 Found 191 hits with Last Name = 'heintzelman' and Initial = 'gr'
Found 191 hits with Last Name = 'heintzelman' and Initial = 'gr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50161342
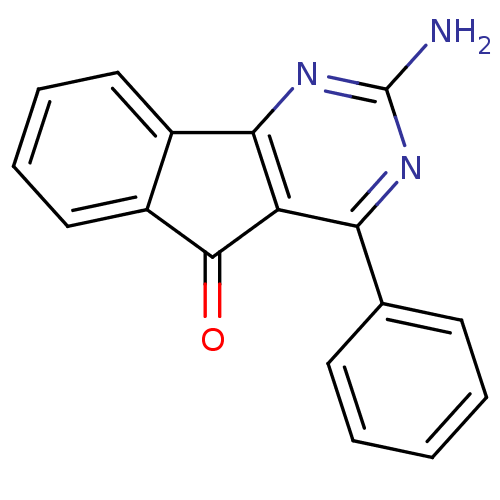
(2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...)Show InChI InChI=1S/C17H11N3O/c18-17-19-14(10-6-2-1-3-7-10)13-15(20-17)11-8-4-5-9-12(11)16(13)21/h1-9H,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50317007
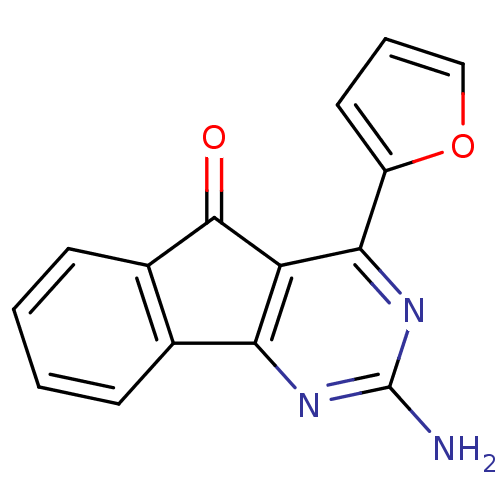
(2-amino-4-(furan-2-yl)-5H-indeno[1,2-d]pyrimidin-5...)Show InChI InChI=1S/C15H9N3O2/c16-15-17-12-8-4-1-2-5-9(8)14(19)11(12)13(18-15)10-6-3-7-20-10/h1-7H,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50317008
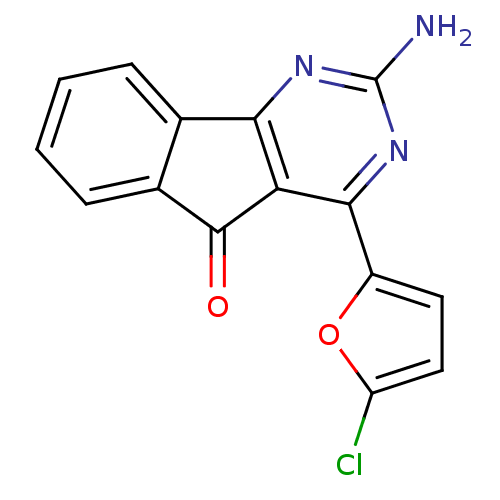
(2-amino-4-(5-chlorofuran-2-yl)-5H-indeno[1,2-d]pyr...)Show InChI InChI=1S/C15H8ClN3O2/c16-10-6-5-9(21-10)13-11-12(18-15(17)19-13)7-3-1-2-4-8(7)14(11)20/h1-6H,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156774

(CHEMBL3792888)Show InChI InChI=1S/C19H19N3O2/c20-10-18(14-3-1-13(12-23)2-4-14)19(24)22-17-6-5-16-11-21-8-7-15(16)9-17/h1-9,11,18,23H,10,12,20H2,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50317007

(2-amino-4-(furan-2-yl)-5H-indeno[1,2-d]pyrimidin-5...)Show InChI InChI=1S/C15H9N3O2/c16-15-17-12-8-4-1-2-5-9(8)14(19)11(12)13(18-15)10-6-3-7-20-10/h1-7H,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156799

