 Found 301 hits with Last Name = 'heimbrook' and Initial = 'dc'
Found 301 hits with Last Name = 'heimbrook' and Initial = 'dc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181139
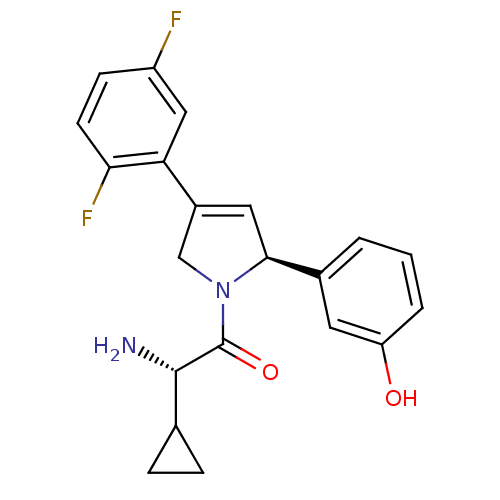
((S)-2-amino-2-cyclopropyl-1-((S)-4-(2,5-difluoroph...)Show SMILES N[C@@H](C1CC1)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:10| Show InChI InChI=1S/C21H20F2N2O2/c22-15-6-7-18(23)17(10-15)14-9-19(13-2-1-3-16(26)8-13)25(11-14)21(27)20(24)12-4-5-12/h1-3,6-10,12,19-20,26H,4-5,11,24H2/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM16179
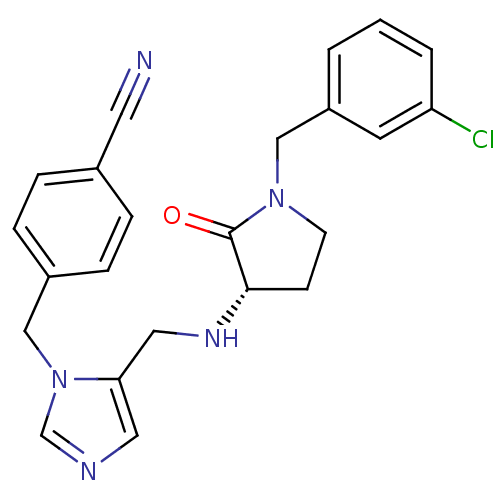
(4-{[5-({[(3S)-1-(3-chlorobenzyl)-2-oxopyrrolidin-3...)Show SMILES Clc1cccc(CN2CC[C@H](NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 |r| Show InChI InChI=1S/C23H22ClN5O/c24-20-3-1-2-19(10-20)15-28-9-8-22(23(28)30)27-13-21-12-26-16-29(21)14-18-6-4-17(11-25)5-7-18/h1-7,10,12,16,22,27H,8-9,13-15H2/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012304

(2-{2-[2-[2-(2,2-Dimethyl-propionylamino)-3-(3H-imi...)Show SMILES CCCCC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C48H72N12O7/c1-10-11-12-15-32(18-28(2)3)56-44(64)38(20-33-23-49-26-53-33)57-40(61)25-52-46(66)41(29(4)5)60-42(62)30(6)55-43(63)37(19-31-22-51-36-17-14-13-16-35(31)36)58-45(65)39(21-34-24-50-27-54-34)59-47(67)48(7,8)9/h13-14,16-17,22-24,26-30,32,37-39,41,51H,10-12,15,18-21,25H2,1-9H3,(H,49,53)(H,50,54)(H,52,66)(H,55,63)(H,56,64)(H,57,61)(H,58,65)(H,59,67)(H,60,62)/t30-,32+,37-,38-,39-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14025

((1R,2R,5R)-30-oxo-19,24-dioxa-2,6,10,12-tetraazahe...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCOc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C25H23N5O3/c26-11-17-2-1-16-9-24(17)33-19-3-4-23-20(10-19)22(6-8-32-23)30-7-5-21(25(30)31)28-13-18-12-27-15-29(18)14-16/h1-4,9-10,12,15,21-22,28H,5-8,13-14H2/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14018
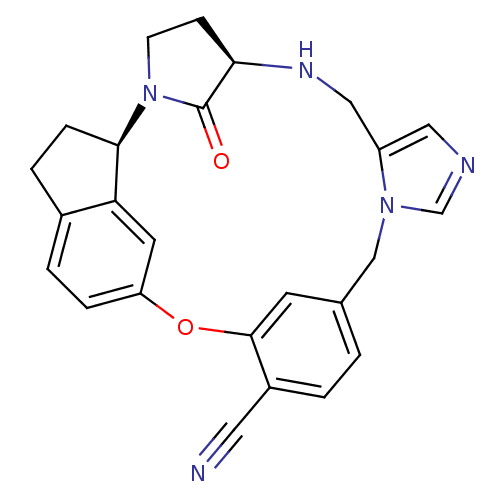
((17R, 20R)-19,20,21,22-Tetrahydro-19-oxo-17H-15,-1...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C25H23N5O2/c26-11-18-2-1-16-9-24(18)32-20-5-3-17-4-6-23(21(17)10-20)30-8-7-22(25(30)31)28-13-19-12-27-15-29(19)14-16/h1-3,5,9-10,12,15,22-23,28H,4,6-8,13-14H2/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14023

