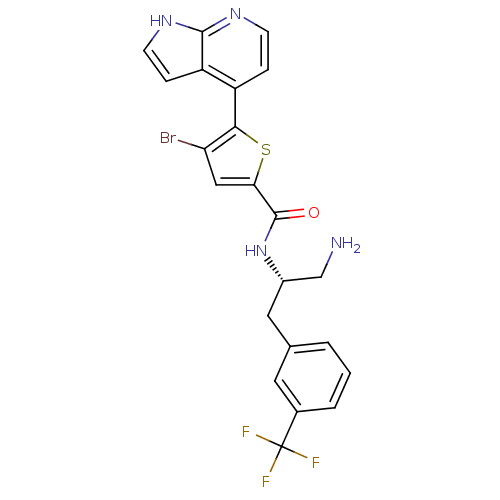
(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099

(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183
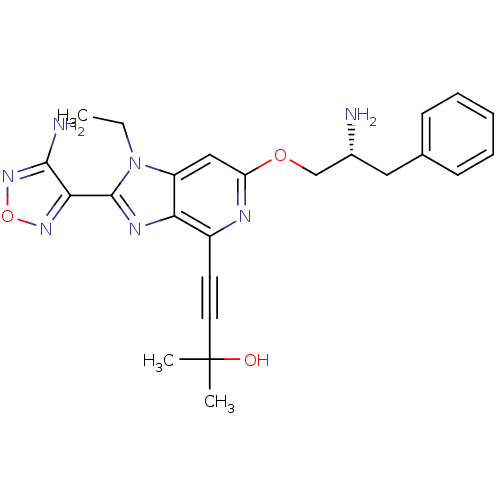
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192
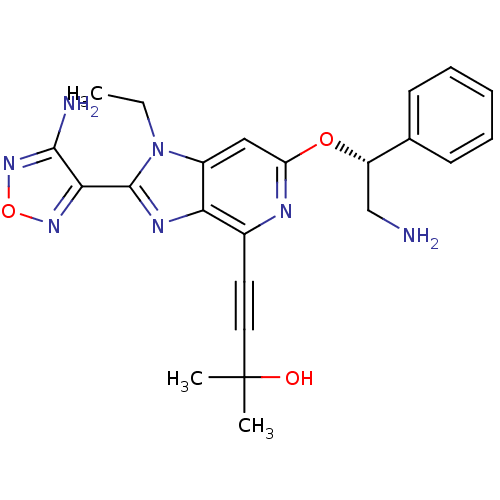
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278836
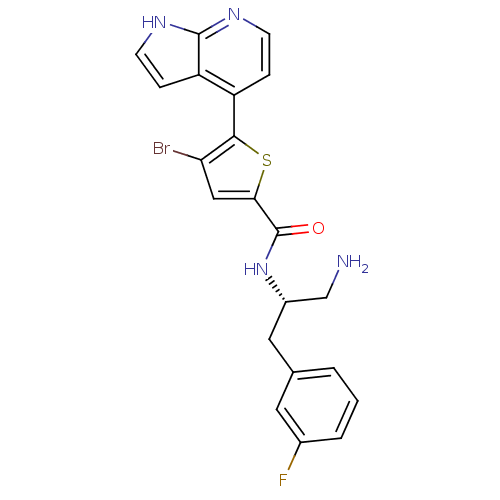
(CHEMBL523586 | N-((S)-1-amino-3-(3-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1cccc(F)c1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-4-6-25-20-16(15)5-7-26-20)21(28)27-14(11-24)9-12-2-1-3-13(23)8-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083761
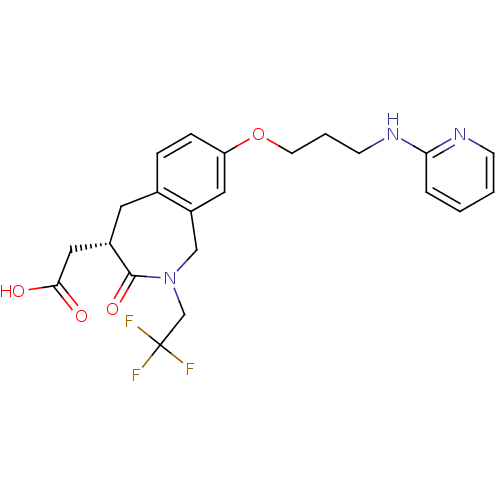
(CHEMBL87429 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013
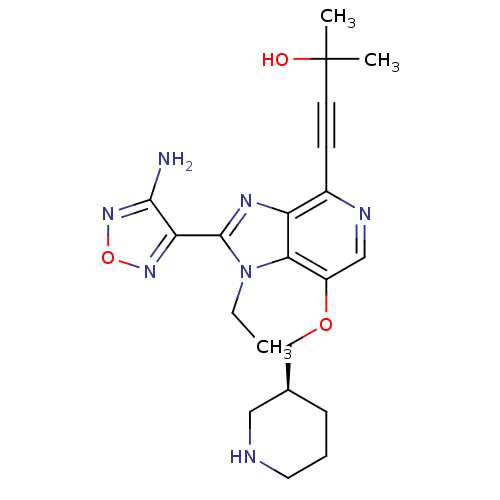
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083763
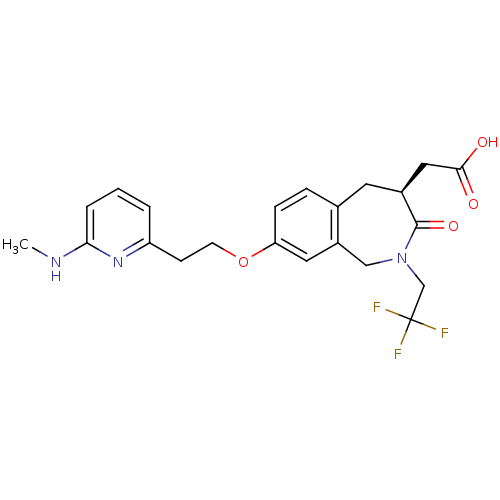