(CHEMBL3793004)Show InChI InChI=1S/C18H17N3O/c19-11-17(13-4-2-1-3-5-13)18(22)21-16-7-6-15-12-20-9-8-14(15)10-16/h1-10,12,17H,11,19H2,(H,21,22)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156784

(CHEMBL3792673)Show InChI InChI=1S/C19H19N3O/c1-13-2-4-14(5-3-13)18(11-20)19(23)22-17-7-6-16-12-21-9-8-15(16)10-17/h2-10,12,18H,11,20H2,1H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156788
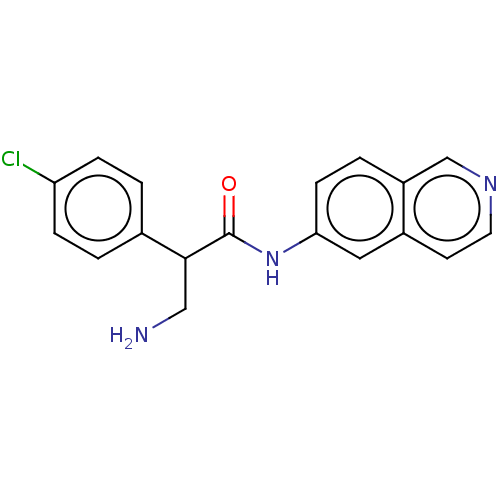
(CHEMBL3794104)Show InChI InChI=1S/C18H16ClN3O/c19-15-4-1-12(2-5-15)17(10-20)18(23)22-16-6-3-14-11-21-8-7-13(14)9-16/h1-9,11,17H,10,20H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50317011
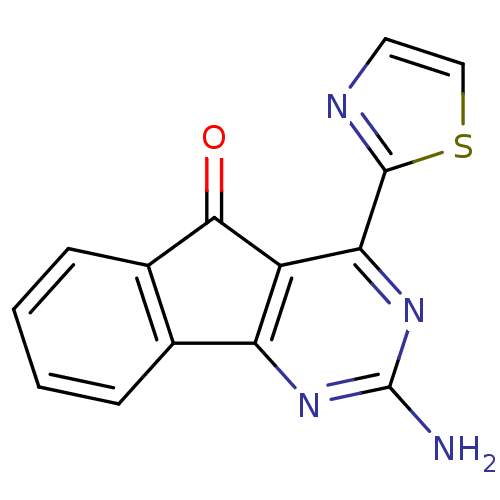
(2-amino-4-(thiazol-2-yl)-5H-indeno[1,2-d]pyrimidin...)Show InChI InChI=1S/C14H8N4OS/c15-14-17-10-7-3-1-2-4-8(7)12(19)9(10)11(18-14)13-16-5-6-20-13/h1-6H,(H2,15,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50317008

(2-amino-4-(5-chlorofuran-2-yl)-5H-indeno[1,2-d]pyr...)Show InChI InChI=1S/C15H8ClN3O2/c16-10-6-5-9(21-10)13-11-12(18-15(17)19-13)7-3-1-2-4-8(7)14(11)20/h1-6H,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156782

(CHEMBL3792663)Show InChI InChI=1S/C18H17N3O2/c19-10-17(12-2-5-16(22)6-3-12)18(23)21-15-4-1-14-11-20-8-7-13(14)9-15/h1-9,11,17,22H,10,19H2,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50317010
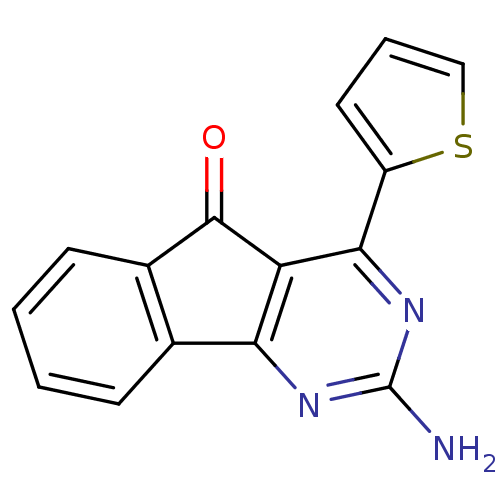
(2-amino-4-(thiophen-2-yl)-5H-indeno[1,2-d]pyrimidi...)Show InChI InChI=1S/C15H9N3OS/c16-15-17-12-8-4-1-2-5-9(8)14(19)11(12)13(18-15)10-6-3-7-20-10/h1-7H,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156732

