

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302101 (3-(2-(2-ethyl-2-phenylhydrazinyl)-3,4-dioxocyclobu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR2 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302098 (3-(2-(2,2-diethylhydrazinyl)-3,4-dioxocyclobut-1-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR2 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302104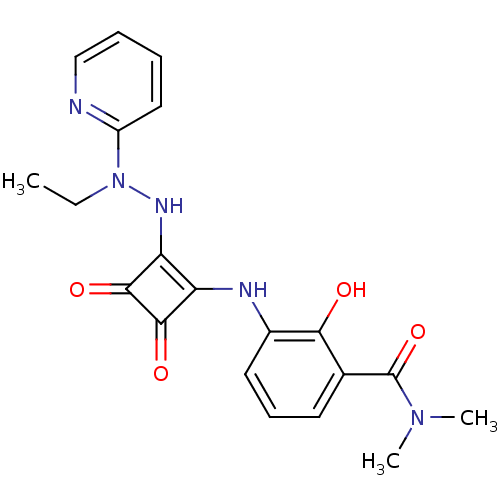 (3-(2-(2-ethyl-2-(pyridin-2-yl)hydrazinyl)-3,4-diox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR2 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302103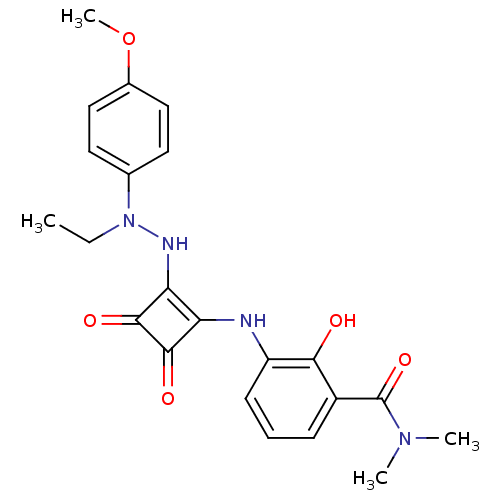 (3-(2-(2-ethyl-2-(4-methoxyphenyl)hydrazinyl)-3,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR2 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302106 (3-(2-(2-ethyl-2-(4-fluorobenzoyl)hydrazinyl)-3,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR2 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302100 (3-(2-(2-acetyl-2-ethylhydrazinyl)-3,4-dioxocyclobu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR2 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302102 (3-(2-(2-ethyl-2-(4-fluorophenyl)hydrazinyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR2 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50302101 (3-(2-(2-ethyl-2-phenylhydrazinyl)-3,4-dioxocyclobu...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR1 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50302104 (3-(2-(2-ethyl-2-(pyridin-2-yl)hydrazinyl)-3,4-diox...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR1 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50302103 (3-(2-(2-ethyl-2-(4-methoxyphenyl)hydrazinyl)-3,4-d...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR1 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302105 (3-(2-(2-ethyl-2-(4-fluorobenzyl)hydrazinyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR2 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50302098 (3-(2-(2,2-diethylhydrazinyl)-3,4-dioxocyclobut-1-e...