 Found 159 hits with Last Name = 'herbst' and Initial = 'r'
Found 159 hits with Last Name = 'herbst' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027641

(1-(2-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...)Show InChI InChI=1S/C12H13NO3S/c1-7(17)11(14)13-9-5-3-2-4-8(9)6-10(13)12(15)16/h2-5,7,10,17H,6H2,1H3,(H,15,16)/t7-,10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027639
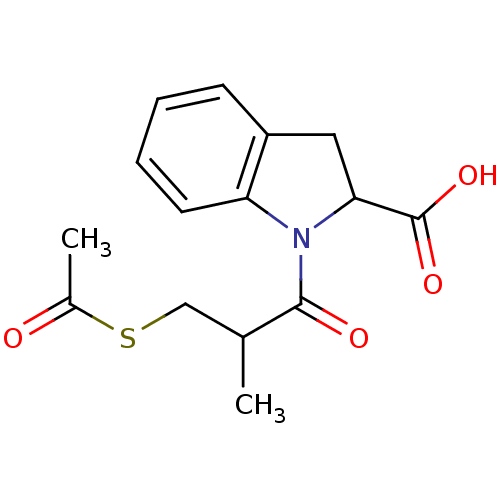
(1-(3-Acetylsulfanyl-2-methyl-propionyl)-2,3-dihydr...)Show InChI InChI=1S/C15H17NO4S/c1-9(8-21-10(2)17)14(18)16-12-6-4-3-5-11(12)7-13(16)15(19)20/h3-6,9,13H,7-8H2,1-2H3,(H,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50404972
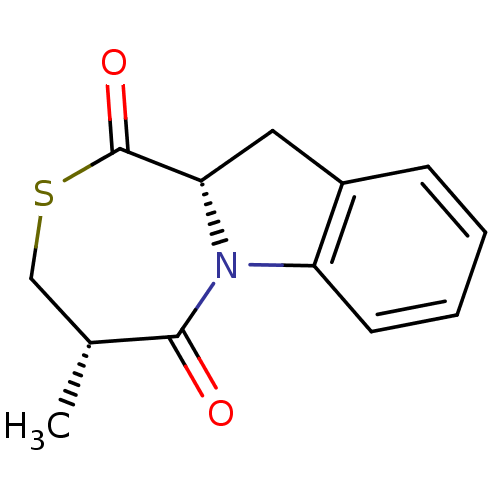
(CHEMBL2112667)Show InChI InChI=1S/C13H13NO2S/c1-8-7-17-13(16)11-6-9-4-2-3-5-10(9)14(11)12(8)15/h2-5,8,11H,6-7H2,1H3/t8-,11+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027637
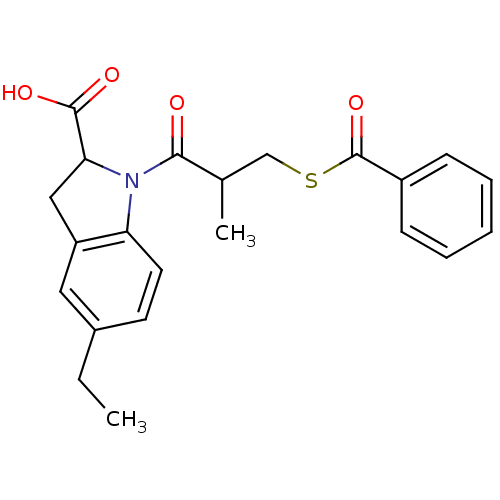
(1-(3-Benzoylsulfanyl-2-methyl-propionyl)-5-ethyl-2...)Show SMILES CCc1ccc2N(C(Cc2c1)C(O)=O)C(=O)C(C)CSC(=O)c1ccccc1 Show InChI InChI=1S/C22H23NO4S/c1-3-15-9-10-18-17(11-15)12-19(21(25)26)23(18)20(24)14(2)13-28-22(27)16-7-5-4-6-8-16/h4-11,14,19H,3,12-13H2,1-2H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50404973
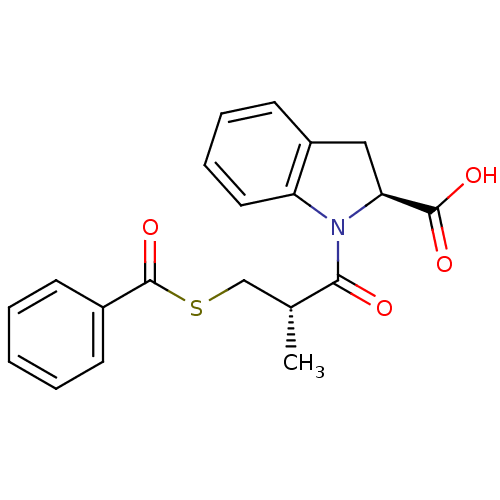
(CHEMBL1979078)Show SMILES C[C@H](CSC(=O)c1ccccc1)C(=O)N1[C@@H](Cc2ccccc12)C(O)=O Show InChI InChI=1S/C20H19NO4S/c1-13(12-26-20(25)14-7-3-2-4-8-14)18(22)21-16-10-6-5-9-15(16)11-17(21)19(23)24/h2-10,13,17H,11-12H2,1H3,(H,23,24)/t13-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50404969
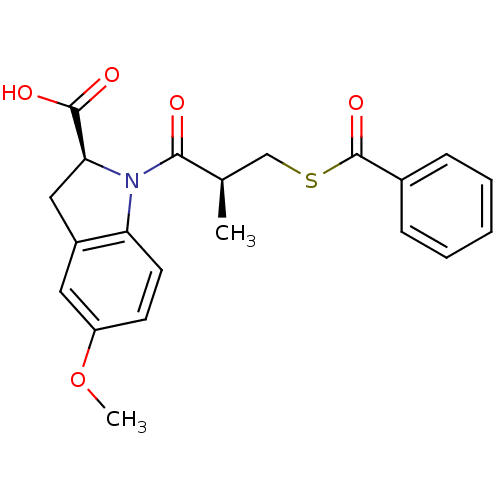
(CHEMBL2111853)Show SMILES COc1ccc2N([C@@H](Cc2c1)C(O)=O)C(=O)[C@H](C)CSC(=O)c1ccccc1 |r| Show InChI InChI=1S/C21H21NO5S/c1-13(12-28-21(26)14-6-4-3-5-7-14)19(23)22-17-9-8-16(27-2)10-15(17)11-18(22)20(24)25/h3-10,13,18H,11-12H2,1-2H3,(H,24,25)/t13-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027644