((1R,2R,5R)-30-oxo-19-oxa-2,6,10,12-tetraazahexacyc...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCCc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C26H25N5O2/c27-12-19-5-4-17-10-25(19)33-21-7-6-18-2-1-3-24(22(18)11-21)31-9-8-23(26(31)32)29-14-20-13-28-16-30(20)15-17/h4-7,10-11,13,16,23-24,29H,1-3,8-9,14-15H2/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50103360
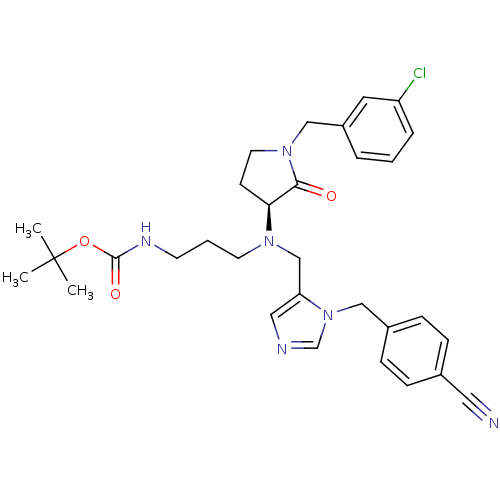
((3-{[1-(3-Chloro-benzyl)-2-oxo-pyrrolidin-3-yl]-[3...)Show SMILES CC(C)(C)OC(=O)NCCCN(Cc1cncn1Cc1ccc(cc1)C#N)[C@H]1CCN(Cc2cccc(Cl)c2)C1=O Show InChI InChI=1S/C31H37ClN6O3/c1-31(2,3)41-30(40)35-13-5-14-36(28-12-15-37(29(28)39)20-25-6-4-7-26(32)16-25)21-27-18-34-22-38(27)19-24-10-8-23(17-33)9-11-24/h4,6-11,16,18,22,28H,5,12-15,19-21H2,1-3H3,(H,35,40)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14014
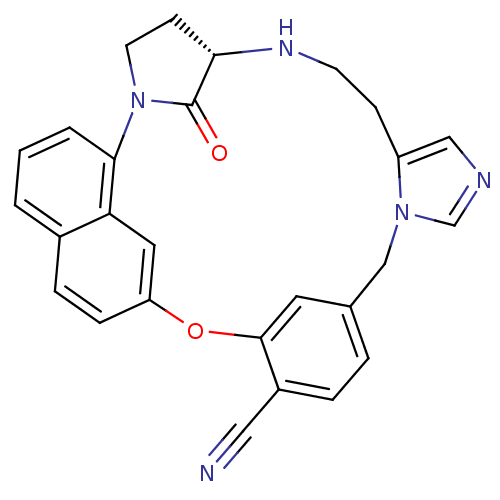
((5S)-31-oxo-20-oxa-2,6,11,13-tetraazahexacyclo[19....)Show SMILES O=C1[C@@H]2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CCN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C27H23N5O2/c28-14-20-5-4-18-12-26(20)34-22-7-6-19-2-1-3-25(23(19)13-22)32-11-9-24(27(32)33)30-10-8-21-15-29-17-31(21)16-18/h1-7,12-13,15,17,24,30H,8-11,16H2/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181138

((S)-2-amino-1-((S)-4-(2,5-difluorophenyl)-2-(3-hyd...)Show SMILES CC(C)[C@H](N)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:9| Show InChI InChI=1S/C21H22F2N2O2/c1-12(2)20(24)21(27)25-11-14(17-10-15(22)6-7-18(17)23)9-19(25)13-4-3-5-16(26)8-13/h3-10,12,19-20,26H,11,24H2,1-2H3/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012313

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CC(C)CC(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C Show InChI InChI=1S/C44H64N12O7/c1-24(2)13-30(14-25(3)4)53-42(61)37(17-32-20-46-23-50-32)54-38(58)21-48-44(63)39(26(5)6)56-40(59)27(7)51-41(60)35(15-29-18-47-34-12-10-9-11-33(29)34)55-43(62)36(52-28(8)57)16-31-19-45-22-49-31/h9-12,18-20,22-27,30,35-37,39,47H,13-17,21H2,1-8H3,(H,45,49)(H,46,50)(H,48,63)(H,51,60)(H,52,57)(H,53,61)(H,54,58)(H,55,62)(H,56,59)/t27-,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for mitogenic inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14015

((5R)-31-oxo-20-oxa-2,6,11,13-tetraazahexacyclo[19....)Show SMILES O=C1[C@H]2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CCN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C27H23N5O2/c28-14-20-5-4-18-12-26(20)34-22-7-6-19-2-1-3-25(23(19)13-22)32-11-9-24(27(32)33)30-10-8-21-15-29-17-31(21)16-18/h1-7,12-13,15,17,24,30H,8-11,16H2/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14017
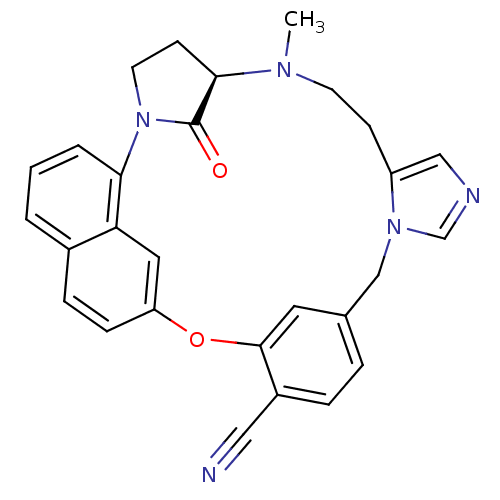
((5R)-6-methyl-31-oxo-20-oxa-2,6,11,13-tetraazahexa...)Show SMILES CN1CCc2cncn2Cc2ccc(C#N)c(Oc3ccc4cccc(N5CC[C@@H]1C5=O)c4c3)c2 |r| Show InChI InChI=1S/C28H25N5O2/c1-31-11-9-22-16-30-18-32(22)17-19-5-6-21(15-29)27(13-19)35-23-8-7-20-3-2-4-25(24(20)14-23)33-12-10-26(31)28(33)34/h2-8,13-14,16,18,26H,9-12,17H2,1H3/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50103360