(CHEMBL86992 | [(S)-8-[2-(6-Methylamino-pyridin-2-y...)Show SMILES CNc1cccc(CCOc2ccc3C[C@@H](CC(O)=O)C(=O)N(CC(F)(F)F)Cc3c2)n1 Show InChI InChI=1S/C22H24F3N3O4/c1-26-19-4-2-3-17(27-19)7-8-32-18-6-5-14-9-15(11-20(29)30)21(31)28(12-16(14)10-18)13-22(23,24)25/h2-6,10,15H,7-9,11-13H2,1H3,(H,26,27)(H,29,30)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083764
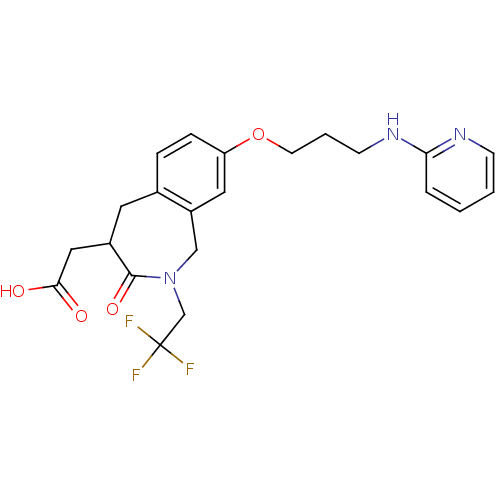
(CHEMBL421533 | [3-Oxo-8-[3-(pyridin-2-ylamino)-pro...)Show SMILES OC(=O)CC1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316185
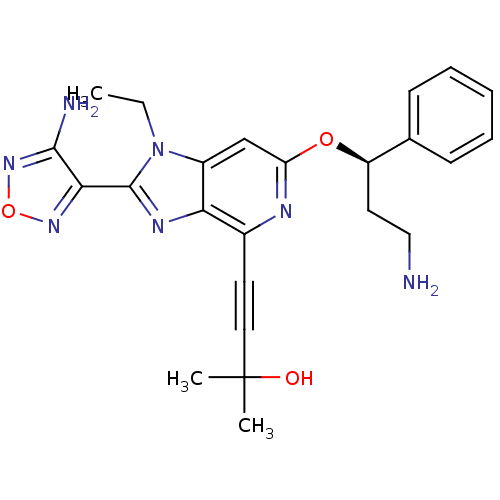
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083762
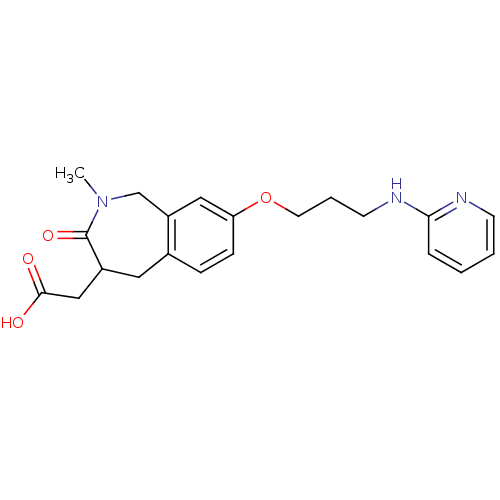
(CHEMBL314022 | {2-Methyl-3-oxo-8-[3-(pyridin-2-yla...)Show InChI InChI=1S/C21H25N3O4/c1-24-14-17-12-18(28-10-4-9-23-19-5-2-3-8-22-19)7-6-15(17)11-16(21(24)27)13-20(25)26/h2-3,5-8,12,16H,4,9-11,13-14H2,1H3,(H,22,23)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278837