(CHEMBL3793497)Show InChI InChI=1S/C22H19N3O/c23-13-21(18-6-5-15-3-1-2-4-16(15)11-18)22(26)25-20-8-7-19-14-24-10-9-17(19)12-20/h1-12,14,21H,13,23H2,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156683

(CHEMBL3793788)Show SMILES NCC(C(=O)Nc1ccc2cnccc2c1)c1ccc(COC(=O)c2cccnc2)cc1 Show InChI InChI=1S/C25H22N4O3/c26-13-23(24(30)29-22-8-7-20-14-28-11-9-19(20)12-22)18-5-3-17(4-6-18)16-32-25(31)21-2-1-10-27-15-21/h1-12,14-15,23H,13,16,26H2,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156787

(CHEMBL3793404)Show InChI InChI=1S/C18H15Cl2N3O/c19-13-2-4-15(17(20)8-13)16(9-21)18(24)23-14-3-1-12-10-22-6-5-11(12)7-14/h1-8,10,16H,9,21H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50148579
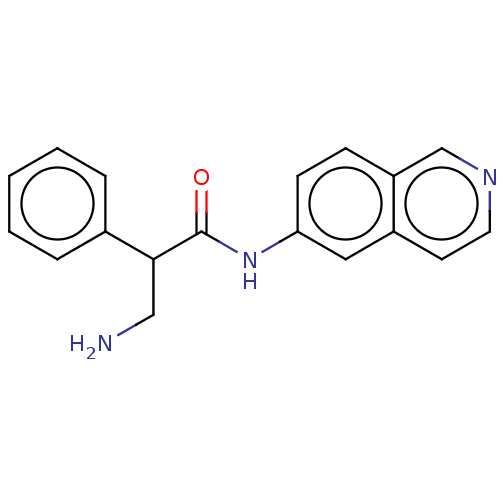
(CHEMBL3770836)Show InChI InChI=1S/C18H17N3O/c19-11-17(13-4-2-1-3-5-13)18(22)21-16-7-6-15-12-20-9-8-14(15)10-16/h1-10,12,17H,11,19H2,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156687

(CHEMBL3793029)Show SMILES NCC(C(=O)Nc1ccc2cnccc2c1)c1ccc(COC(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C27H25N3O3/c28-16-25(27(32)30-24-11-10-23-17-29-13-12-22(23)15-24)21-8-6-20(7-9-21)18-33-26(31)14-19-4-2-1-3-5-19/h1-13,15,17,25H,14,16,18,28H2,(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316893
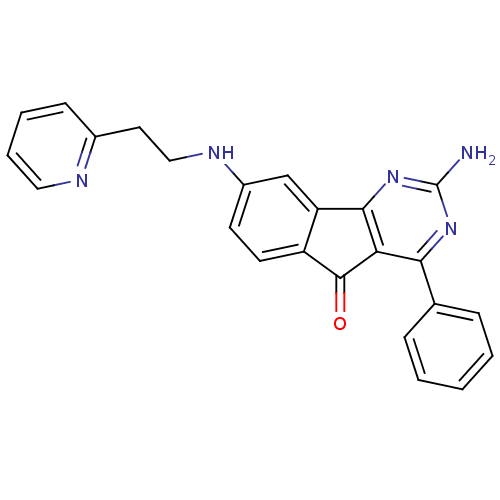
(2-amino-4-phenyl-8-(2-(pyridin-2-yl)ethylamino)-5H...)Show SMILES Nc1nc2-c3cc(NCCc4ccccn4)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C24H19N5O/c25-24-28-21(15-6-2-1-3-7-15)20-22(29-24)19-14-17(9-10-18(19)23(20)30)27-13-11-16-8-4-5-12-26-16/h1-10,12,14,27H,11,13H2,(H2,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonistic activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2868-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.024
BindingDB Entry DOI: 10.7270/Q27S7NXB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316892
(2-amino-4-phenyl-8-(2-(pyridin-4-yl)ethylamino)-5H...)Show SMILES Nc1nc2-c3cc(NCCc4ccncc4)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C24H19N5O/c25-24-28-21(16-4-2-1-3-5-16)20-22(29-24)19-14-17(6-7-18(19)23(20)30)27-13-10-15-8-11-26-12-9-15/h1-9,11-12,14,27H,10,13H2,(H2,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonistic activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2868-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.024
BindingDB Entry DOI: 10.7270/Q27S7NXB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156789