((3-{[1-(3-Chloro-benzyl)-2-oxo-pyrrolidin-3-yl]-[3...)Show SMILES CC(C)(C)OC(=O)NCCCN(Cc1cncn1Cc1ccc(cc1)C#N)[C@H]1CCN(Cc2cccc(Cl)c2)C1=O Show InChI InChI=1S/C31H37ClN6O3/c1-31(2,3)41-30(40)35-13-5-14-36(28-12-15-37(29(28)39)20-25-6-4-7-26(32)16-25)21-27-18-34-22-38(27)19-24-10-8-23(17-33)9-11-24/h4,6-11,16,18,22,28H,5,12-15,19-21H2,1-3H3,(H,35,40)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase using purified recombinant human enzyme |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181137
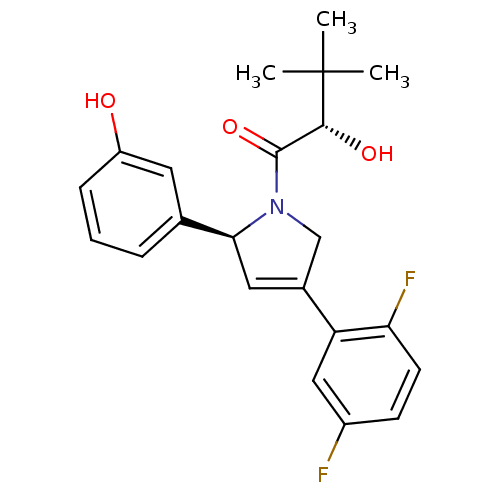
((S)-1-((S)-4-(2,5-difluorophenyl)-2-(3-hydroxyphen...)Show SMILES CC(C)(C)[C@H](O)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:10| Show InChI InChI=1S/C22H23F2NO3/c1-22(2,3)20(27)21(28)25-12-14(17-11-15(23)7-8-18(17)24)10-19(25)13-5-4-6-16(26)9-13/h4-11,19-20,26-27H,12H2,1-3H3/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14008
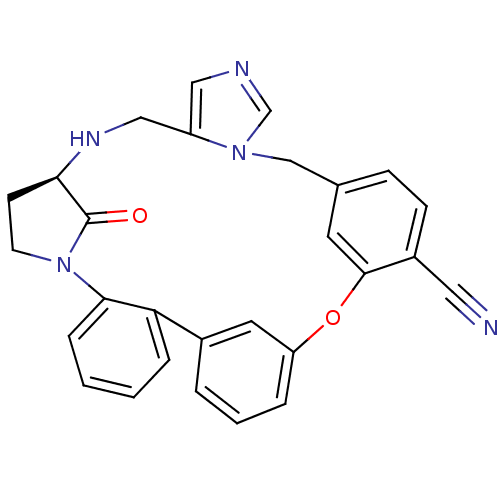
((11R)-32-oxo-25-oxa-8,12,16,18-tetraazahexacyclo[2...)Show SMILES O=C1[C@H]2CCN1c1ccccc1-c1cccc(Oc3cc(Cn4cncc4CN2)ccc3C#N)c1 |r| Show InChI InChI=1S/C28H23N5O2/c29-14-21-9-8-19-12-27(21)35-23-5-3-4-20(13-23)24-6-1-2-7-26(24)33-11-10-25(28(33)34)31-16-22-15-30-18-32(22)17-19/h1-9,12-13,15,18,25,31H,10-11,16-17H2/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14022
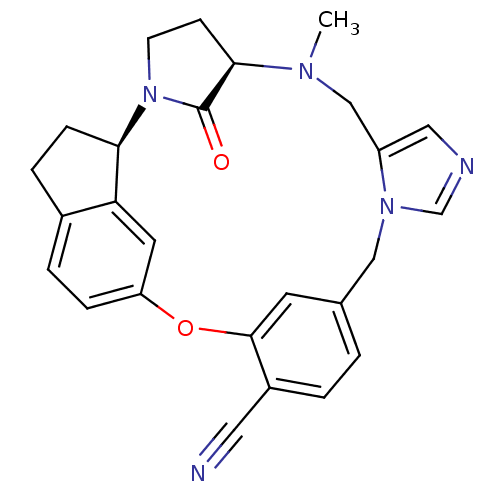
((1R,2R,5R)-6-methyl-29-oxo-19-oxa-2,6,10,12-tetraa...)Show SMILES CN1Cc2cncn2Cc2ccc(C#N)c(Oc3ccc4CC[C@@H](N5CC[C@@H]1C5=O)c4c3)c2 |r| Show InChI InChI=1S/C26H25N5O2/c1-29-15-20-13-28-16-30(20)14-17-2-3-19(12-27)25(10-17)33-21-6-4-18-5-7-23(22(18)11-21)31-9-8-24(29)26(31)32/h2-4,6,10-11,13,16,23-24H,5,7-9,14-15H2,1H3/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012318

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CCCC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C Show InChI InChI=1S/C44H64N12O7/c1-8-9-12-30(15-25(2)3)53-42(61)37(18-32-21-46-24-50-32)54-38(58)22-48-44(63)39(26(4)5)56-40(59)27(6)51-41(60)35(16-29-19-47-34-14-11-10-13-33(29)34)55-43(62)36(52-28(7)57)17-31-20-45-23-49-31/h10-11,13-14,19-21,23-27,30,35-37,39,47H,8-9,12,15-18,22H2,1-7H3,(H,45,49)(H,46,50)(H,48,63)(H,51,60)(H,52,57)(H,53,61)(H,54,58)(H,55,62)(H,56,59)/t27-,30+,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14007
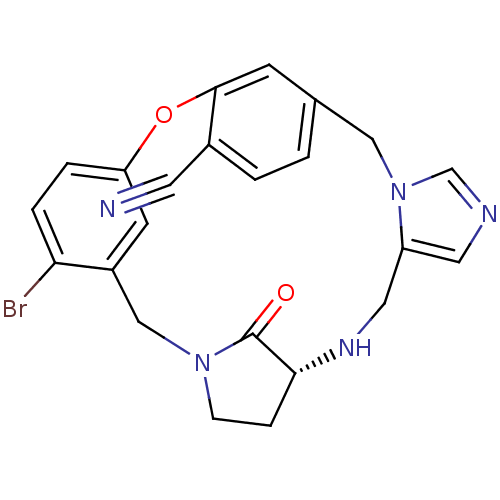
((6R)-24-bromo-27-oxo-20-oxa-3,7,11,13-tetraazapent...)Show SMILES Brc1ccc2Oc3cc(Cn4cncc4CN[C@@H]4CCN(Cc1c2)C4=O)ccc3C#N |r| Show InChI InChI=1S/C23H20BrN5O2/c24-20-4-3-19-8-17(20)13-28-6-5-21(23(28)30)27-11-18-10-26-14-29(18)12-15-1-2-16(9-25)22(7-15)31-19/h1-4,7-8,10,14,21,27H,5-6,11-13H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14016