(1-(3-Benzoylsulfanyl-propionyl)-2,3-dihydro-1H-ind...)Show InChI InChI=1S/C19H17NO4S/c21-17(10-11-25-19(24)13-6-2-1-3-7-13)20-15-9-5-4-8-14(15)12-16(20)18(22)23/h1-9,16H,10-12H2,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027634

(1-(2-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...)Show InChI InChI=1S/C12H13NO3S/c1-7(17)11(14)13-9-5-3-2-4-8(9)6-10(13)12(15)16/h2-5,7,10,17H,6H2,1H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027630

(1-(3-Benzoylsulfanyl-2-methyl-propionyl)-2,3-dihyd...)Show SMILES CC(CSC(=O)c1ccccc1)C(=O)N1C(Cc2ccccc12)C(O)=O Show InChI InChI=1S/C20H19NO4S/c1-13(12-26-20(25)14-7-3-2-4-8-14)18(22)21-16-10-6-5-9-15(16)11-17(21)19(23)24/h2-10,13,17H,11-12H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027631
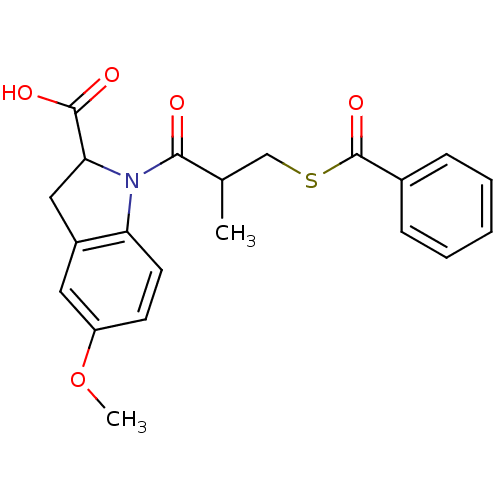
(1-(3-Benzoylsulfanyl-2-methyl-propionyl)-5-methoxy...)Show SMILES COc1ccc2N(C(Cc2c1)C(O)=O)C(=O)C(C)CSC(=O)c1ccccc1 Show InChI InChI=1S/C21H21NO5S/c1-13(12-28-21(26)14-6-4-3-5-7-14)19(23)22-17-9-8-16(27-2)10-15(17)11-18(22)20(24)25/h3-10,13,18H,11-12H2,1-2H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027631

