

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50305799 (5-((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 by cell-cell fusion assay | Bioorg Med Chem Lett 20: 704-8 (2010) Article DOI: 10.1016/j.bmcl.2009.11.072 BindingDB Entry DOI: 10.7270/Q22R3RSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50329256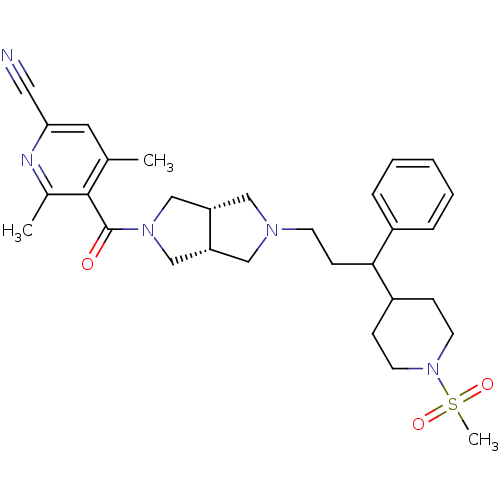 (4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of CCR5 by cell-cell fusion inhibition assay | Bioorg Med Chem Lett 20: 6802-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.118 BindingDB Entry DOI: 10.7270/Q29G5N1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334986 (4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 20: 1674-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.080 BindingDB Entry DOI: 10.7270/Q23B6131 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50305800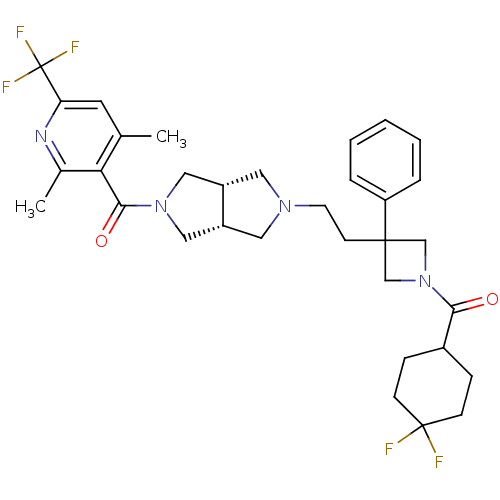 (((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 by cell-cell fusion assay | Bioorg Med Chem Lett 20: 704-8 (2010) Article DOI: 10.1016/j.bmcl.2009.11.072 BindingDB Entry DOI: 10.7270/Q22R3RSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27614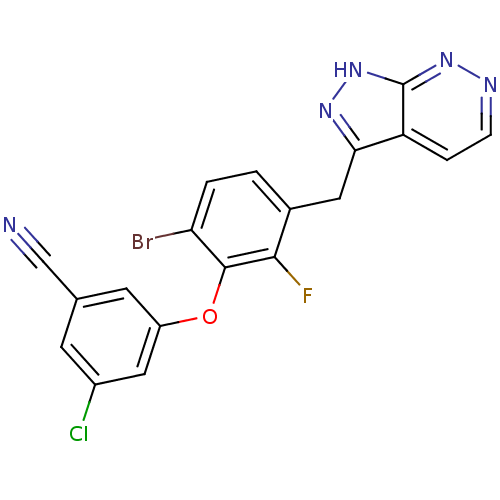 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | n/a | n/a | 3 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27615 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyrazin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | >25 