((5S)-6-methyl-31-oxo-20-oxa-2,6,11,13-tetraazahexa...)Show SMILES CN1CCc2cncn2Cc2ccc(C#N)c(Oc3ccc4cccc(N5CC[C@H]1C5=O)c4c3)c2 |r| Show InChI InChI=1S/C28H25N5O2/c1-31-11-9-22-16-30-18-32(22)17-19-5-6-21(15-29)27(13-19)35-23-8-7-20-3-2-4-25(24(20)14-23)33-12-10-26(31)28(33)34/h2-8,13-14,16,18,26H,9-12,17H2,1H3/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14024
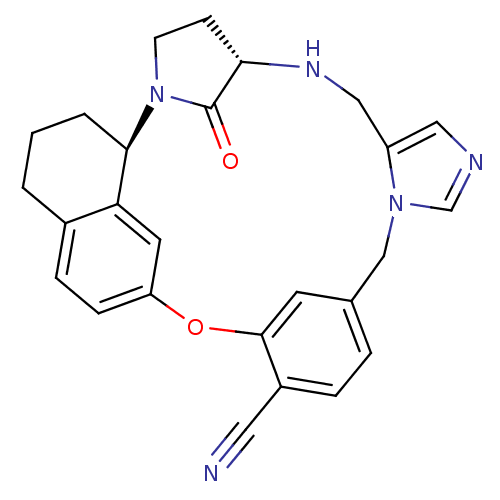
((1R,2R,5S)-30-oxo-19-oxa-2,6,10,12-tetraazahexacyc...)Show SMILES O=C1[C@@H]2CCN1[C@@H]1CCCc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C26H25N5O2/c27-12-19-5-4-17-10-25(19)33-21-7-6-18-2-1-3-24(22(18)11-21)31-9-8-23(26(31)32)29-14-20-13-28-16-30(20)15-17/h4-7,10-11,13,16,23-24,29H,1-3,8-9,14-15H2/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50103333
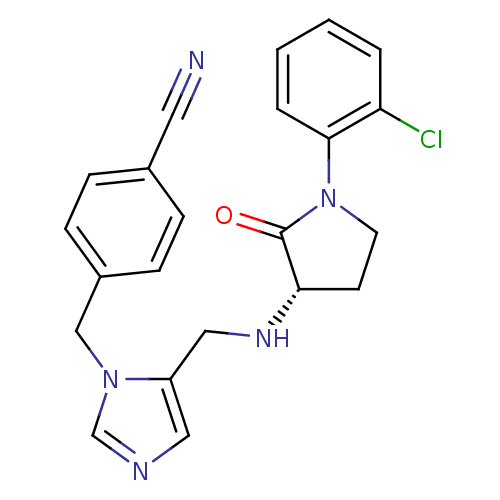
(4-(5-{[1-(2-Chloro-phenyl)-2-oxo-pyrrolidin-3-ylam...)Show SMILES Clc1ccccc1N1CC[C@H](NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C22H20ClN5O/c23-19-3-1-2-4-21(19)28-10-9-20(22(28)29)26-13-18-12-25-15-27(18)14-17-7-5-16(11-24)6-8-17/h1-8,12,15,20,26H,9-10,13-14H2/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase using purified recombinant human enzyme |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14010
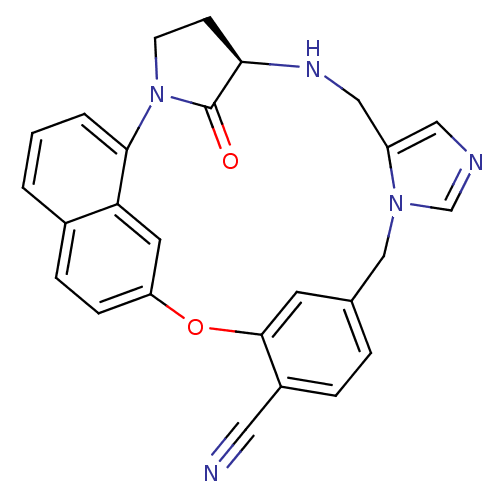
((20R)-19,20,21,22-Tetrahydro-19-oxo-5H-18,20-ethan...)Show SMILES O=C1[C@H]2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C26H21N5O2/c27-12-19-5-4-17-10-25(19)33-21-7-6-18-2-1-3-24(22(18)11-21)31-9-8-23(26(31)32)29-14-20-13-28-16-30(20)15-17/h1-7,10-11,13,16,23,29H,8-9,14-15H2/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012306

(Ac-His-Trp-Ala-Val-Gly-His-Leu-Met-NH2 | CHEMBL274...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)C(N)=O Show InChI InChI=1S/C46H66N14O9S/c1-24(2)14-34(43(66)57-33(40(47)63)12-13-70-7)58-45(68)37(17-30-20-49-23-53-30)56-38(62)21-51-46(69)39(25(3)4)60-41(64)26(5)54-42(65)35(15-28-18-50-32-11-9-8-10-31(28)32)59-44(67)36(55-27(6)61)16-29-19-48-22-52-29/h8-11,18-20,22-26,33-37,39,50H,12-17,21H2,1-7H3,(H2,47,63)(H,48,52)(H,49,53)(H,51,69)(H,54,65)(H,55,61)(H,56,62)(H,57,66)(H,58,68)(H,59,67)(H,60,64)/t26-,33-,34-,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012304

(2-{2-[2-[2-(2,2-Dimethyl-propionylamino)-3-(3H-imi...)Show SMILES CCCCC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C48H72N12O7/c1-10-11-12-15-32(18-28(2)3)56-44(64)38(20-33-23-49-26-53-33)57-40(61)25-52-46(66)41(29(4)5)60-42(62)30(6)55-43(63)37(19-31-22-51-36-17-14-13-16-35(31)36)58-45(65)39(21-34-24-50-27-54-34)59-47(67)48(7,8)9/h13-14,16-17,22-24,26-30,32,37-39,41,51H,10-12,15,18-21,25H2,1-9H3,(H,49,53)(H,50,54)(H,52,66)(H,55,63)(H,56,64)(H,57,61)(H,58,65)(H,59,67)(H,60,62)/t30-,32+,37-,38-,39-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM16179

(4-{[5-({[(3S)-1-(3-chlorobenzyl)-2-oxopyrrolidin-3...)Show SMILES Clc1cccc(CN2CC[C@H](NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 |r| Show InChI InChI=1S/C23H22ClN5O/c24-20-3-1-2-19(10-20)15-28-9-8-22(23(28)30)27-13-21-12-26-16-29(21)14-18-6-4-17(11-25)5-7-18/h1-7,10,12,16,22,27H,8-9,13-15H2/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase using purified recombinant human enzyme |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14005
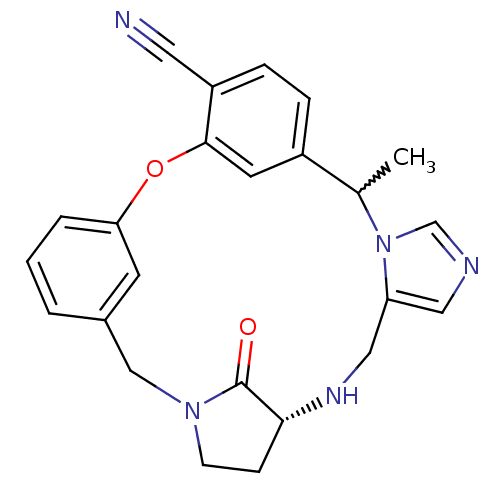
((20R)-19,20,21,22-Tetrahydro-5-methyl-19-oxo-17H-1...)Show SMILES CC1c2ccc(C#N)c(Oc3cccc(CN4CC[C@@H](NCc5cncn15)C4=O)c3)c2 |r,w:1.0| Show InChI InChI=1S/C24H23N5O2/c1-16-18-5-6-19(11-25)23(10-18)31-21-4-2-3-17(9-21)14-28-8-7-22(24(28)30)27-13-20-12-26-15-29(16)20/h2-6,9-10,12,15-16,22,27H,7-8,13-14H2,1H3/t16?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012317