(1-(3-Benzoylsulfanyl-2-methyl-propionyl)-5-methoxy...)Show SMILES COc1ccc2N(C(Cc2c1)C(O)=O)C(=O)C(C)CSC(=O)c1ccccc1 Show InChI InChI=1S/C21H21NO5S/c1-13(12-28-21(26)14-6-4-3-5-7-14)19(23)22-17-9-8-16(27-2)10-15(17)11-18(22)20(24)25/h3-10,13,18H,11-12H2,1-2H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027632
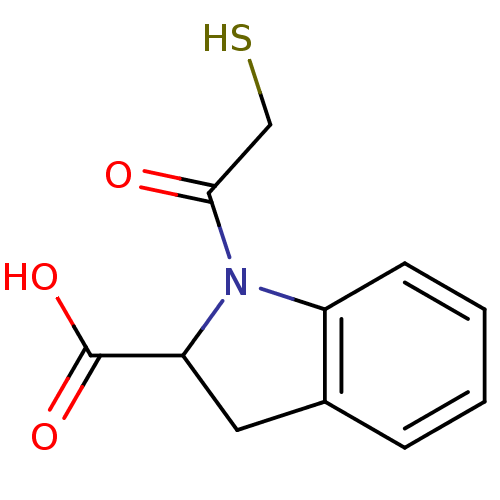
(1-(2-Mercapto-acetyl)-2,3-dihydro-1H-indole-2-carb...)Show InChI InChI=1S/C11H11NO3S/c13-10(6-16)12-8-4-2-1-3-7(8)5-9(12)11(14)15/h1-4,9,16H,5-6H2,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027634

(1-(2-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...)Show InChI InChI=1S/C12H13NO3S/c1-7(17)11(14)13-9-5-3-2-4-8(9)6-10(13)12(15)16/h2-5,7,10,17H,6H2,1H3,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50404968

(CHEMBL2111854)Show SMILES C[C@H](CSC(=O)c1ccccc1)C(=O)N1[C@@H](Cc2ccccc12)C(O)=O |r| Show InChI InChI=1S/C20H19NO4S/c1-13(12-26-20(25)14-7-3-2-4-8-14)18(22)21-16-10-6-5-9-15(16)11-17(21)19(23)24/h2-10,13,17H,11-12H2,1H3,(H,23,24)/t13-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50404967

(CHEMBL2111855)Show SMILES C[C@@H](CSC(=O)c1ccccc1)C(=O)N1[C@@H](Cc2ccccc12)C(O)=O |r| Show InChI InChI=1S/C20H19NO4S/c1-13(12-26-20(25)14-7-3-2-4-8-14)18(22)21-16-10-6-5-9-15(16)11-17(21)19(23)24/h2-10,13,17H,11-12H2,1H3,(H,23,24)/t13-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027630

(1-(3-Benzoylsulfanyl-2-methyl-propionyl)-2,3-dihyd...)Show SMILES CC(CSC(=O)c1ccccc1)C(=O)N1C(Cc2ccccc12)C(O)=O Show InChI InChI=1S/C20H19NO4S/c1-13(12-26-20(25)14-7-3-2-4-8-14)18(22)21-16-10-6-5-9-15(16)11-17(21)19(23)24/h2-10,13,17H,11-12H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293756
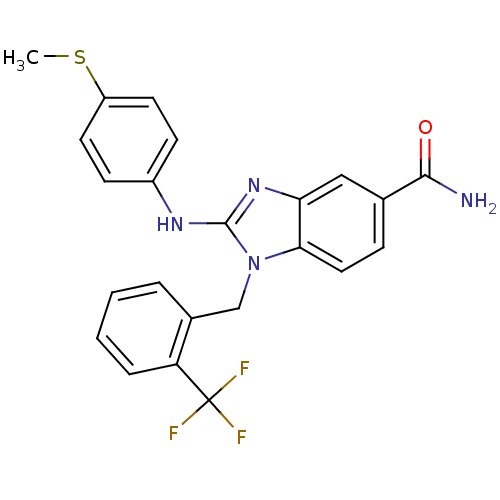
(2-(4-(methylthio)phenylamino)-1-(2-(trifluoromethy...)Show SMILES CSc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4OS/c1-32-17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50404967