(CHEMBL496690 | N-((S)-1-amino-3-(4-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1ccc(F)cc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-5-7-25-20-16(15)6-8-26-20)21(28)27-14(11-24)9-12-1-3-13(23)4-2-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50059133
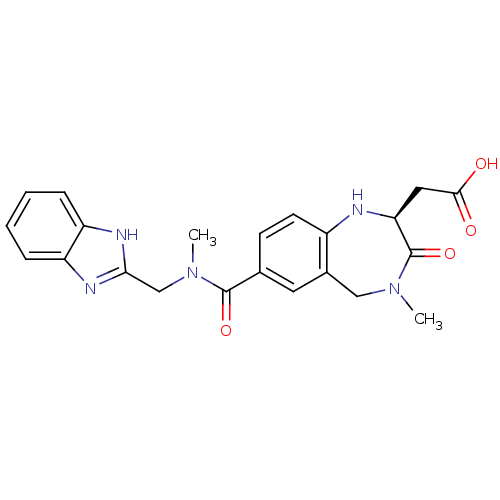
(CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...)Show SMILES CN(Cc1nc2ccccc2[nH]1)C(=O)c1ccc2N[C@@H](CC(O)=O)C(=O)N(C)Cc2c1 Show InChI InChI=1S/C22H23N5O4/c1-26-11-14-9-13(7-8-15(14)23-18(22(26)31)10-20(28)29)21(30)27(2)12-19-24-16-5-3-4-6-17(16)25-19/h3-9,18,23H,10-12H2,1-2H3,(H,24,25)(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278833
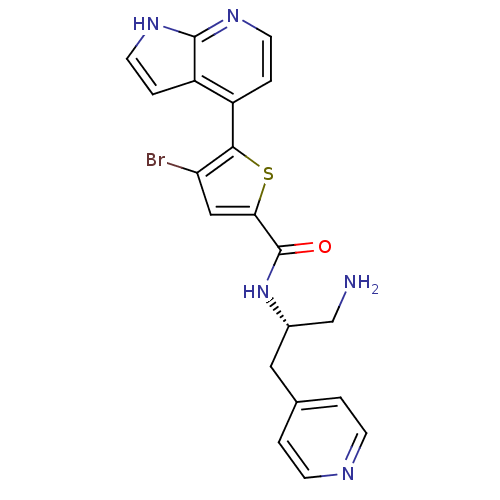
(CHEMBL498051 | N-((S)-1-amino-3-(pyridin-4-yl)prop...)Show SMILES NC[C@H](Cc1ccncc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H18BrN5OS/c21-16-10-17(20(27)26-13(11-22)9-12-1-5-23-6-2-12)28-18(16)14-3-7-24-19-15(14)4-8-25-19/h1-8,10,13H,9,11,22H2,(H,24,25)(H,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278100
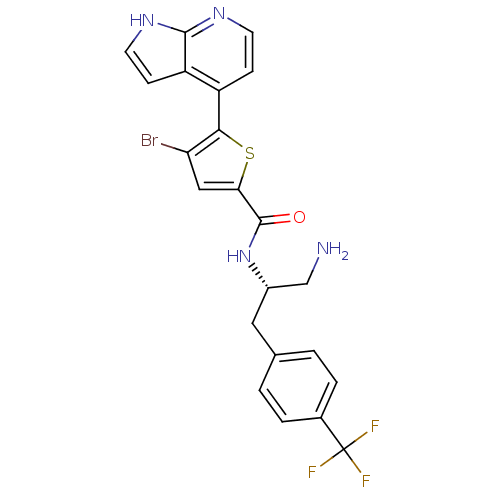
(CHEMBL482537 | N-((S)-1-amino-3-(4-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccc(cc1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-5-7-28-20-16(15)6-8-29-20)21(31)30-14(11-27)9-12-1-3-13(4-2-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50126595
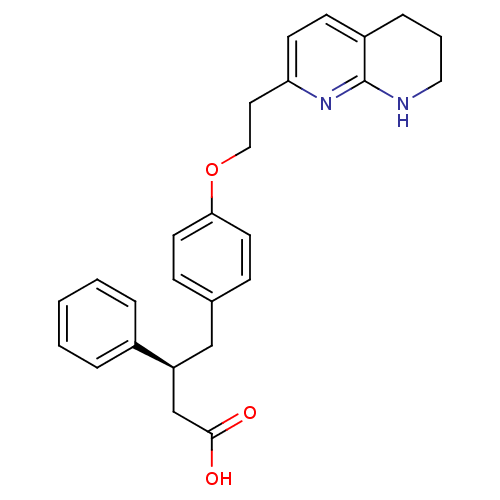
(3-Phenyl-4-{4-[2-(5,6,7,8-tetrahydro-[1,8]naphthyr...)Show SMILES OC(=O)C[C@H](Cc1ccc(OCCc2ccc3CCCNc3n2)cc1)c1ccccc1 Show InChI InChI=1S/C26H28N2O3/c29-25(30)18-22(20-5-2-1-3-6-20)17-19-8-12-24(13-9-19)31-16-14-23-11-10-21-7-4-15-27-26(21)28-23/h1-3,5-6,8-13,22H,4,7,14-18H2,(H,27,28)(H,29,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphav/beta 3 vitronectin receptor in HEK cells |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50126595