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CCCCC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C Show InChI InChI=1S/C45H66N12O7/c1-8-9-10-13-31(16-26(2)3)54-43(62)38(19-33-22-47-25-51-33)55-39(59)23-49-45(64)40(27(4)5)57-41(60)28(6)52-42(61)36(17-30-20-48-35-15-12-11-14-34(30)35)56-44(63)37(53-29(7)58)18-32-21-46-24-50-32/h11-12,14-15,20-22,24-28,31,36-38,40,48H,8-10,13,16-19,23H2,1-7H3,(H,46,50)(H,47,51)(H,49,64)(H,52,61)(H,53,58)(H,54,62)(H,55,59)(H,56,63)(H,57,60)/t28-,31+,36-,37-,38-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM5284
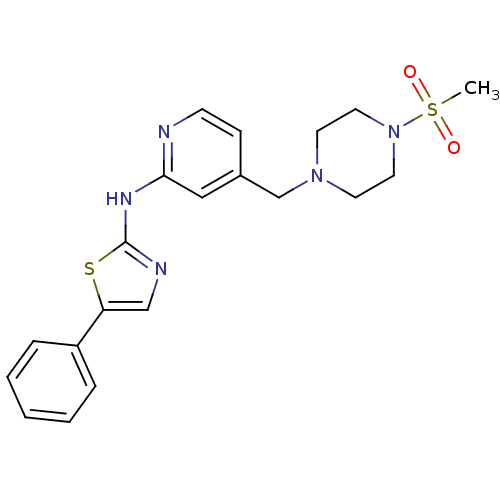
(4-{[4-(Methylsulfonyl)piperazin-1-yl]methyl}-N-(5-...)Show SMILES CS(=O)(=O)N1CCN(Cc2ccnc(Nc3ncc(s3)-c3ccccc3)c2)CC1 Show InChI InChI=1S/C20H23N5O2S2/c1-29(26,27)25-11-9-24(10-12-25)15-16-7-8-21-19(13-16)23-20-22-14-18(28-20)17-5-3-2-4-6-17/h2-8,13-14H,9-12,15H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories
| Assay Description
Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... |
J Med Chem 47: 6363-72 (2004)
Article DOI: 10.1021/jm049697f
BindingDB Entry DOI: 10.7270/Q2PG1PXD |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM24060
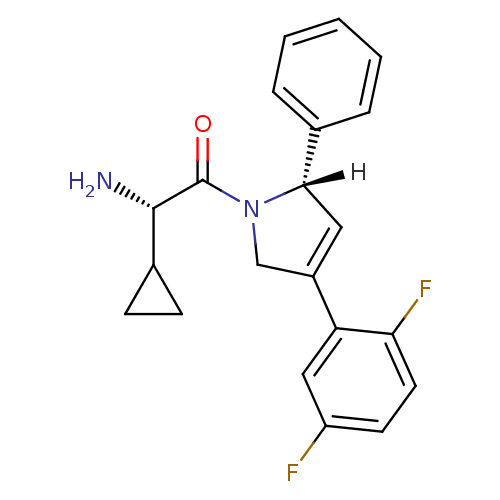
((2S)-2-amino-2-cyclopropyl-1-[(2S)-4-(2,5-difluoro...)Show SMILES [H][C@]1(C=C(CN1C(=O)[C@@H](N)C1CC1)c1cc(F)ccc1F)c1ccccc1 |r,c:2| Show InChI InChI=1S/C21H20F2N2O/c22-16-8-9-18(23)17(11-16)15-10-19(13-4-2-1-3-5-13)25(12-15)21(26)20(24)14-6-7-14/h1-5,8-11,14,19-20H,6-7,12,24H2/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Merck Research Laboratories
| Assay Description
The kinesin motor domain is incubated with microtubules, 1 mM ATP (1: 1 MgCl2 : Na-ATP), and compound at 23°C in buffer. After reaction was term... |
Bioorg Med Chem Lett 16: 1775-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.030
BindingDB Entry DOI: 10.7270/Q27W69HG |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14012

((5S)-7,31-dioxo-20-oxa-2,6,11,13-tetraazahexacyclo...)Show SMILES O=C1[C@@H]2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CC(=O)N2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C27H21N5O3/c28-13-19-5-4-17-10-25(19)35-21-7-6-18-2-1-3-24(22(18)12-21)32-9-8-23(27(32)34)30-26(33)11-20-14-29-16-31(20)15-17/h1-7,10,12,14,16,23H,8-9,11,15H2,(H,30,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14013
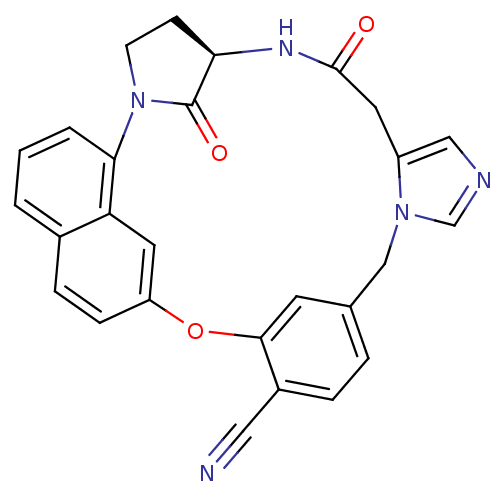
((5R)-7,31-dioxo-20-oxa-2,6,11,13-tetraazahexacyclo...)Show SMILES O=C1[C@H]2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CC(=O)N2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C27H21N5O3/c28-13-19-5-4-17-10-25(19)35-21-7-6-18-2-1-3-24(22(18)12-21)32-9-8-23(27(32)34)30-26(33)11-20-14-29-16-31(20)15-17/h1-7,10,12,14,16,23H,8-9,11,15H2,(H,30,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012311