(CHEMBL2111855)Show SMILES C[C@@H](CSC(=O)c1ccccc1)C(=O)N1[C@@H](Cc2ccccc12)C(O)=O |r| Show InChI InChI=1S/C20H19NO4S/c1-13(12-26-20(25)14-7-3-2-4-8-14)18(22)21-16-10-6-5-9-15(16)11-17(21)19(23)24/h2-10,13,17H,11-12H2,1H3,(H,23,24)/t13-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50404970

(CHEMBL2112668)Show SMILES C[C@@H](CSC(=O)c1ccccc1)C(=O)N1[C@H](Cc2ccccc12)C(O)=O |r| Show InChI InChI=1S/C20H19NO4S/c1-13(12-26-20(25)14-7-3-2-4-8-14)18(22)21-16-10-6-5-9-15(16)11-17(21)19(23)24/h2-10,13,17H,11-12H2,1H3,(H,23,24)/t13-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027636

(Bis-(1-Propionyl-2,3-dihydro-1H-indole-2-carboxyli...)Show SMILES C[C@H](CSC[C@@H](C)C(=O)N1[C@@H](Cc2ccccc12)C(O)=O)C(=O)N1[C@@H](Cc2ccccc12)C(O)=O Show InChI InChI=1S/C26H28N2O6S/c1-15(23(29)27-19-9-5-3-7-17(19)11-21(27)25(31)32)13-35-14-16(2)24(30)28-20-10-6-4-8-18(20)12-22(28)26(33)34/h3-10,15-16,21-22H,11-14H2,1-2H3,(H,31,32)(H,33,34)/t15-,16-,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293755
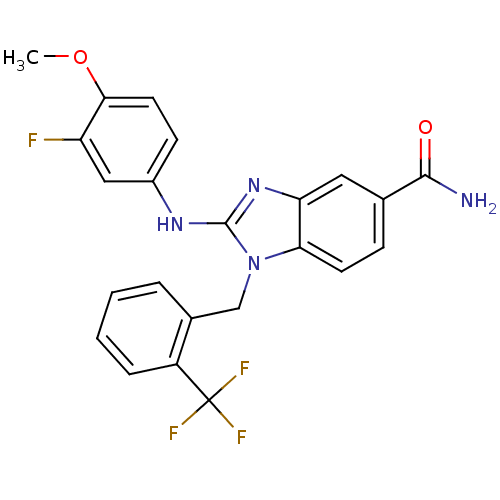
(2-(3-fluoro-4-methoxyphenylamino)-1-(2-(trifluorom...)Show SMILES COc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1F Show InChI InChI=1S/C23H18F4N4O2/c1-33-20-9-7-15(11-17(20)24)29-22-30-18-10-13(21(28)32)6-8-19(18)31(22)12-14-4-2-3-5-16(14)23(25,26)27/h2-11H,12H2,1H3,(H2,28,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293761