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR1 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50302106 (3-(2-(2-ethyl-2-(4-fluorobenzoyl)hydrazinyl)-3,4-d...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR1 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50302100 (3-(2-(2-acetyl-2-ethylhydrazinyl)-3,4-dioxocyclobu...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR1 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50302102 (3-(2-(2-ethyl-2-(4-fluorophenyl)hydrazinyl)-3,4-di...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR1 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50302105 (3-(2-(2-ethyl-2-(4-fluorobenzyl)hydrazinyl)-3,4-di...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]IL-8 from human CXCR1 expressed in mouse BaF3 cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343131 (2-(2-cyanopyrimidin-5-yl)-N-(3-ethylphenyl)-4-o-to...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343129 (2-(4-cyanopyridin-3-yl)-N-(3-ethylphenyl)-4-o-toly...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343132 (2-(3,5-dimethylisoxazol-4-yl)-N-(3-ethylphenyl)-4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322836 (CHEMBL1210244 | rac-N-(3-methyl-1-(piperidin-1-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4700-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.143 BindingDB Entry DOI: 10.7270/Q2PR7W6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343120 (4-(2,6-dimethylphenyl)-N-(3-ethylphenyl)-2-(pyridi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343128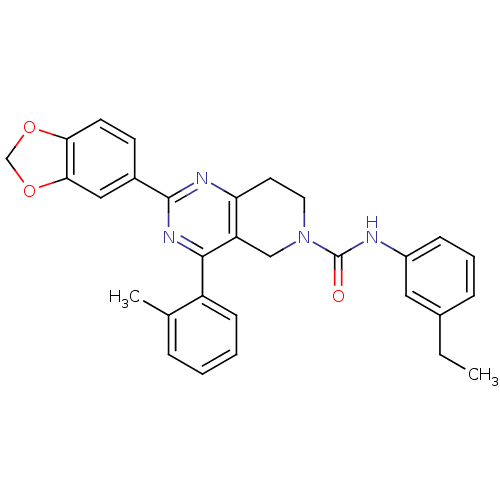 (2-(benzo[d][1,3]dioxol-5-yl)-N-(3-ethylphenyl)-4-o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322839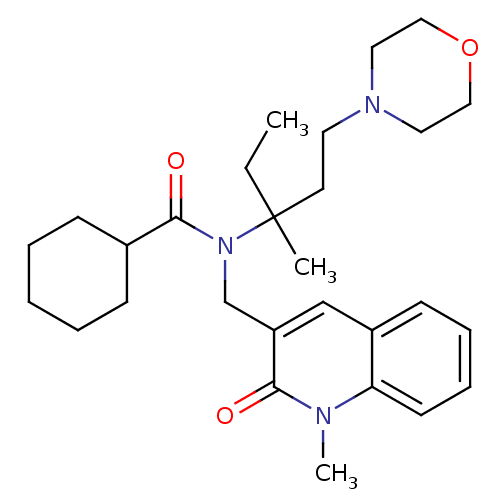 (CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4700-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.143 BindingDB Entry DOI: 10.7270/Q2PR7W6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343130 (CHEMBL1771458 | N-(3-ethylphenyl)-2-(pyrimidin-5-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343127 (CHEMBL1771455 | N-(3-ethylphenyl)-2-(quinolin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322837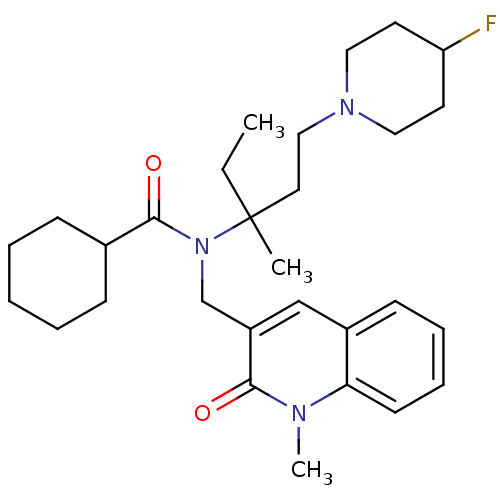 (CHEMBL1210311 | rac-N-(1-(4-fluoropiperidin-1-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4700-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.143 BindingDB Entry DOI: 10.7270/Q2PR7W6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343094 (2-(4-cyanophenyl)-N-(3-ethylphenyl)-4-o-tolyl-7,8-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322842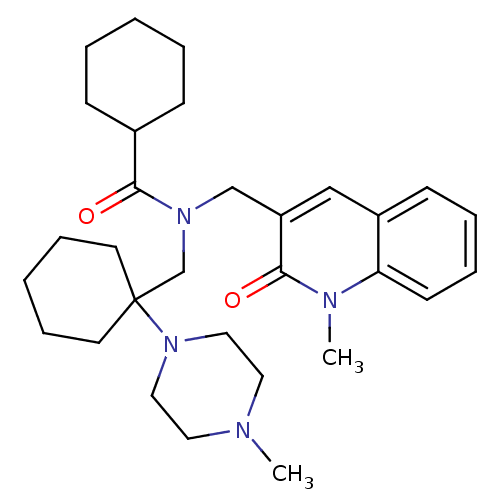 (CHEMBL1210177 | N-((1-methyl-2-oxo-1,2-dihydroquin...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4700-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.143 BindingDB Entry DOI: 10.7270/Q2PR7W6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322838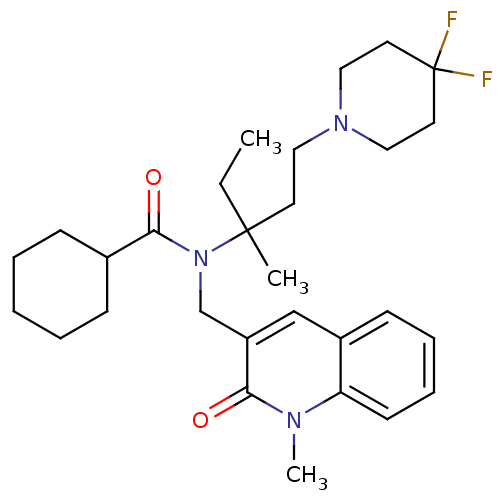 (CHEMBL1210312 | rac-N-(1-(4,4-difluoropiperidin-1-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4700-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.143 BindingDB Entry DOI: 10.7270/Q2PR7W6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322839 (CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4700-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.143 BindingDB Entry DOI: 10.7270/Q2PR7W6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322839 (CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4700-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.143 BindingDB Entry DOI: 10.7270/Q2PR7W6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343095 (CHEMBL1771452 | N-(3-ethylphenyl)-2-(4-fluoropheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343109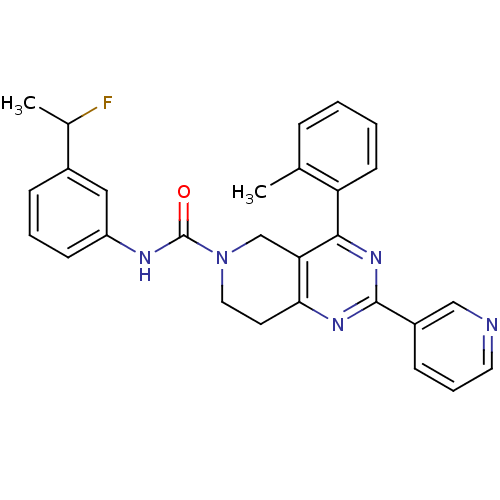 (CHEMBL1771244 | N-(3-(1-fluoroethyl)phenyl)-2-(pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343125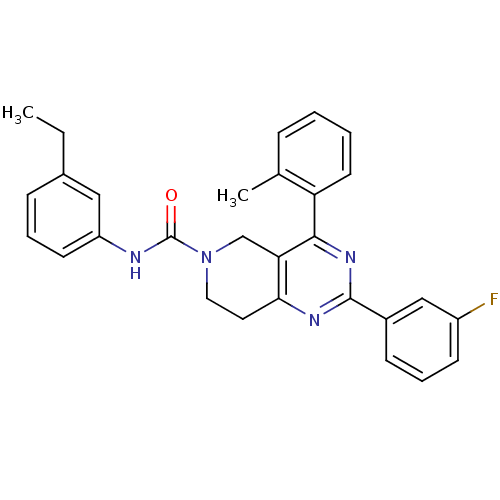 (CHEMBL1771453 | N-(3-ethylphenyl)-2-(3-fluoropheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322834 (CHEMBL1210242 | N-((1-methyl-2-oxo-1,2-dihydroquin...