(CHEMBL3793505)Show InChI InChI=1S/C18H16ClN3O/c19-15-3-1-2-13(8-15)17(10-20)18(23)22-16-5-4-14-11-21-7-6-12(14)9-16/h1-9,11,17H,10,20H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156685
(CHEMBL3792412)Show SMILES Cc1cc(C)cc(c1)C(=O)OCc1ccc(cc1)C(CN)C(=O)Nc1ccc2cnccc2c1 Show InChI InChI=1S/C28H27N3O3/c1-18-11-19(2)13-24(12-18)28(33)34-17-20-3-5-21(6-4-20)26(15-29)27(32)31-25-8-7-23-16-30-10-9-22(23)14-25/h3-14,16,26H,15,17,29H2,1-2H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50317003
(2-amino-8-((morpholinoamino)methyl)-4-phenyl-5H-in...)Show SMILES Nc1nc2-c3cc(CNN4CCOCC4)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C22H21N5O2/c23-22-25-19(15-4-2-1-3-5-15)18-20(26-22)17-12-14(6-7-16(17)21(18)28)13-24-27-8-10-29-11-9-27/h1-7,12,24H,8-11,13H2,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156691

(CHEMBL3792435)Show SMILES NCC(C(=O)Nc1ccc2cnccc2c1)c1ccc(OC(=O)CN)cc1 Show InChI InChI=1S/C20H20N4O3/c21-10-18(13-2-5-17(6-3-13)27-19(25)11-22)20(26)24-16-4-1-15-12-23-8-7-14(15)9-16/h1-9,12,18H,10-11,21-22H2,(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156795

(CHEMBL3794270)Show InChI InChI=1S/C18H16FN3O/c19-17-4-2-1-3-15(17)16(10-20)18(23)22-14-6-5-13-11-21-8-7-12(13)9-14/h1-9,11,16H,10,20H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156794

(CHEMBL3792666)Show InChI InChI=1S/C18H16FN3O/c19-15-3-1-2-13(8-15)17(10-20)18(23)22-16-5-4-14-11-21-7-6-12(14)9-16/h1-9,11,17H,10,20H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156780
(CHEMBL3794298)Show InChI InChI=1S/C19H19N3O2/c1-12-8-14(3-5-18(12)23)17(10-20)19(24)22-16-4-2-15-11-21-7-6-13(15)9-16/h2-9,11,17,23H,10,20H2,1H3,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156793
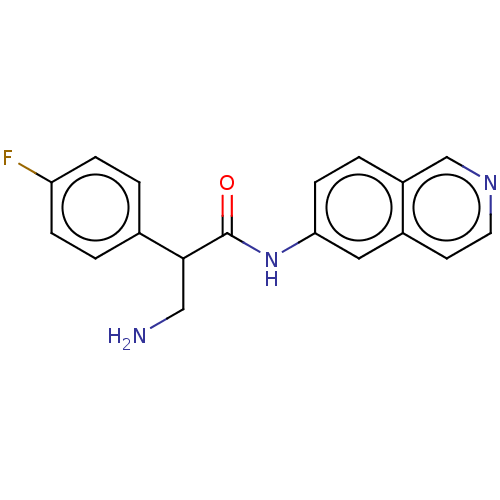
(CHEMBL3792921)Show InChI InChI=1S/C18H16FN3O/c19-15-4-1-12(2-5-15)17(10-20)18(23)22-16-6-3-14-11-21-8-7-13(14)9-16/h1-9,11,17H,10,20H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156786