(2-(4-(methylthio)phenylamino)-1-(2-(trifluoromethy...)Show SMILES CSc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(O)=O)cc1 Show InChI InChI=1S/C23H18F3N3O2S/c1-32-17-9-7-16(8-10-17)27-22-28-19-12-14(21(30)31)6-11-20(19)29(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H,27,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293752
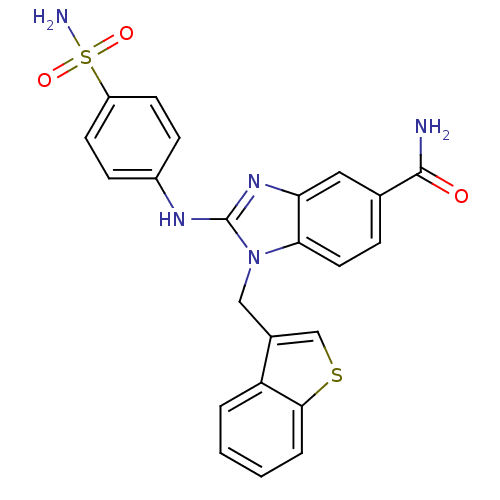
(1-(benzo[b]thiophen-3-ylmethyl)-2-(4-sulfamoylphen...)Show SMILES NC(=O)c1ccc2n(Cc3csc4ccccc34)c(Nc3ccc(cc3)S(N)(=O)=O)nc2c1 Show InChI InChI=1S/C23H19N5O3S2/c24-22(29)14-5-10-20-19(11-14)27-23(26-16-6-8-17(9-7-16)33(25,30)31)28(20)12-15-13-32-21-4-2-1-3-18(15)21/h1-11,13H,12H2,(H2,24,29)(H,26,27)(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293762
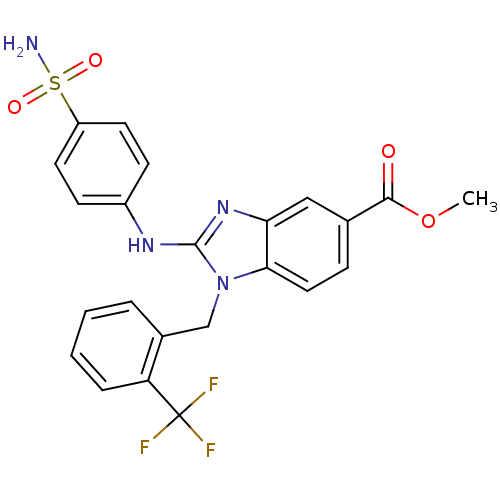
(CHEMBL558477 | methyl 2-(4-sulfamoylphenylamino)-1...)Show SMILES COC(=O)c1ccc2n(Cc3ccccc3C(F)(F)F)c(Nc3ccc(cc3)S(N)(=O)=O)nc2c1 Show InChI InChI=1S/C23H19F3N4O4S/c1-34-21(31)14-6-11-20-19(12-14)29-22(28-16-7-9-17(10-8-16)35(27,32)33)30(20)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H,28,29)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293760
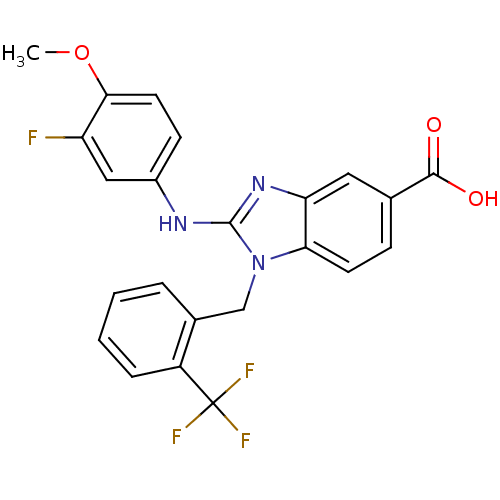
(2-(3-fluoro-4-methoxyphenylamino)-1-(2-(trifluorom...)Show SMILES COc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(O)=O)cc1F Show InChI InChI=1S/C23H17F4N3O3/c1-33-20-9-7-15(11-17(20)24)28-22-29-18-10-13(21(31)32)6-8-19(18)30(22)12-14-4-2-3-5-16(14)23(25,26)27/h2-11H,12H2,1H3,(H,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293769

(CHEMBL538431 | N-(2-methoxyphenyl)-2-(4-sulfamoylp...)Show SMILES COc1ccccc1NC(=O)c1ccc2n(Cc3ccccc3C(F)(F)F)c(Nc3ccc(cc3)S(N)(=O)=O)nc2c1 Show InChI InChI=1S/C29H24F3N5O4S/c1-41-26-9-5-4-8-23(26)35-27(38)18-10-15-25-24(16-18)36-28(34-20-11-13-21(14-12-20)42(33,39)40)37(25)17-19-6-2-3-7-22(19)29(30,31)32/h2-16H,17H2,1H3,(H,34,36)(H,35,38)(H2,33,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293754
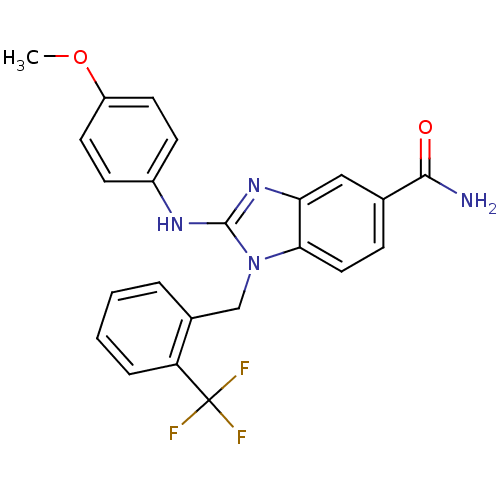
(2-(4-methoxyphenylamino)-1-(2-(trifluoromethyl)ben...)Show SMILES COc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4O2/c1-32-17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50404967

(CHEMBL2111855)Show SMILES C[C@@H](CSC(=O)c1ccccc1)C(=O)N1[C@@H](Cc2ccccc12)C(O)=O |r| Show InChI InChI=1S/C20H19NO4S/c1-13(12-26-20(25)14-7-3-2-4-8-14)18(22)21-16-10-6-5-9-15(16)11-17(21)19(23)24/h2-10,13,17H,11-12H2,1H3,(H,23,24)/t13-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50293754

