

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006828 (2-(2-{[1-(3-{2-[2-(2-Amino-3-hydroxy-propionylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV-Protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM881 (L-685,434 derivative | L-689,502 | N-(2(R)-Hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006829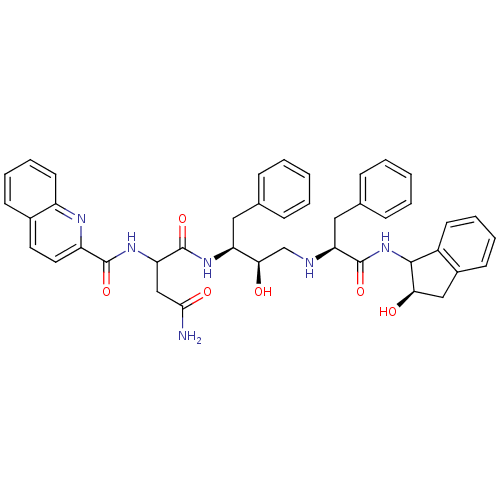 (CHEMBL315253 | N*1*-{1-Benzyl-2-hydroxy-3-[1-(2-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV-Protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283119 (CHEMBL69604 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(1R,2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006834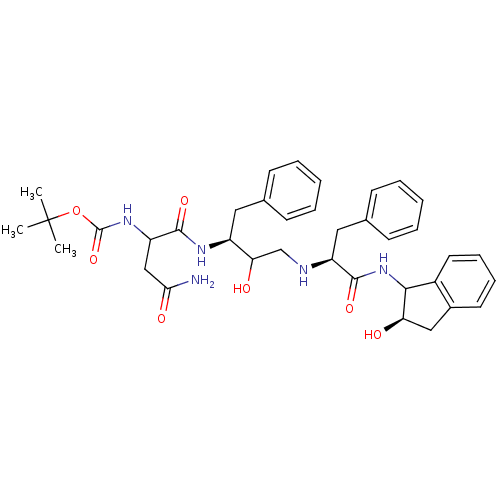 ((1-{1-Benzyl-2-hydroxy-3-[1-(2-hydroxy-indan-1-ylc...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV-Protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006826 (2-Amino-N*1*-{1-benzyl-2-hydroxy-3-[1-(2-hydroxy-i...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV-Protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006824 (CHEMBL82338 | [(1S,2R)-1-Benzyl-3-((3S,4aS,8aS)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV-Protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006831 (CHEMBL316216 | {1-Benzyl-2-hydroxy-3-[3-(2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV-Protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50406675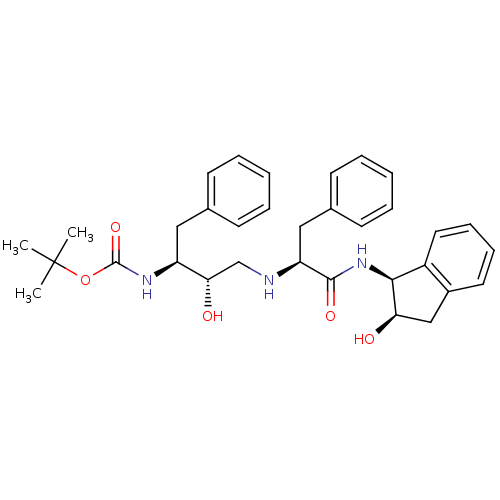 (CHEMBL2114201) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50406677 (CHEMBL2114200) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50406676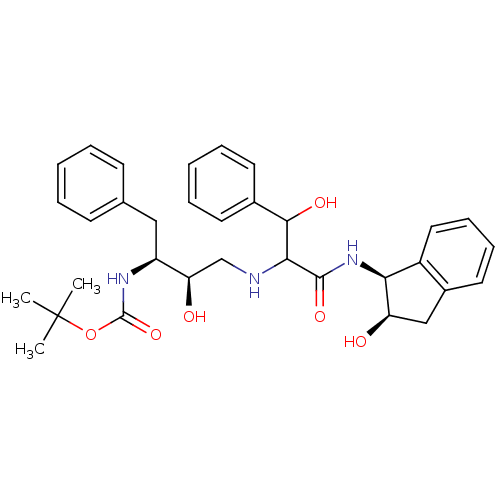 (CHEMBL2115206) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50406676 (CHEMBL2115206) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50406678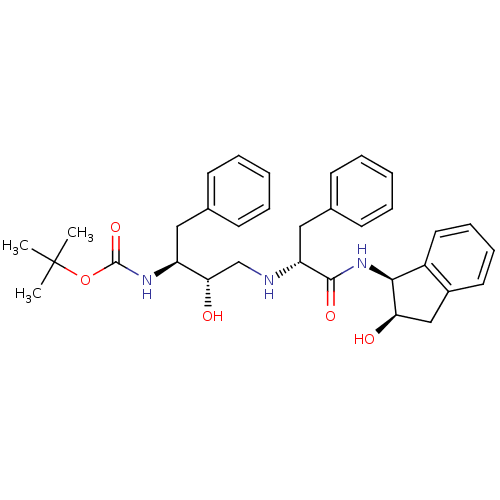 (CHEMBL2115205) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006825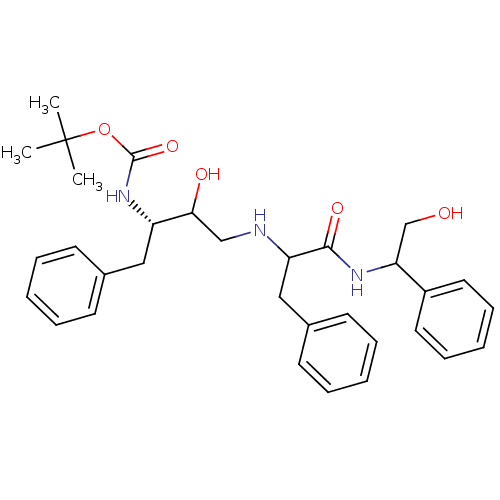 (CHEMBL431023 | {1-Benzyl-2-hydroxy-3-[1-(2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006835 (2-(3-Amino-2-hydroxy-4-phenyl-butylamino)-N-(2-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV-Protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006832 (CHEMBL313993 | [1-Benzyl-3-(1-tert-butylcarbamoyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV-Protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||