(CHEMBL3793489)Show InChI InChI=1S/C19H19N3O/c1-13-4-2-3-5-17(13)18(11-20)19(23)22-16-7-6-15-12-21-9-8-14(15)10-16/h2-10,12,18H,11,20H2,1H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156733
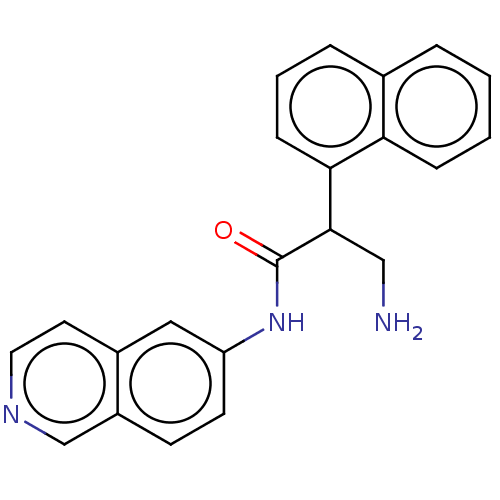
(CHEMBL3793296)Show InChI InChI=1S/C22H19N3O/c23-13-21(20-7-3-5-15-4-1-2-6-19(15)20)22(26)25-18-9-8-17-14-24-11-10-16(17)12-18/h1-12,14,21H,13,23H2,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50161342

(2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...)Show InChI InChI=1S/C17H11N3O/c18-17-19-14(10-6-2-1-3-7-10)13-15(20-17)11-8-4-5-9-12(11)16(13)21/h1-9H,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156791
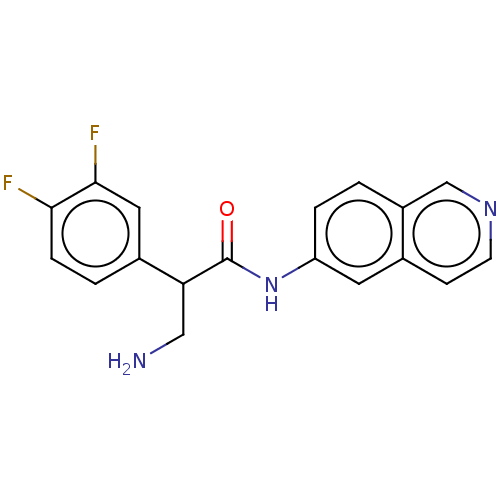
(CHEMBL3793969)Show InChI InChI=1S/C18H15F2N3O/c19-16-4-2-12(8-17(16)20)15(9-21)18(24)23-14-3-1-13-10-22-6-5-11(13)7-14/h1-8,10,15H,9,21H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156790
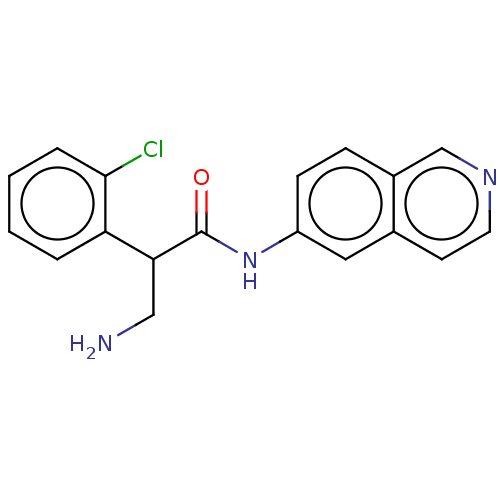
(CHEMBL3792549)Show InChI InChI=1S/C18H16ClN3O/c19-17-4-2-1-3-15(17)16(10-20)18(23)22-14-6-5-13-11-21-8-7-12(13)9-14/h1-9,11,16H,10,20H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156783
(CHEMBL3792734)Show InChI InChI=1S/C18H17N3O2/c19-10-17(13-2-1-3-16(22)9-13)18(23)21-15-5-4-14-11-20-7-6-12(14)8-15/h1-9,11,17,22H,10,19H2,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156777