(2-(4-methoxyphenylamino)-1-(2-(trifluoromethyl)ben...)Show SMILES COc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4O2/c1-32-17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 preincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293753

(2-(4-(methylsulfonyl)phenylamino)-1-(2-(trifluorom...)Show SMILES CS(=O)(=O)c1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4O3S/c1-34(32,33)17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293749
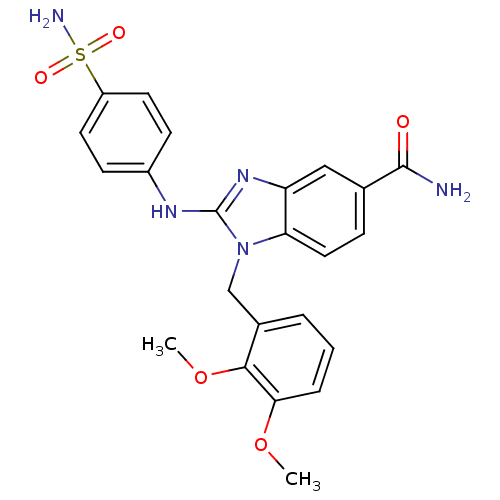
(1-(2,3-dimethoxybenzyl)-2-(4-sulfamoylphenylamino)...)Show SMILES COc1cccc(Cn2c(Nc3ccc(cc3)S(N)(=O)=O)nc3cc(ccc23)C(N)=O)c1OC Show InChI InChI=1S/C23H23N5O5S/c1-32-20-5-3-4-15(21(20)33-2)13-28-19-11-6-14(22(24)29)12-18(19)27-23(28)26-16-7-9-17(10-8-16)34(25,30)31/h3-12H,13H2,1-2H3,(H2,24,29)(H,26,27)(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293765

(2-(4-methoxyphenylamino)-N-methyl-1-(2-(trifluorom...)Show SMILES CNC(=O)c1ccc2n(Cc3ccccc3C(F)(F)F)c(Nc3ccc(OC)cc3)nc2c1 Show InChI InChI=1S/C24H21F3N4O2/c1-28-22(32)15-7-12-21-20(13-15)30-23(29-17-8-10-18(33-2)11-9-17)31(21)14-16-5-3-4-6-19(16)24(25,26)27/h3-13H,14H2,1-2H3,(H,28,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293770
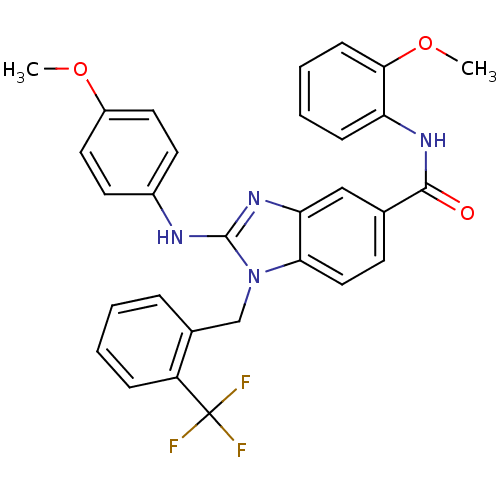
(CHEMBL563993 | N-(2-methoxyphenyl)-2-(4-methoxyphe...)Show SMILES COc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(=O)Nc2ccccc2OC)cc1 Show InChI InChI=1S/C30H25F3N4O3/c1-39-22-14-12-21(13-15-22)34-29-36-25-17-19(28(38)35-24-9-5-6-10-27(24)40-2)11-16-26(25)37(29)18-20-7-3-4-8-23(20)30(31,32)33/h3-17H,18H2,1-2H3,(H,34,36)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293758
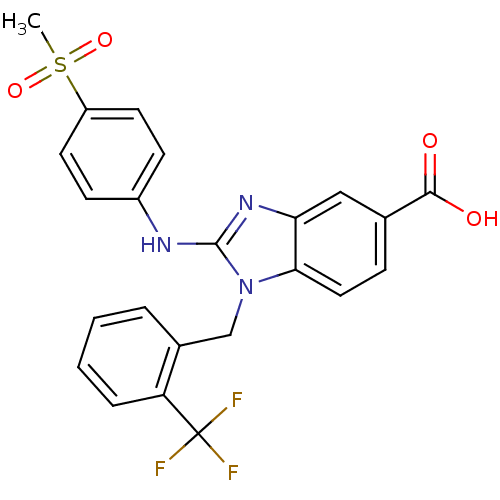
(2-(4-(methylsulfonyl)phenylamino)-1-(2-(trifluorom...)Show SMILES CS(=O)(=O)c1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(O)=O)cc1 Show InChI InChI=1S/C23H18F3N3O4S/c1-34(32,33)17-9-7-16(8-10-17)27-22-28-19-12-14(21(30)31)6-11-20(19)29(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H,27,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50293756