(3-Phenyl-4-{4-[2-(5,6,7,8-tetrahydro-[1,8]naphthyr...)Show SMILES OC(=O)C[C@H](Cc1ccc(OCCc2ccc3CCCNc3n2)cc1)c1ccccc1 Show InChI InChI=1S/C26H28N2O3/c29-25(30)18-22(20-5-2-1-3-6-20)17-19-8-12-24(13-9-19)31-16-14-23-11-10-21-7-4-15-27-26(21)28-23/h1-3,5-6,8-13,22H,4,7,14-18H2,(H,27,28)(H,29,30)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta5 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50078714
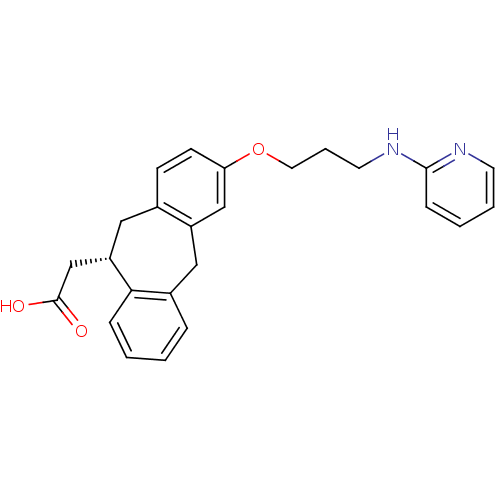
(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta3 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316182
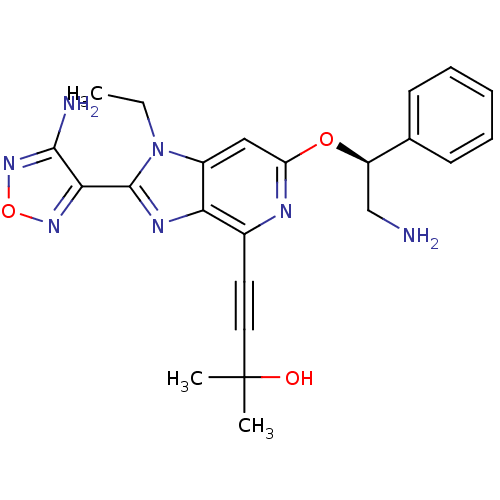
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278770