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4700-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.143 BindingDB Entry DOI: 10.7270/Q2PR7W6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50348617 (CHEMBL1801361) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4704-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.016 BindingDB Entry DOI: 10.7270/Q2ZS2WVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50348615 (CHEMBL1801359) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4704-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.016 BindingDB Entry DOI: 10.7270/Q2ZS2WVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302098 (3-(2-(2,2-diethylhydrazinyl)-3,4-dioxocyclobut-1-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 expressed in CHO cells assessed as inhibition of IL-8-induced chemotaxis | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343121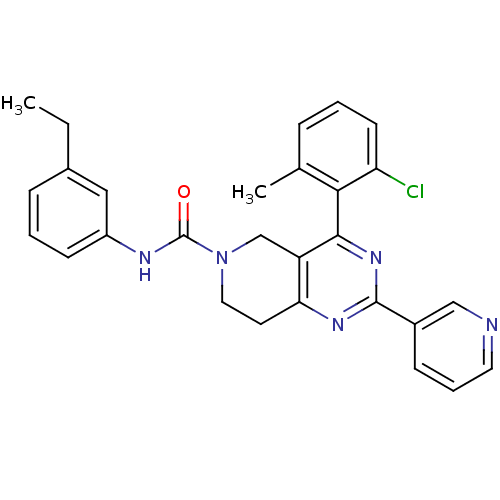 (4-(2-chloro-6-methylphenyl)-N-(3-ethylphenyl)-2-(p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50348616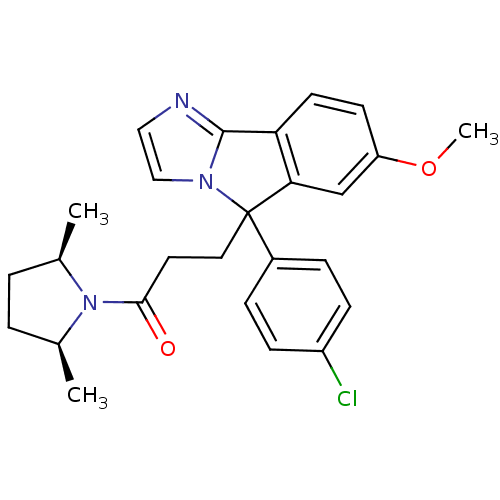 (CHEMBL1801360) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4704-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.016 BindingDB Entry DOI: 10.7270/Q2ZS2WVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343122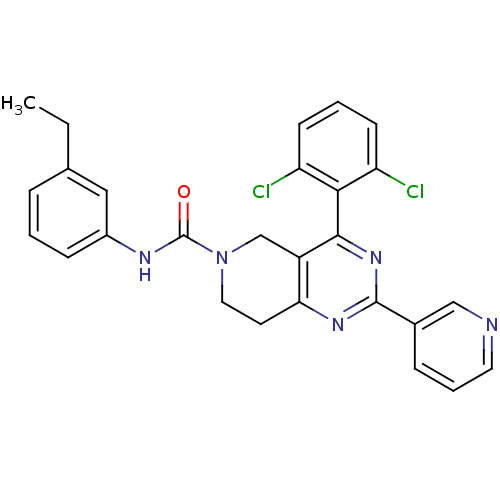 (4-(2,6-dichlorophenyl)-N-(3-ethylphenyl)-2-(pyridi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302101 (3-(2-(2-ethyl-2-phenylhydrazinyl)-3,4-dioxocyclobu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 expressed in CHO cells assessed as inhibition of IL-8-induced chemotaxis | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343090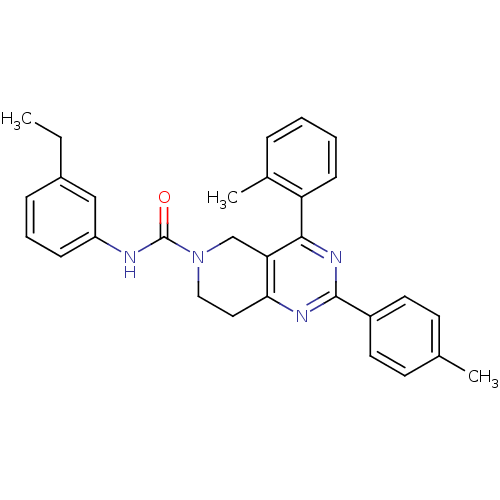 (CHEMBL1771446 | N-(3-ethylphenyl)-4-o-tolyl-2-p-to...