(2-(4-(methylthio)phenylamino)-1-(2-(trifluoromethy...)Show SMILES CSc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4OS/c1-32-17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 preincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293744

(2-(4-sulfamoylphenylamino)-1-(2-(trifluoromethyl)b...)Show SMILES NC(=O)c1ccc2n(Cc3ccccc3C(F)(F)F)c(Nc3ccc(cc3)S(N)(=O)=O)nc2c1 Show InChI InChI=1S/C22H18F3N5O3S/c23-22(24,25)17-4-2-1-3-14(17)12-30-19-10-5-13(20(26)31)11-18(19)29-21(30)28-15-6-8-16(9-7-15)34(27,32)33/h1-11H,12H2,(H2,26,31)(H,28,29)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293779
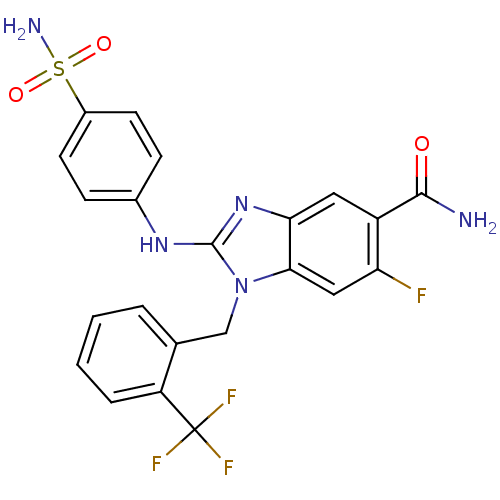
(6-fluoro-2-(4-sulfamoylphenylamino)-1-(2-(trifluor...)Show SMILES NC(=O)c1cc2nc(Nc3ccc(cc3)S(N)(=O)=O)n(Cc3ccccc3C(F)(F)F)c2cc1F Show InChI InChI=1S/C22H17F4N5O3S/c23-17-10-19-18(9-15(17)20(27)32)30-21(29-13-5-7-14(8-6-13)35(28,33)34)31(19)11-12-3-1-2-4-16(12)22(24,25)26/h1-10H,11H2,(H2,27,32)(H,29,30)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50293753

(2-(4-(methylsulfonyl)phenylamino)-1-(2-(trifluorom...)Show SMILES CS(=O)(=O)c1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4O3S/c1-34(32,33)17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 preincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50293754

(2-(4-methoxyphenylamino)-1-(2-(trifluoromethyl)ben...)Show SMILES COc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4O2/c1-32-17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 coincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293766
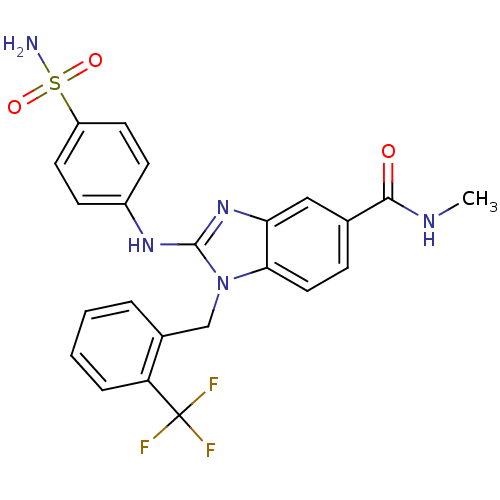
(CHEMBL562353 | N-methyl-2-(4-sulfamoylphenylamino)...)Show SMILES CNC(=O)c1ccc2n(Cc3ccccc3C(F)(F)F)c(Nc3ccc(cc3)S(N)(=O)=O)nc2c1 Show InChI InChI=1S/C23H20F3N5O3S/c1-28-21(32)14-6-11-20-19(12-14)30-22(29-16-7-9-17(10-8-16)35(27,33)34)31(20)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H,28,32)(H,29,30)(H2,27,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50293763
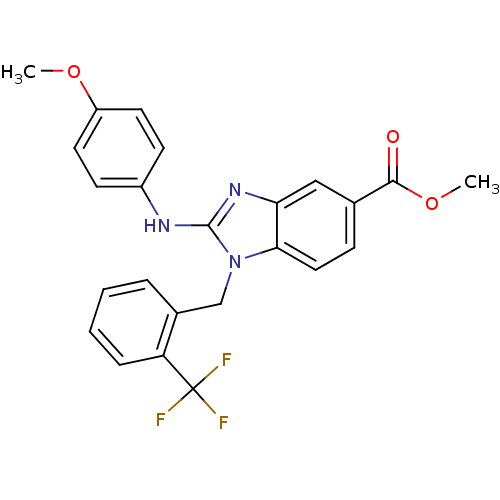
(CHEMBL551230 | methyl 2-(4-methoxyphenylamino)-1-(...)Show SMILES COC(=O)c1ccc2n(Cc3ccccc3C(F)(F)F)c(Nc3ccc(OC)cc3)nc2c1 Show InChI InChI=1S/C24H20F3N3O3/c1-32-18-10-8-17(9-11-18)28-23-29-20-13-15(22(31)33-2)7-12-21(20)30(23)14-16-5-3-4-6-19(16)24(25,26)27/h3-13H,14H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human kinesin spindle protein by endpoint assay |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50293756