(CHEMBL470597 | N-((S)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278770

(CHEMBL470597 | N-((S)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50078714

(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaIIb-beta3 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50078714

(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099

(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098

(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098

(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278771
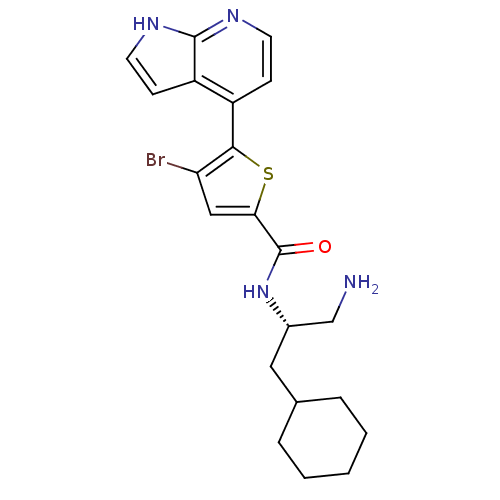
(CHEMBL470598 | N-((S)-1-amino-3-cyclohexylpropan-2...)Show SMILES NC[C@H](CC1CCCCC1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H25BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h6-9,11,13-14H,1-5,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278771

(CHEMBL470598 | N-((S)-1-amino-3-cyclohexylpropan-2...)Show SMILES NC[C@H](CC1CCCCC1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H25BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h6-9,11,13-14H,1-5,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SMYD3
(Homo sapiens (Human)) | BDBM50509592

(CHEMBL4460946)Show SMILES FC(F)(F)CCCN[C@H]1CC[C@H](CS(=O)(=O)N2CCC(CC2)NC(=O)c2cc(on2)C2CC2)CC1 |r,wU:8.7,wD:11.11,(68.22,-17.58,;66.9,-16.8,;66.91,-15.26,;68.21,-16.02,;65.56,-17.55,;64.24,-16.77,;62.91,-17.52,;61.59,-16.74,;60.24,-17.5,;60.23,-19.04,;58.9,-19.8,;57.56,-19.01,;56.23,-19.78,;54.89,-19,;54.11,-17.66,;55.66,-17.66,;53.56,-19.78,;52.22,-18.99,;50.9,-19.78,;50.9,-21.32,;52.22,-22.07,;53.56,-21.32,;49.57,-22.08,;48.23,-21.32,;48.23,-19.78,;46.9,-22.09,;46.74,-23.62,;45.24,-23.94,;44.46,-22.62,;45.49,-21.47,;44.62,-25.35,;43.37,-26.26,;44.78,-26.88,;57.57,-17.48,;58.91,-16.72,)| Show InChI InChI=1S/C23H35F3N4O4S/c24-23(25,26)10-1-11-27-18-6-2-16(3-7-18)15-35(32,33)30-12-8-19(9-13-30)28-22(31)20-14-21(34-29-20)17-4-5-17/h14,16-19,27H,1-13,15H2,(H,28,31)/t16-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Cheng-Prusoff equation analysis |
ACS Med Chem Lett 11: 133-140 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00493
BindingDB Entry DOI: 10.7270/Q2W66Q2M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316189
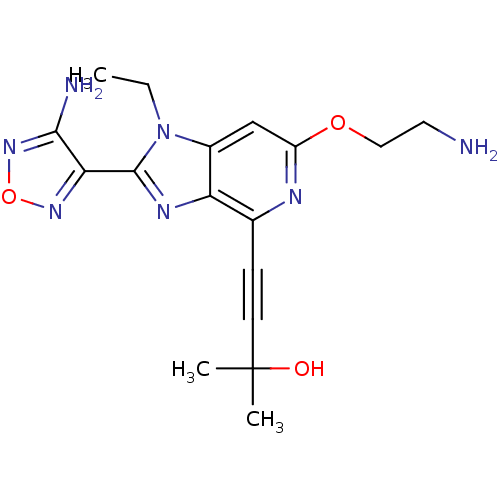
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(2-aminoetho...)Show SMILES CCn1c(nc2c(nc(OCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C17H21N7O3/c1-4-24-11-9-12(26-8-7-18)20-10(5-6-17(2,3)25)13(11)21-16(24)14-15(19)23-27-22-14/h9,25H,4,7-8,18H2,1-3H3,(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50126594