(CHEMBL3793516)Show InChI InChI=1S/C19H19N3O2/c1-24-17-6-3-13(4-7-17)18(11-20)19(23)22-16-5-2-15-12-21-9-8-14(15)10-16/h2-10,12,18H,11,20H2,1H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50148578
(CHEMBL3770730)Show InChI InChI=1S/C17H15N3O/c18-16(12-4-2-1-3-5-12)17(21)20-15-7-6-14-11-19-9-8-13(14)10-15/h1-11,16H,18H2,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156797
(CHEMBL3793538)Show InChI InChI=1S/C19H19N3O/c20-10-8-18(14-4-2-1-3-5-14)19(23)22-17-7-6-16-13-21-11-9-15(16)12-17/h1-7,9,11-13,18H,8,10,20H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50317014
(2-amino-4-(3-methoxyphenyl)-5H-indeno[1,2-d]pyrimi...)Show InChI InChI=1S/C18H13N3O2/c1-23-11-6-4-5-10(9-11)15-14-16(21-18(19)20-15)12-7-2-3-8-13(12)17(14)22/h2-9H,1H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156684

(CHEMBL3793688)Show SMILES CCCCC(=O)OCc1ccc(cc1)C(CN)C(=O)Nc1ccc2cnccc2c1 Show InChI InChI=1S/C24H27N3O3/c1-2-3-4-23(28)30-16-17-5-7-18(8-6-17)22(14-25)24(29)27-21-10-9-20-15-26-12-11-19(20)13-21/h5-13,15,22H,2-4,14,16,25H2,1H3,(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156688

(CHEMBL3792969)Show SMILES NCC(C(=O)Nc1ccc2cnccc2c1)c1ccc(COC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C26H23N3O3/c27-15-24(25(30)29-23-11-10-22-16-28-13-12-21(22)14-23)19-8-6-18(7-9-19)17-32-26(31)20-4-2-1-3-5-20/h1-14,16,24H,15,17,27H2,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50317003