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50302104 (3-(2-(2-ethyl-2-(pyridin-2-yl)hydrazinyl)-3,4-diox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi PharmaTech Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 expressed in CHO cells assessed as inhibition of IL-8-induced chemotaxis | Bioorg Med Chem Lett 19: 5741-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.014 BindingDB Entry DOI: 10.7270/Q25H7GB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343103 (CHEMBL1771238 | N-(3-ethylphenyl)-2-(pyridin-3-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50348614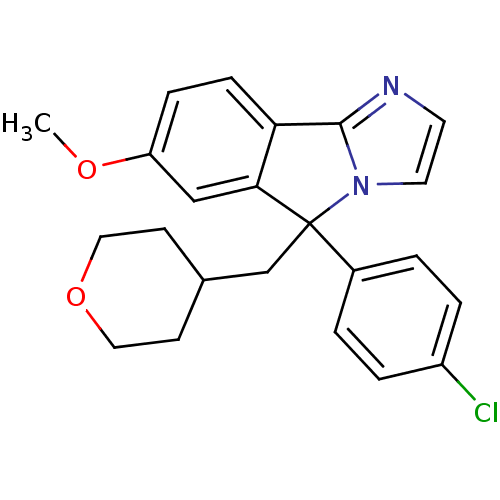 (CHEMBL1801358) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4704-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.016 BindingDB Entry DOI: 10.7270/Q2ZS2WVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343089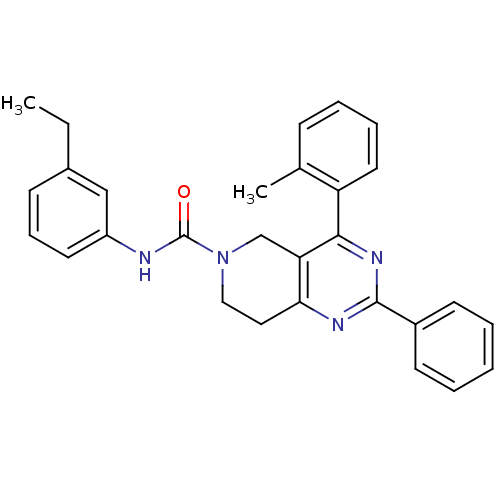 (CHEMBL1771445 | N-(3-ethylphenyl)-2-phenyl-4-o-tol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50348612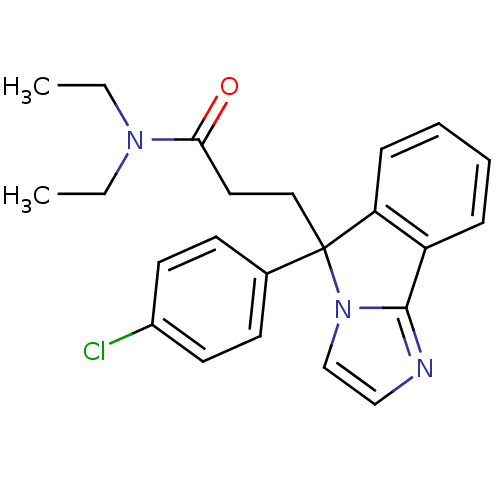 (CHEMBL1801337) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4704-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.016 BindingDB Entry DOI: 10.7270/Q2ZS2WVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50348591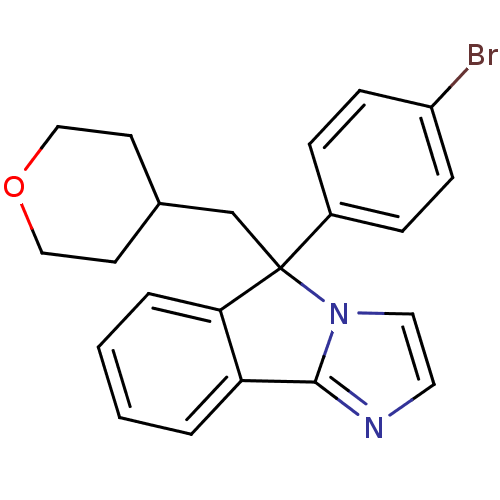 (CHEMBL1801272) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4704-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.016 BindingDB Entry DOI: 10.7270/Q2ZS2WVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50348611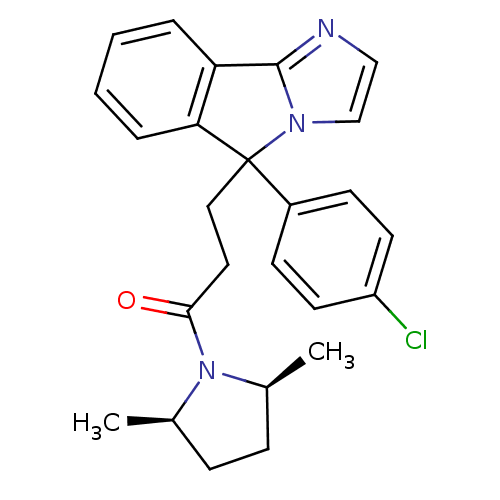 (CHEMBL1801336) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human recombinant NPS receptor expressed in CHOK1 cells assessed as inhibition of NPS-induced calcium mobilization by FLIPR as... | Bioorg Med Chem Lett 20: 4704-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.016 BindingDB Entry DOI: 10.7270/Q2ZS2WVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 125 total ) | Next | Last >> |