(3-Phenyl-4-{4-[3-(pyridin-2-ylamino)-propoxy]-phen...)Show InChI InChI=1S/C24H26N2O3/c27-24(28)18-21(20-7-2-1-3-8-20)17-19-10-12-22(13-11-19)29-16-6-15-26-23-9-4-5-14-25-23/h1-5,7-14,21H,6,15-18H2,(H,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta3 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50126597
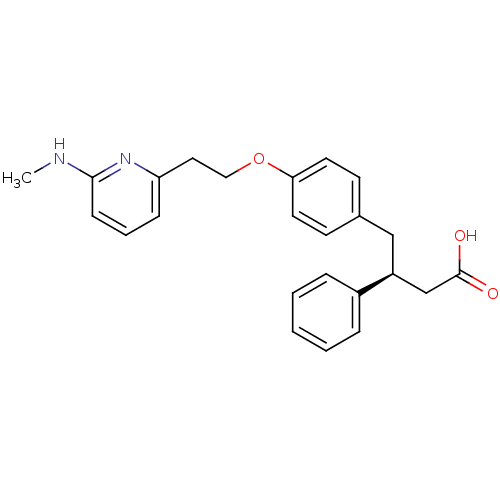
(4-{4-[2-(6-Methylamino-pyridin-2-yl)-ethoxy]-pheny...)Show SMILES CNc1cccc(CCOc2ccc(C[C@@H](CC(O)=O)c3ccccc3)cc2)n1 Show InChI InChI=1S/C24H26N2O3/c1-25-23-9-5-8-21(26-23)14-15-29-22-12-10-18(11-13-22)16-20(17-24(27)28)19-6-3-2-4-7-19/h2-13,20H,14-17H2,1H3,(H,25,26)(H,27,28)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta3 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278693

((+/-)-N-(1-amino-3-phenylpropan-2-yl)-4-bromo-5-(1...)Show SMILES NCC(Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50126596
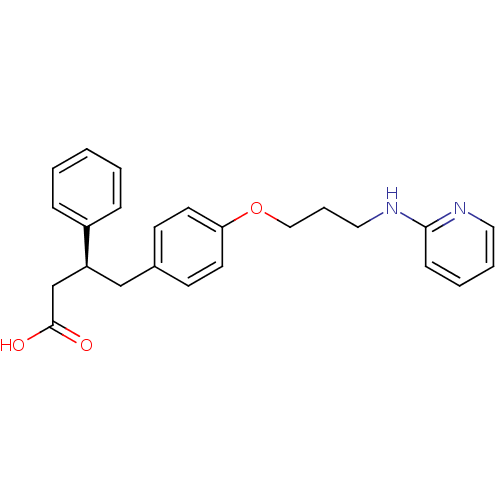
(3-Phenyl-4-{4-[3-(pyridin-2-ylamino)-propoxy]-phen...)Show SMILES OC(=O)C[C@H](Cc1ccc(OCCCNc2ccccn2)cc1)c1ccccc1 Show InChI InChI=1S/C24H26N2O3/c27-24(28)18-21(20-7-2-1-3-8-20)17-19-10-12-22(13-11-19)29-16-6-15-26-23-9-4-5-14-25-23/h1-5,7-14,21H,6,15-18H2,(H,25,26)(H,27,28)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphav/beta 3 vitronectin receptor in HEK cells |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278100