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27614 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | 4 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50305801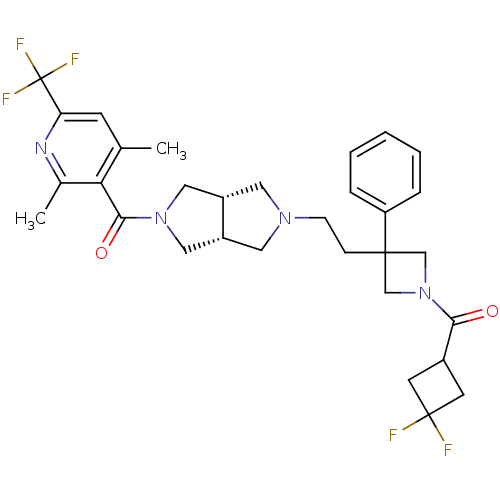 (((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbony...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 by cell-cell fusion assay | Bioorg Med Chem Lett 20: 704-8 (2010) Article DOI: 10.1016/j.bmcl.2009.11.072 BindingDB Entry DOI: 10.7270/Q22R3RSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318447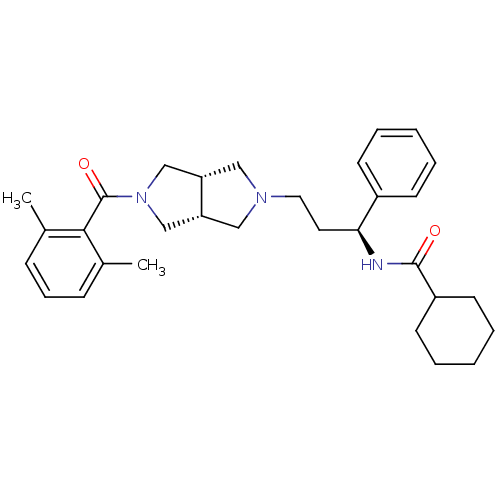 (CHEMBL1096764 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310739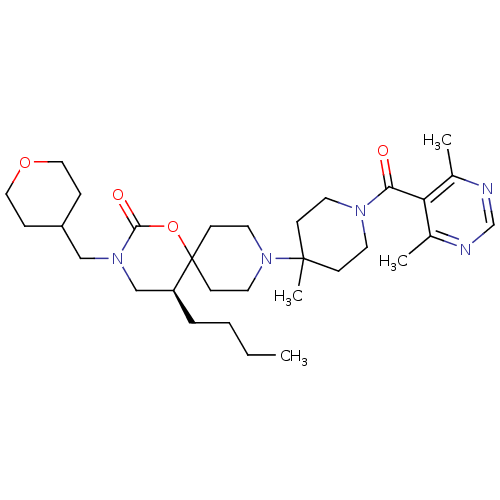 ((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Displacement of [128I]-RANTES from CCR5 receptor receptors expressed in CHO cells | Bioorg Med Chem Lett 20: 1830-3 (2010) Article DOI: 10.1016/j.bmcl.2010.02.004 BindingDB Entry DOI: 10.7270/Q25Q4W7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318446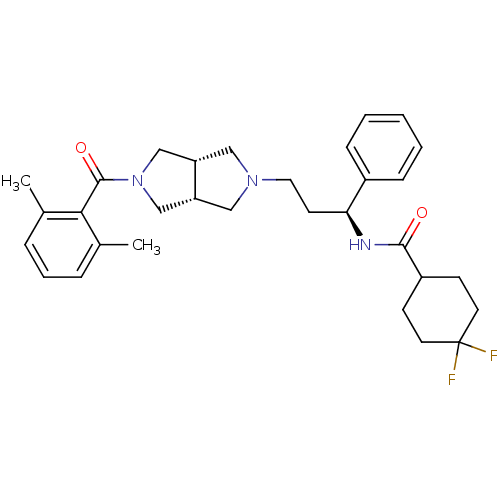 (CHEMBL1096765 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27613 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | 2 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50305794 (((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 by cell-cell fusion assay | Bioorg Med Chem Lett 20: 704-8 (2010) Article DOI: 10.1016/j.bmcl.2009.11.072 BindingDB Entry DOI: 10.7270/Q22R3RSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50312846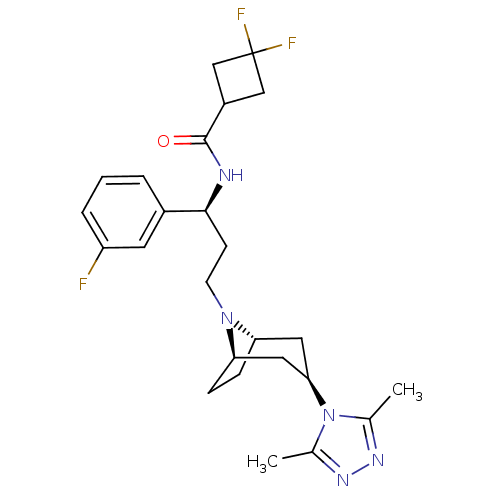 (CHEMBL1087535 | N-((S)-3-((1R,3S,5S)-3-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 20: 1674-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.080 BindingDB Entry DOI: 10.7270/Q23B6131 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50312848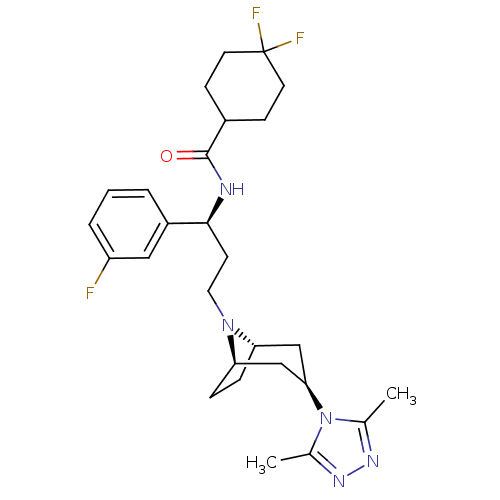 (CHEMBL1087153 | N-((S)-3-((1R,3S,5S)-3-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 20: 1674-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.080 BindingDB Entry DOI: 10.7270/Q23B6131 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319434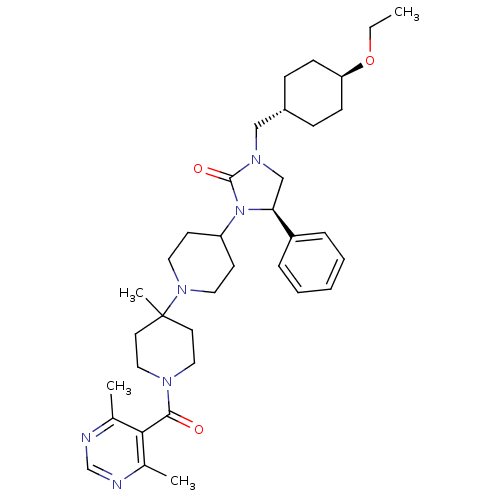 ((R)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50312852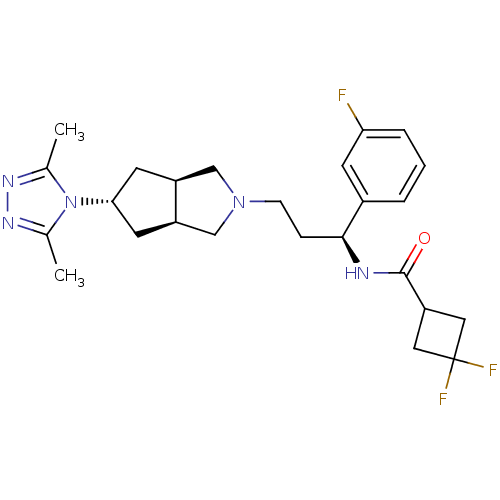 (CHEMBL1081476 | Exo-N-((S)-3-(5-(3,5-dimethyl-4H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 20: 1674-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.080 BindingDB Entry DOI: 10.7270/Q23B6131 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50312850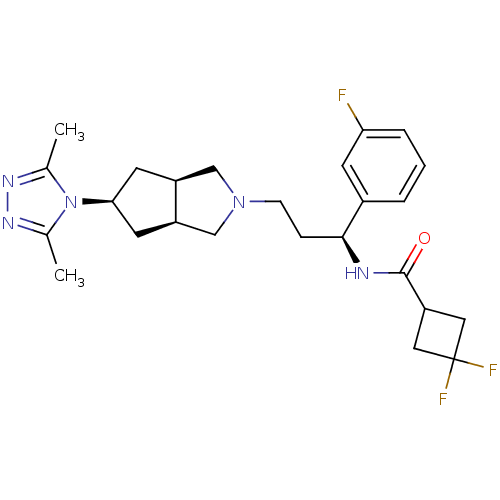 (CHEMBL1082191 | Endo-N-((S)-3-(5-(3,5-dimethyl-4H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 20: 1674-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.080 BindingDB Entry DOI: 10.7270/Q23B6131 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50115528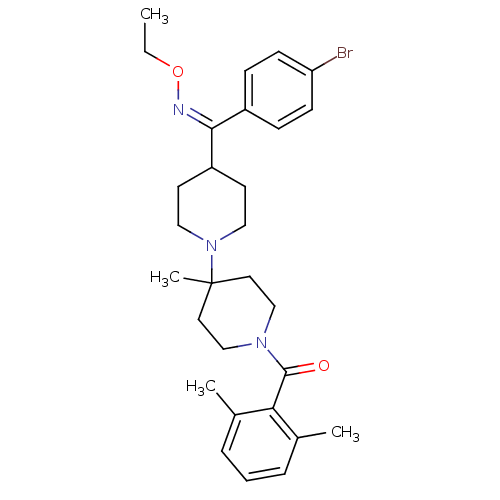 ((Z)-(4-((4-bromophenyl)(ethoxyimino)methyl)-4'-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318439 (CHEMBL1097815 | N-((S)-3-((3aR,6aS)-5-(4-fluoro-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318451 (CHEMBL1096445 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482847 (CHEMBL1258135) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 6020-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.068 BindingDB Entry DOI: 10.7270/Q2T72M88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482849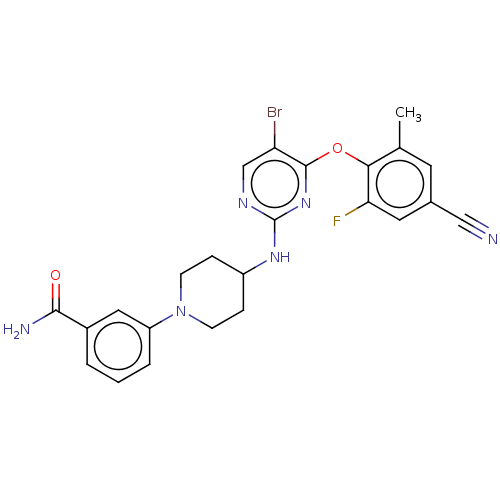 (CHEMBL1258364) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 6020-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.068 BindingDB Entry DOI: 10.7270/Q2T72M88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482849 (CHEMBL1258364) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 6020-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.068 BindingDB Entry DOI: 10.7270/Q2T72M88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482847 (CHEMBL1258135) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 6020-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.068 BindingDB Entry DOI: 10.7270/Q2T72M88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310731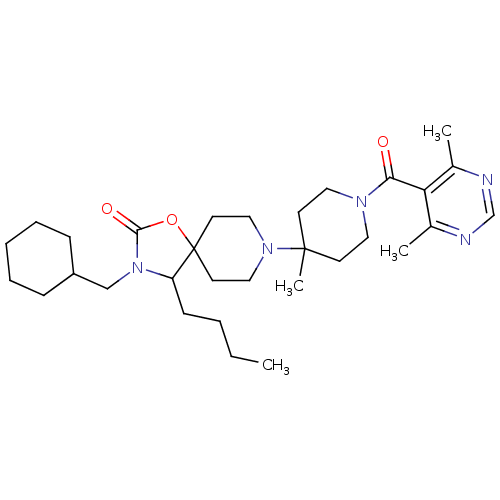 (4-butyl-3-(cyclohexylmethyl)-8-(1-(4,6-dimethylpyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50312845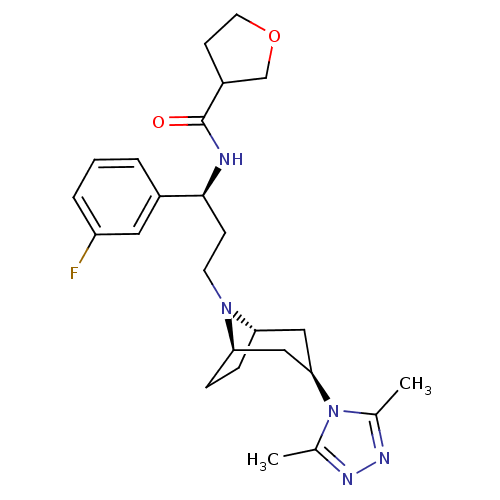 (CHEMBL1087534 | N-((S)-3-((1R,3S,5S)-3-(3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 20: 1674-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.080 