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CCCC[C@@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(C)=O)C(C)C Show InChI InChI=1S/C44H64N12O7/c1-8-9-12-30(15-25(2)3)53-42(61)37(18-32-21-46-24-50-32)54-38(58)22-48-44(63)39(26(4)5)56-40(59)27(6)51-41(60)35(16-29-19-47-34-14-11-10-13-33(29)34)55-43(62)36(52-28(7)57)17-31-20-45-23-49-31/h10-11,13-14,19-21,23-27,30,35-37,39,47H,8-9,12,15-18,22H2,1-7H3,(H,45,49)(H,46,50)(H,48,63)(H,51,60)(H,52,57)(H,53,61)(H,54,58)(H,55,62)(H,56,59)/t27-,30-,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14003
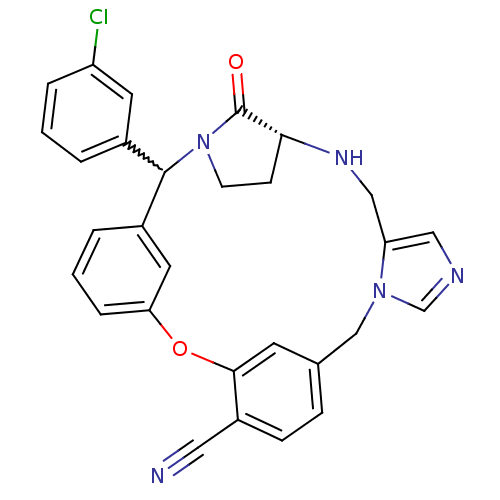
((20R)-17-(3-Chlorophenyl)-19,20,21,22-tetrahydro-1...)Show SMILES Clc1cccc(c1)C1N2CC[C@@H](NCc3cncn3Cc3ccc(C#N)c(Oc4cccc1c4)c3)C2=O |r,w:7.7| Show InChI InChI=1S/C29H24ClN5O2/c30-23-5-1-3-20(12-23)28-21-4-2-6-25(13-21)37-27-11-19(7-8-22(27)14-31)17-34-18-32-15-24(34)16-33-26-9-10-35(28)29(26)36/h1-8,11-13,15,18,26,28,33H,9-10,16-17H2/t26-,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50103322

(4-(5-{[[1-(3-Chloro-benzyl)-2-oxo-pyrrolidin-3-yl]...)Show SMILES Clc1cccc(CN2CC[C@H](N(CCCc3ccccc3)Cc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 Show InChI InChI=1S/C32H32ClN5O/c33-29-10-4-8-28(18-29)22-37-17-15-31(32(37)39)36(16-5-9-25-6-2-1-3-7-25)23-30-20-35-24-38(30)21-27-13-11-26(19-34)12-14-27/h1-4,6-8,10-14,18,20,24,31H,5,9,15-17,21-23H2/t31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase using purified recombinant human enzyme |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM24054
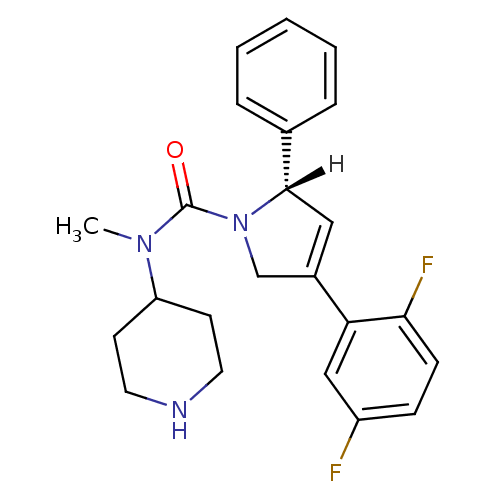
((2S)-4-(2,5-difluorophenyl)-N-methyl-2-phenyl-N-(p...)Show SMILES [H][C@]1(C=C(CN1C(=O)N(C)C1CCNCC1)c1cc(F)ccc1F)c1ccccc1 |r,c:2| Show InChI InChI=1S/C23H25F2N3O/c1-27(19-9-11-26-12-10-19)23(29)28-15-17(20-14-18(24)7-8-21(20)25)13-22(28)16-5-3-2-4-6-16/h2-8,13-14,19,22,26H,9-12,15H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Merck Research Laboratories
| Assay Description
The kinesin motor domain is incubated with microtubules, 1 mM ATP (1: 1 MgCl2 : Na-ATP), and compound at 23°C in buffer. After reaction was term... |
Bioorg Med Chem Lett 16: 1775-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.030
BindingDB Entry DOI: 10.7270/Q27W69HG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012307

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CCC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C Show InChI InChI=1S/C43H62N12O7/c1-8-11-29(14-24(2)3)52-41(60)36(17-31-20-45-23-49-31)53-37(57)21-47-43(62)38(25(4)5)55-39(58)26(6)50-40(59)34(15-28-18-46-33-13-10-9-12-32(28)33)54-42(61)35(51-27(7)56)16-30-19-44-22-48-30/h9-10,12-13,18-20,22-26,29,34-36,38,46H,8,11,14-17,21H2,1-7H3,(H,44,48)(H,45,49)(H,47,62)(H,50,59)(H,51,56)(H,52,60)(H,53,57)(H,54,61)(H,55,58)/t26-,29+,34-,35-,36-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50103318