(CHEMBL482537 | N-((S)-1-amino-3-(4-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccc(cc1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-5-7-28-20-16(15)6-8-29-20)21(31)30-14(11-27)9-12-1-3-13(4-2-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278100

(CHEMBL482537 | N-((S)-1-amino-3-(4-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccc(cc1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-5-7-28-20-16(15)6-8-29-20)21(31)30-14(11-27)9-12-1-3-13(4-2-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316182

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SMYD3
(Homo sapiens (Human)) | BDBM50509581
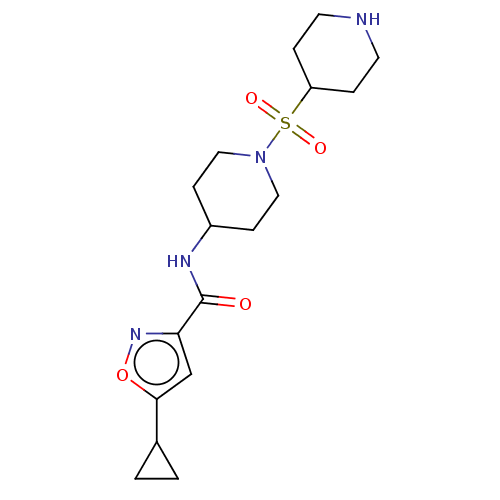
(CHEMBL4535915)Show SMILES O=C(NC1CCN(CC1)S(=O)(=O)C1CCNCC1)c1cc(on1)C1CC1 Show InChI InChI=1S/C17H26N4O4S/c22-17(15-11-16(25-20-15)12-1-2-12)19-13-5-9-21(10-6-13)26(23,24)14-3-7-18-8-4-14/h11-14,18H,1-10H2,(H,19,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Cheng-Prusoff equation analysis |
ACS Med Chem Lett 11: 133-140 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00493
BindingDB Entry DOI: 10.7270/Q2W66Q2M |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SMYD3
(Homo sapiens (Human)) | BDBM50509609

(CHEMBL4476167)Show SMILES FC(F)(F)CCCN[C@H]1CC[C@@H](CC1)S(=O)(=O)N1CCC(CC1)NC(=O)c1cc(on1)C1CC1 |r,wU:11.14,wD:8.7,(25.04,-22.84,;23.7,-23.6,;23.69,-25.14,;25.02,-24.36,;22.37,-22.82,;21.03,-23.58,;19.7,-22.8,;18.36,-23.56,;17.04,-22.78,;17.06,-21.23,;15.72,-20.44,;14.38,-21.21,;14.36,-22.76,;15.7,-23.54,;13.04,-20.42,;12.27,-19.09,;13.82,-19.08,;11.71,-21.2,;10.38,-20.42,;9.06,-21.2,;9.06,-22.74,;10.38,-23.5,;11.71,-22.74,;7.73,-23.51,;6.39,-22.75,;6.39,-21.21,;5.06,-23.51,;4.91,-25.04,;3.4,-25.36,;2.62,-24.04,;3.65,-22.89,;2.78,-26.77,;1.54,-27.68,;2.95,-28.3,)| Show InChI InChI=1S/C22H33F3N4O4S/c23-22(24,25)10-1-11-26-16-4-6-18(7-5-16)34(31,32)29-12-8-17(9-13-29)27-21(30)19-14-20(33-28-19)15-2-3-15/h14-18,26H,1-13H2,(H,27,30)/t16-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Cheng-Prusoff equation analysis |
ACS Med Chem Lett 11: 133-140 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00493
BindingDB Entry DOI: 10.7270/Q2W66Q2M |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SMYD3
(Homo sapiens (Human)) | BDBM50509609