BindingDB Entry DOI: 10.7270/Q23B6131 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318436 (CHEMBL1097169 | N-((S)-3-((3aR,6aS)-5-(2,4-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318448 (CHEMBL1096768 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482853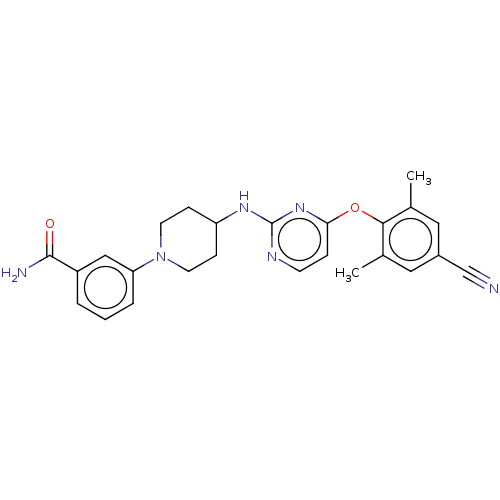 (CHEMBL1258814) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 6020-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.068 BindingDB Entry DOI: 10.7270/Q2T72M88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27611 (3-[6-bromo-2-fluoro-3-({6-methyl-7-oxo-1H,6H,7H-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | 4 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27612 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319451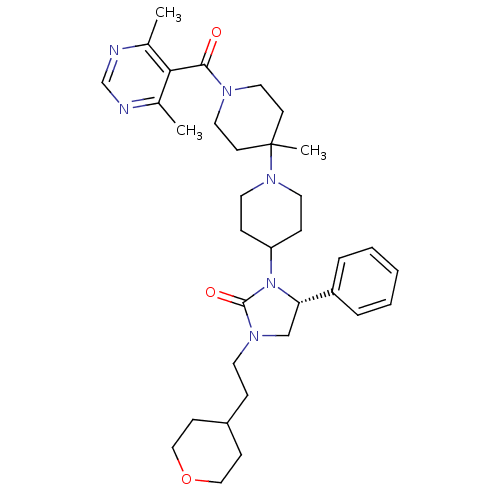 ((R)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50329255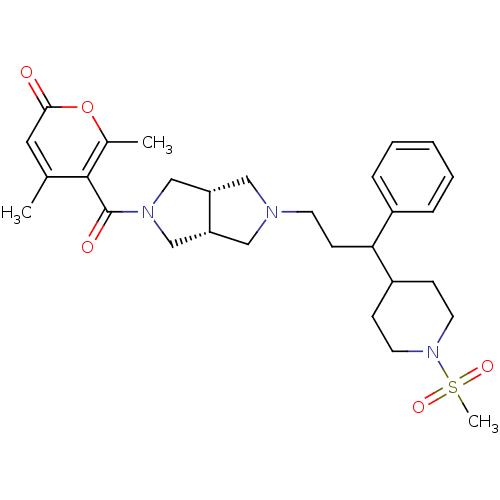 (4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of CCR5 by cell-cell fusion inhibition assay | Bioorg Med Chem Lett 20: 6802-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.118 BindingDB Entry DOI: 10.7270/Q29G5N1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50312843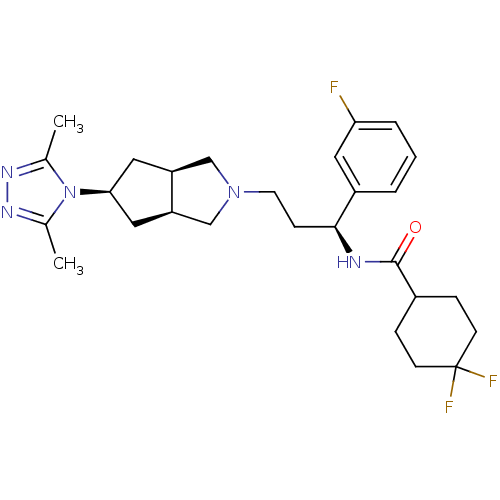 (CHEMBL1082202 | Endo-N-((S)-3-(5-(3,5-dimethyl-4H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Displacement of [128I]RANTES from human CCR5 receptor coexpressed with Galphai6 in CHO cells after 2 hrs by scintillation