(2-amino-8-((morpholinoamino)methyl)-4-phenyl-5H-in...)Show SMILES Nc1nc2-c3cc(CNN4CCOCC4)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C22H21N5O2/c23-22-25-19(15-4-2-1-3-5-15)18-20(26-22)17-12-14(6-7-16(17)21(18)28)13-24-27-8-10-29-11-9-27/h1-7,12,24H,8-11,13H2,(H2,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156711
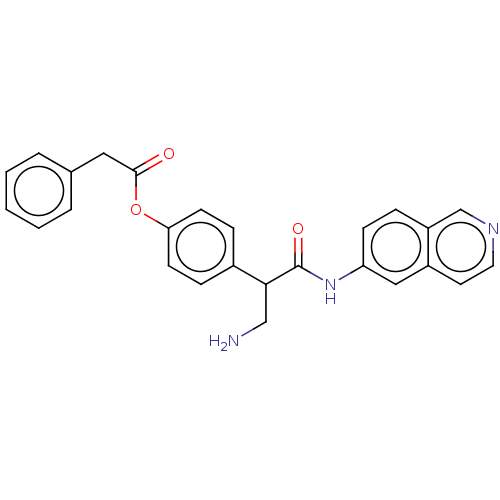
(CHEMBL3792599)Show SMILES NCC(C(=O)Nc1ccc2cnccc2c1)c1ccc(OC(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C26H23N3O3/c27-16-24(26(31)29-22-9-6-21-17-28-13-12-20(21)15-22)19-7-10-23(11-8-19)32-25(30)14-18-4-2-1-3-5-18/h1-13,15,17,24H,14,16,27H2,(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156734
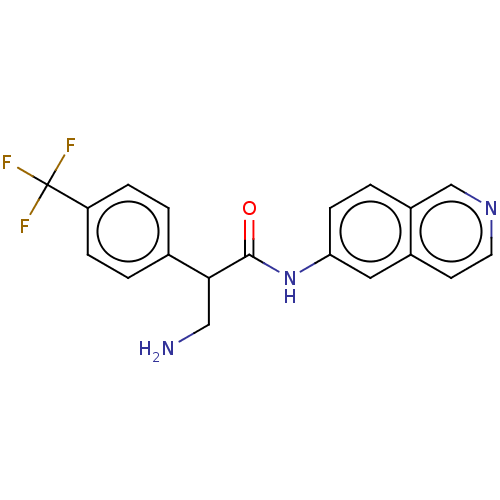
(CHEMBL3793235)Show SMILES NCC(C(=O)Nc1ccc2cnccc2c1)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C19H16F3N3O/c20-19(21,22)15-4-1-12(2-5-15)17(10-23)18(26)25-16-6-3-14-11-24-8-7-13(14)9-16/h1-9,11,17H,10,23H2,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156792
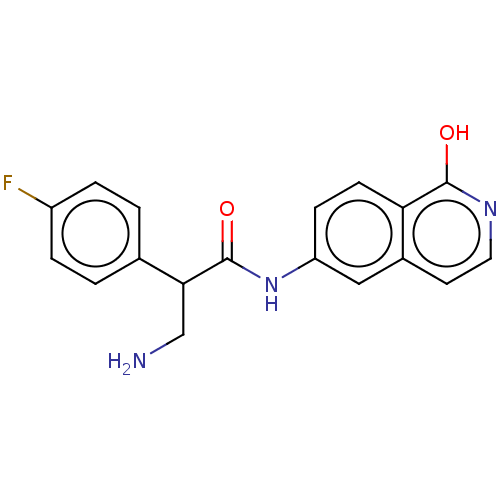
(CHEMBL3792581)Show InChI InChI=1S/C18H16FN3O2/c19-13-3-1-11(2-4-13)16(10-20)18(24)22-14-5-6-15-12(9-14)7-8-21-17(15)23/h1-9,16H,10,20H2,(H,21,23)(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156778

(CHEMBL3793153)Show InChI InChI=1S/C19H19N3O2/c1-24-17-4-2-3-14(10-17)18(11-20)19(23)22-16-6-5-15-12-21-8-7-13(15)9-16/h2-10,12,18H,11,20H2,1H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156776

(CHEMBL3792593)Show SMILES NCC(C(=O)Nc1ccc2cnccc2c1)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C25H23N3O2/c26-15-24(25(29)28-22-9-6-21-16-27-13-12-20(21)14-22)19-7-10-23(11-8-19)30-17-18-4-2-1-3-5-18/h1-14,16,24H,15,17,26H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50156796