(CHEMBL4476167)Show SMILES FC(F)(F)CCCN[C@H]1CC[C@@H](CC1)S(=O)(=O)N1CCC(CC1)NC(=O)c1cc(on1)C1CC1 |r,wU:11.14,wD:8.7,(25.04,-22.84,;23.7,-23.6,;23.69,-25.14,;25.02,-24.36,;22.37,-22.82,;21.03,-23.58,;19.7,-22.8,;18.36,-23.56,;17.04,-22.78,;17.06,-21.23,;15.72,-20.44,;14.38,-21.21,;14.36,-22.76,;15.7,-23.54,;13.04,-20.42,;12.27,-19.09,;13.82,-19.08,;11.71,-21.2,;10.38,-20.42,;9.06,-21.2,;9.06,-22.74,;10.38,-23.5,;11.71,-22.74,;7.73,-23.51,;6.39,-22.75,;6.39,-21.21,;5.06,-23.51,;4.91,-25.04,;3.4,-25.36,;2.62,-24.04,;3.65,-22.89,;2.78,-26.77,;1.54,-27.68,;2.95,-28.3,)| Show InChI InChI=1S/C22H33F3N4O4S/c23-22(24,25)10-1-11-26-16-4-6-18(7-5-16)34(31,32)29-12-8-17(9-13-29)27-21(30)19-14-20(33-28-19)15-2-3-15/h14-18,26H,1-13H2,(H,27,30)/t16-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Lineweaver-Burk plot analysis |
ACS Med Chem Lett 11: 133-140 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00493
BindingDB Entry DOI: 10.7270/Q2W66Q2M |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083760
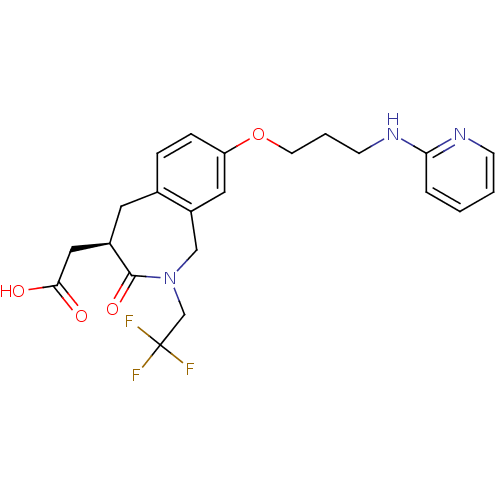
(CHEMBL86991 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...)Show SMILES OC(=O)C[C@H]1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278603
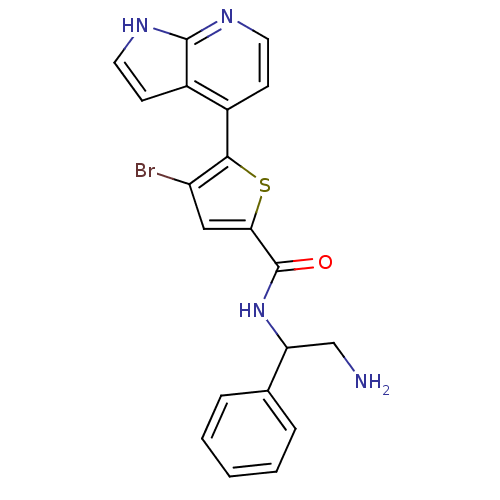
((+/-)-N-(2-amino-1-phenylethyl)-4-bromo-5-(1H-pyrr...)Show SMILES NCC(NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12)c1ccccc1 Show InChI InChI=1S/C20H17BrN4OS/c21-15-10-17(20(26)25-16(11-22)12-4-2-1-3-5-12)27-18(15)13-6-8-23-19-14(13)7-9-24-19/h1-10,16H,11,22H2,(H,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data