(4-(5-{[1-(2-Chloro-benzyl)-2-oxo-pyrrolidin-3-ylam...)Show SMILES Clc1ccccc1CN1CC[C@H](NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C23H22ClN5O/c24-21-4-2-1-3-19(21)15-28-10-9-22(23(28)30)27-13-20-12-26-16-29(20)14-18-7-5-17(11-25)6-8-18/h1-8,12,16,22,27H,9-10,13-15H2/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM24061
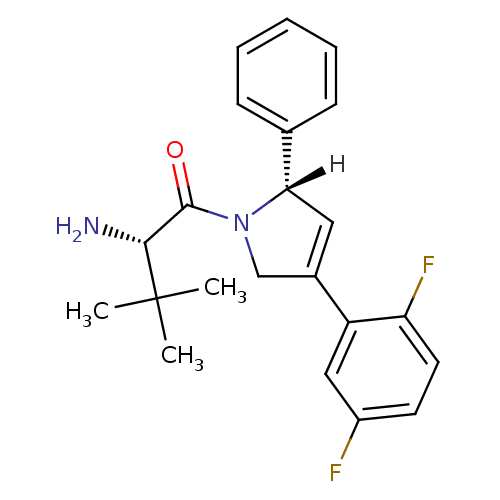
((2S)-2-amino-1-[(2S)-4-(2,5-difluorophenyl)-2-phen...)Show SMILES [H][C@]1(C=C(CN1C(=O)[C@@H](N)C(C)(C)C)c1cc(F)ccc1F)c1ccccc1 |r,c:2| Show InChI InChI=1S/C22H24F2N2O/c1-22(2,3)20(25)21(27)26-13-15(17-12-16(23)9-10-18(17)24)11-19(26)14-7-5-4-6-8-14/h4-12,19-20H,13,25H2,1-3H3/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Merck Research Laboratories
| Assay Description
The kinesin motor domain is incubated with microtubules, 1 mM ATP (1: 1 MgCl2 : Na-ATP), and compound at 23°C in buffer. After reaction was term... |
Bioorg Med Chem Lett 16: 1775-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.030
BindingDB Entry DOI: 10.7270/Q27W69HG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012308

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CCCCCC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C Show InChI InChI=1S/C46H68N12O7/c1-8-9-10-11-14-32(17-27(2)3)55-44(63)39(20-34-23-48-26-52-34)56-40(60)24-50-46(65)41(28(4)5)58-42(61)29(6)53-43(62)37(18-31-21-49-36-16-13-12-15-35(31)36)57-45(64)38(54-30(7)59)19-33-22-47-25-51-33/h12-13,15-16,21-23,25-29,32,37-39,41,49H,8-11,14,17-20,24H2,1-7H3,(H,47,51)(H,48,52)(H,50,65)(H,53,62)(H,54,59)(H,55,63)(H,56,60)(H,57,64)(H,58,61)/t29-,32+,37-,38-,39-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181141
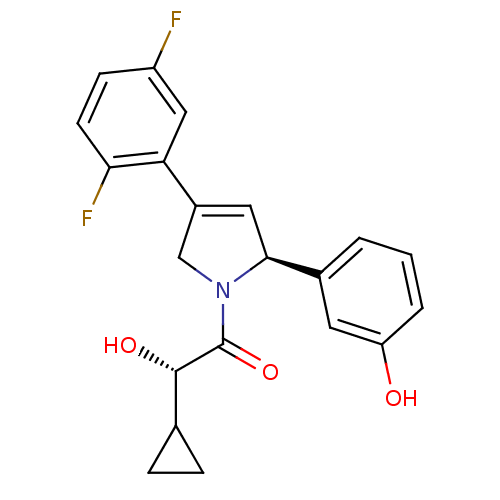
((S)-2-cyclopropyl-1-((S)-4-(2,5-difluorophenyl)-2-...)Show SMILES O[C@@H](C1CC1)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:10| Show InChI InChI=1S/C21H19F2NO3/c22-15-6-7-18(23)17(10-15)14-9-19(13-2-1-3-16(25)8-13)24(11-14)21(27)20(26)12-4-5-12/h1-3,6-10,12,19-20,25-26H,4-5,11H2/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM5282
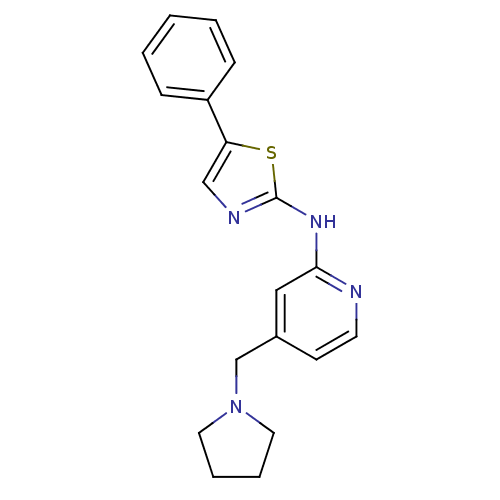
((5-Phenylthiazol-2-yl)(4-pyrrolidin-1-ylmethylpyri...)Show InChI InChI=1S/C19H20N4S/c1-2-6-16(7-3-1)17-13-21-19(24-17)22-18-12-15(8-9-20-18)14-23-10-4-5-11-23/h1-3,6-9,12-13H,4-5,10-11,14H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories
| Assay Description
Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... |
J Med Chem 47: 6363-72 (2004)
Article DOI: 10.1021/jm049697f
BindingDB Entry DOI: 10.7270/Q2PG1PXD |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012318

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CCCC[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C Show InChI InChI=1S/C44H64N12O7/c1-8-9-12-30(15-25(2)3)53-42(61)37(18-32-21-46-24-50-32)54-38(58)22-48-44(63)39(26(4)5)56-40(59)27(6)51-41(60)35(16-29-19-47-34-14-11-10-13-33(29)34)55-43(62)36(52-28(7)57)17-31-20-45-23-49-31/h10-11,13-14,19-21,23-27,30,35-37,39,47H,8-9,12,15-18,22H2,1-7H3,(H,45,49)(H,46,50)(H,48,63)(H,51,60)(H,52,57)(H,53,61)(H,54,58)(H,55,62)(H,56,59)/t27-,30+,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for mitogenic inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012309

(Ac-His-Trp-Ala-Val-D-Ala-His-Leu-Met-NH2 | CHEMBL2...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)C(N)=O Show InChI InChI=1S/C47H68N14O9S/c1-24(2)15-35(44(67)57-34(40(48)63)13-14-71-8)59-46(69)38(18-31-21-50-23-53-31)58-41(64)26(5)55-47(70)39(25(3)4)61-42(65)27(6)54-43(66)36(16-29-19-51-33-12-10-9-11-32(29)33)60-45(68)37(56-28(7)62)17-30-20-49-22-52-30/h9-12,19-27,34-39,51H,13-18H2,1-8H3,(H2,48,63)(H,49,52)(H,50,53)(H,54,66)(H,55,70)(H,56,62)(H,57,67)(H,58,64)(H,59,69)(H,60,68)(H,61,65)/t26-,27+,34+,35+,36+,37+,38+,39+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012297