(2-(4-(methylthio)phenylamino)-1-(2-(trifluoromethy...)Show SMILES CSc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4OS/c1-32-17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 coincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027635
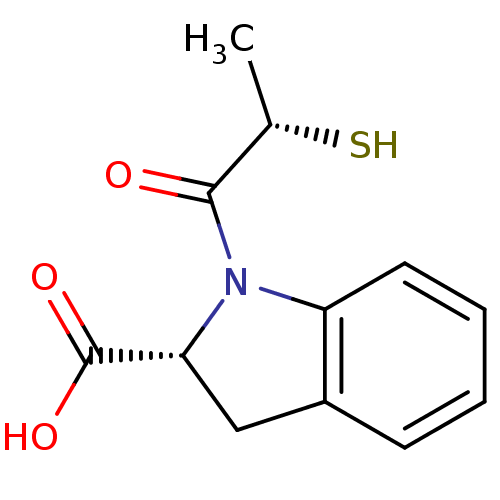
(1-(2-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...)Show InChI InChI=1S/C12H13NO3S/c1-7(17)11(14)13-9-5-3-2-4-8(9)6-10(13)12(15)16/h2-5,7,10,17H,6H2,1H3,(H,15,16)/t7-,10+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50293754

(2-(4-methoxyphenylamino)-1-(2-(trifluoromethyl)ben...)Show SMILES COc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4O2/c1-32-17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 preincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027639

(1-(3-Acetylsulfanyl-2-methyl-propionyl)-2,3-dihydr...)Show InChI InChI=1S/C15H17NO4S/c1-9(8-21-10(2)17)14(18)16-12-6-4-3-5-11(12)7-13(16)15(19)20/h3-6,9,13H,7-8H2,1-2H3,(H,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 26: 394-403 (1983)
BindingDB Entry DOI: 10.7270/Q2T15473 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50293756

(2-(4-(methylthio)phenylamino)-1-(2-(trifluoromethy...)Show SMILES CSc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4OS/c1-32-17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 preincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50293753

(2-(4-(methylsulfonyl)phenylamino)-1-(2-(trifluorom...)Show SMILES CS(=O)(=O)c1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4O3S/c1-34(32,33)17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 coincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50293754

(2-(4-methoxyphenylamino)-1-(2-(trifluoromethyl)ben...)Show SMILES COc1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4O2/c1-32-17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 preincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50293753

(2-(4-(methylsulfonyl)phenylamino)-1-(2-(trifluorom...)Show SMILES CS(=O)(=O)c1ccc(Nc2nc3cc(ccc3n2Cc2ccccc2C(F)(F)F)C(N)=O)cc1 Show InChI InChI=1S/C23H19F3N4O3S/c1-34(32,33)17-9-7-16(8-10-17)28-22-29-19-12-14(21(27)31)6-11-20(19)30(22)13-15-4-2-3-5-18(15)23(24,25)26/h2-12H,13H2,1H3,(H2,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 preincubated with compound |
Bioorg Med Chem Lett 19: 3405-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.040
BindingDB Entry DOI: 10.7270/Q2MS3SS3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data