counting | Bioorg Med Chem Lett 20: 1674-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.080 BindingDB Entry DOI: 10.7270/Q23B6131 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318445 (CHEMBL1096766 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482845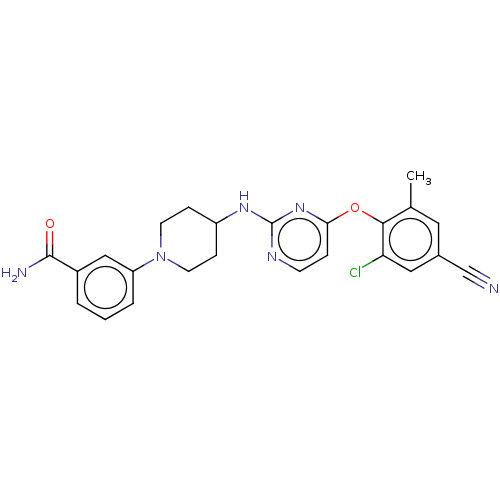 (CHEMBL1257905) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 6020-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.068 BindingDB Entry DOI: 10.7270/Q2T72M88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482845 (CHEMBL1257905) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 6020-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.068 BindingDB Entry DOI: 10.7270/Q2T72M88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27613 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | 18 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318427 (2-(3,3-difluorocyclobutyl)-N-((S)-3-((3aR,6aS)-5-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318441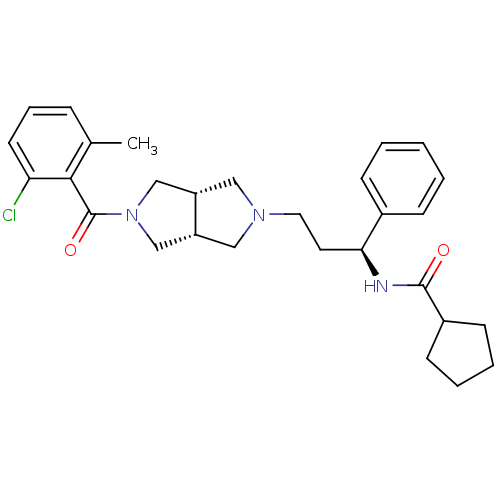 (CHEMBL1096102 | N-((S)-3-((3aR,6aS)-5-(2-chloro-6-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482842 (CHEMBL1257316) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 6020-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.068 BindingDB Entry DOI: 10.7270/Q2T72M88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50305791 (((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 by cell-cell fusion assay | Bioorg Med