(CHEMBL3792781)Show InChI InChI=1S/C18H17N3O2/c19-11-16(12-4-2-1-3-5-12)18(23)21-14-6-7-15-13(10-14)8-9-20-17(15)22/h1-10,16H,11,19H2,(H,20,22)(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50319005
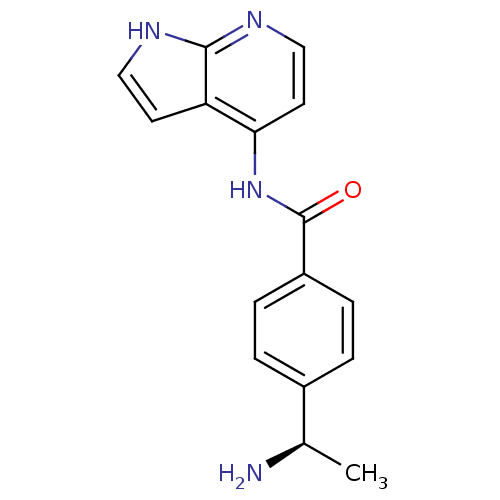
((R)-4-(1-aminoethyl)-N-(1H-pyrrolo[2,3-b]pyridin-4...)Show SMILES C[C@@H](N)c1ccc(cc1)C(=O)Nc1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C16H16N4O/c1-10(17)11-2-4-12(5-3-11)16(21)20-14-7-9-19-15-13(14)6-8-18-15/h2-10H,17H2,1H3,(H2,18,19,20,21)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aerie Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using RSK2 peptide (KKRNRTLTK) as substrate after 180 mins by luminescent kinase assay |
Bioorg Med Chem Lett 26: 2475-80 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.104
BindingDB Entry DOI: 10.7270/Q2VD71CH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316992
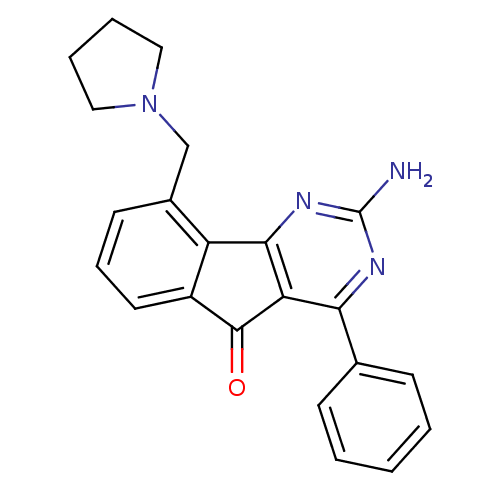
(2-amino-4-phenyl-9-(pyrrolidin-1-ylmethyl)-5H-inde...)Show SMILES Nc1nc2-c3c(cccc3CN3CCCC3)C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C22H20N4O/c23-22-24-19(14-7-2-1-3-8-14)18-20(25-22)17-15(13-26-11-4-5-12-26)9-6-10-16(17)21(18)27/h1-3,6-10H,4-5,11-13H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50317014

(2-amino-4-(3-methoxyphenyl)-5H-indeno[1,2-d]pyrimi...)Show InChI InChI=1S/C18H13N3O2/c1-23-11-6-4-5-10(9-11)15-14-16(21-18(19)20-15)12-7-2-3-8-13(12)17(14)22/h2-9H,1H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A1 receptor |
Bioorg Med Chem Lett 20: 2864-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.042
BindingDB Entry DOI: 10.7270/Q2GB246H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50316899
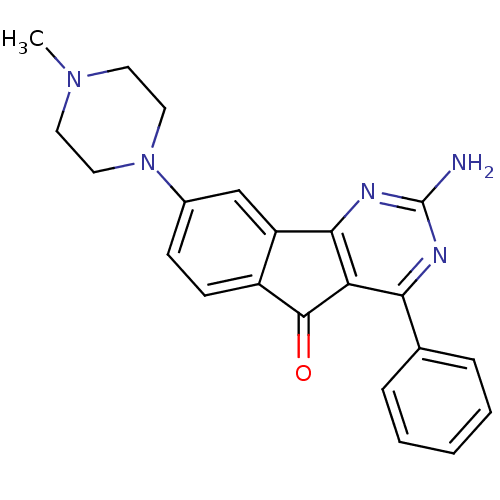
(2-amino-8-(4-methylpiperazin-1-yl)-4-phenyl-5H-ind...)Show SMILES CN1CCN(CC1)c1ccc2C(=O)c3c(nc(N)nc3-c3ccccc3)-c2c1 Show InChI InChI=1S/C22H21N5O/c1-26-9-11-27(12-10-26)15-7-8-16-17(13-15)20-18(21(16)28)19(24-22(23)25-20)14-5-3-2-4-6-14/h2-8,13H,9-12H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonistic activity at adenosine A2A receptor |
Bioorg Med Chem Lett 20: 2868-71 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.024
BindingDB Entry DOI: 10.7270/Q27S7NXB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data