(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-yl)-propio...)Show SMILES CCCCC(CCCC)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C Show InChI InChI=1S/C44H64N12O7/c1-7-9-13-30(14-10-8-2)53-42(61)37(19-32-22-46-25-50-32)54-38(58)23-48-44(63)39(26(3)4)56-40(59)27(5)51-41(60)35(17-29-20-47-34-16-12-11-15-33(29)34)55-43(62)36(52-28(6)57)18-31-21-45-24-49-31/h11-12,15-16,20-22,24-27,30,35-37,39,47H,7-10,13-14,17-19,23H2,1-6H3,(H,45,49)(H,46,50)(H,48,63)(H,51,60)(H,52,57)(H,53,61)(H,54,58)(H,55,62)(H,56,59)/t27-,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for mitogenic inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50103334
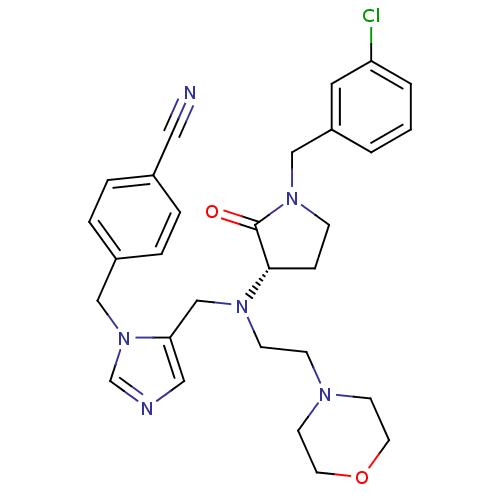
(4-(5-{[[1-(3-Chloro-benzyl)-2-oxo-pyrrolidin-3-yl]...)Show SMILES Clc1cccc(CN2CC[C@H](N(CCN3CCOCC3)Cc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 Show InChI InChI=1S/C29H33ClN6O2/c30-26-3-1-2-25(16-26)20-35-9-8-28(29(35)37)34(11-10-33-12-14-38-15-13-33)21-27-18-32-22-36(27)19-24-6-4-23(17-31)5-7-24/h1-7,16,18,22,28H,8-15,19-21H2/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14009
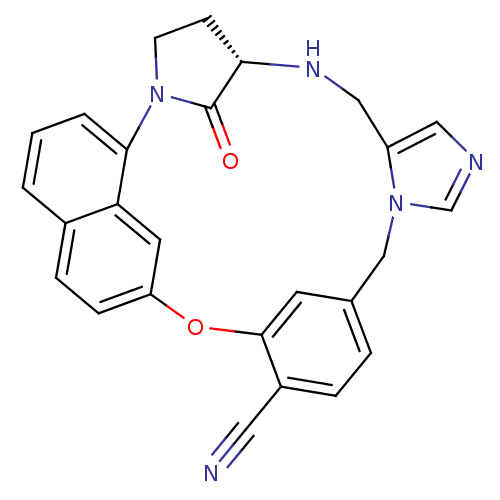
((20S)-19,20,21,22-Tetrahydro-19-oxo-5H-18,20-ethan...)Show SMILES O=C1[C@@H]2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C26H21N5O2/c27-12-19-5-4-17-10-25(19)33-21-7-6-18-2-1-3-24(22(18)11-21)31-9-8-23(26(31)32)29-14-20-13-28-16-30(20)15-17/h1-7,10-11,13,16,23,29H,8-9,14-15H2/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13998
((20R)-19,20,21,22-Tetrahydro-19-oxo-17H-18,20-etha...)Show SMILES O=C1[C@H]2CCN1Cc1cccc(Oc3cc(Cn4cncc4CN2)ccc3C#N)c1 |r| Show InChI InChI=1S/C23H21N5O2/c24-10-18-5-4-17-9-22(18)30-20-3-1-2-16(8-20)13-27-7-6-21(23(27)29)26-12-19-11-25-15-28(19)14-17/h1-5,8-9,11,15,21,26H,6-7,12-14H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14020
((1R,2R,5S)-29-oxo-19-oxa-2,6,10,12-tetraazahexacyc...)Show SMILES O=C1[C@@H]2CCN1[C@@H]1CCc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C25H23N5O2/c26-11-18-2-1-16-9-24(18)32-20-5-3-17-4-6-23(21(17)10-20)30-8-7-22(25(30)31)28-13-19-12-27-15-29(19)14-16/h1-3,5,9-10,12,15,22-23,28H,4,6-8,13-14H2/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM24058

((2S)-2-amino-1-[(2S)-4-(2,5-difluorophenyl)-2-phen...)Show SMILES [H][C@]1(C=C(CN1C(=O)[C@@H](N)C(C)C)c1cc(F)ccc1F)c1ccccc1 |r,c:2| Show InChI InChI=1S/C21H22F2N2O/c1-13(2)20(24)21(26)25-12-15(17-11-16(22)8-9-18(17)23)10-19(25)14-6-4-3-5-7-14/h3-11,13,19-20H,12,24H2,1-2H3/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Merck Research Laboratories
| Assay Description
The kinesin motor domain is incubated with microtubules, 1 mM ATP (1: 1 MgCl2 : Na-ATP), and compound at 23°C in buffer. After reaction was term... |
Bioorg Med Chem Lett 16: 1775-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.030
BindingDB Entry DOI: 10.7270/Q27W69HG |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(MOUSE) | BDBM50012300

(2-[2-[2-(2-{2-[2-[2-Acetylamino-3-(3H-imidazol-4-y...)Show SMILES CCOC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(C)=O)C(C)C Show InChI InChI=1S/C43H60N12O9/c1-8-64-43(63)35(13-23(2)3)54-41(61)34(16-29-19-45-22-49-29)52-36(57)20-47-42(62)37(24(4)5)55-38(58)25(6)50-39(59)32(14-27-17-46-31-12-10-9-11-30(27)31)53-40(60)33(51-26(7)56)15-28-18-44-21-48-28/h9-12,17-19,21-25,32-35,37,46H,8,13-16,20H2,1-7H3,(H,44,48)(H,45,49)(H,47,62)(H,50,59)(H,51,56)(H,52,57)(H,53,60)(H,54,61)(H,55,58)/t25-,32-,33-,34-,35-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against Swiss 3T3 murine fibroblast cells. |
J Med Chem 34: 2102-7 (1991)
BindingDB Entry DOI: 10.7270/Q2X63KXB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data