Chem Lett 20: 704-8 (2010) Article DOI: 10.1016/j.bmcl.2009.11.072 BindingDB Entry DOI: 10.7270/Q22R3RSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310730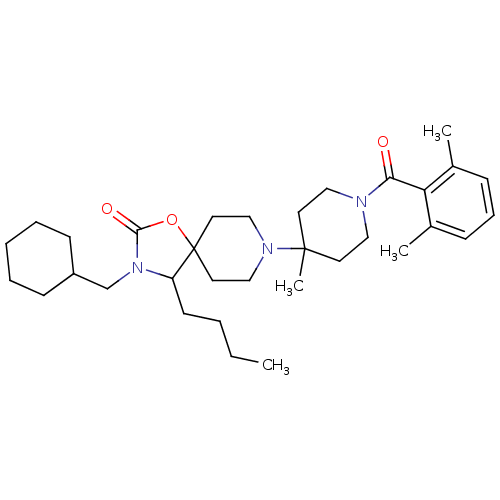 (4-butyl-3-(cyclohexylmethyl)-8-(1-(2,6-dimethylben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310747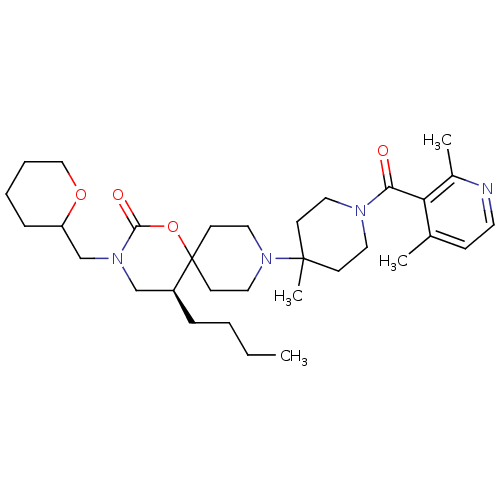 ((5S)-5-butyl-9-(1-(2,4-dimethylnicotinoyl)-4-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254720 ((4-(7-butyl-9-(morpholinosulfonyl)-3,9-diazaspiro[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50329231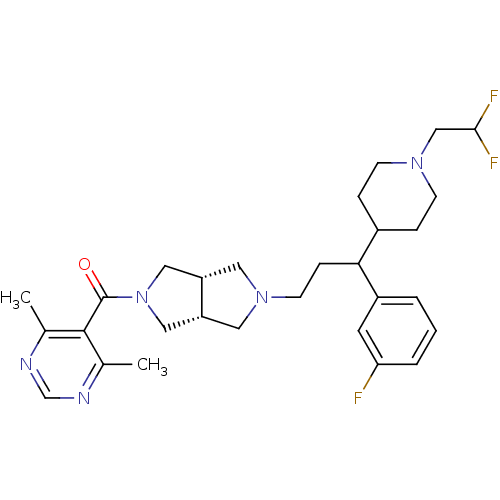 (((3aR,6aS)-5-(3-(1-(2,2-difluoroethyl)piperidin-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of CCR5 by cell-cell fusion inhibition assay | Bioorg Med Chem Lett 20: 6802-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.118 BindingDB Entry DOI: 10.7270/Q29G5N1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318449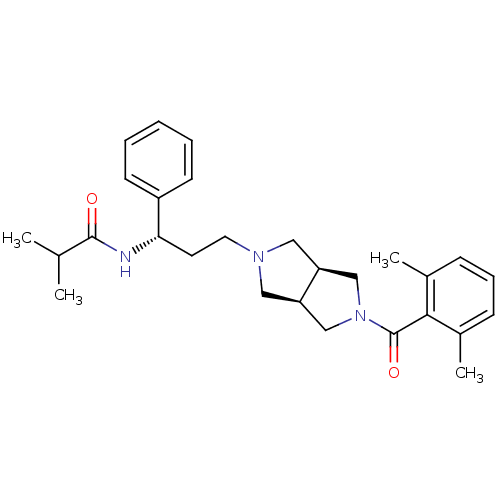 (CHEMBL1096447 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482824 (CHEMBL1258931) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 6020-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.068 BindingDB Entry DOI: 10.7270/Q2T72M88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27611 (3-[6-bromo-2-fluoro-3-({6-methyl-7-oxo-1H,6H,7H-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | 97 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 427 total ) | Next | Last >> |