

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233623 (CHEMBL4104013) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233623 (CHEMBL4104013) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233626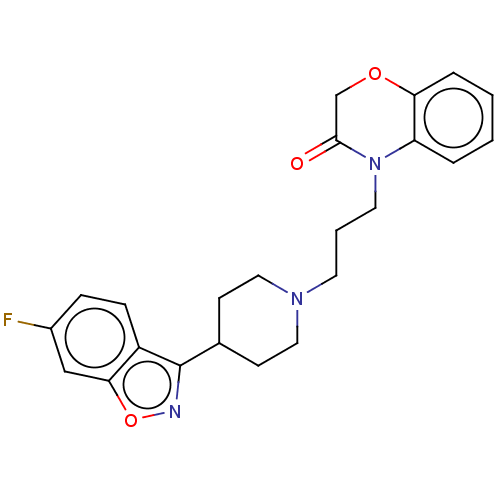 (CHEMBL4062602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233626 (CHEMBL4062602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233626 (CHEMBL4062602) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233626 (CHEMBL4062602) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233625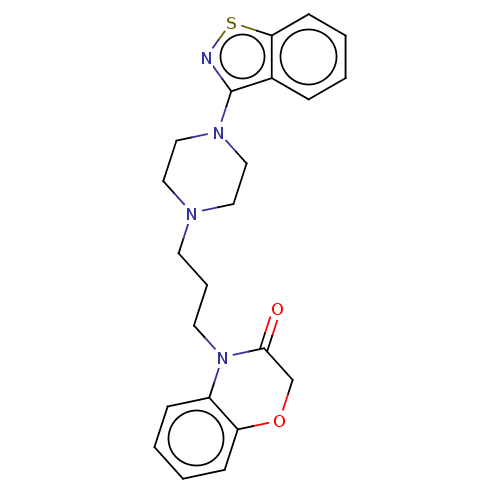 (CHEMBL4090568) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233625 (CHEMBL4090568) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from dopamine D2L receptor (unknown origin) expressed in CHO cell membranes | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443100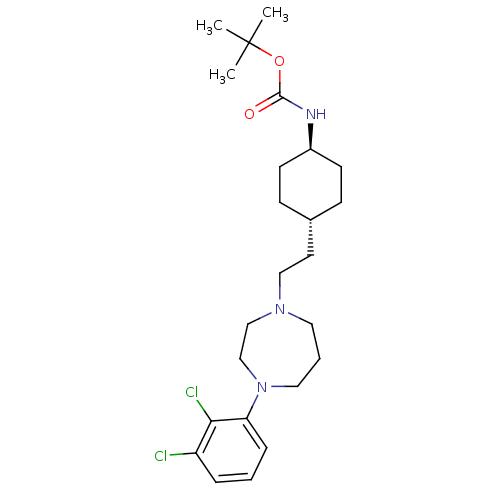 (CHEMBL3085820) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233625 (CHEMBL4090568) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233625 (CHEMBL4090568) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443102 (CHEMBL3085819) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443096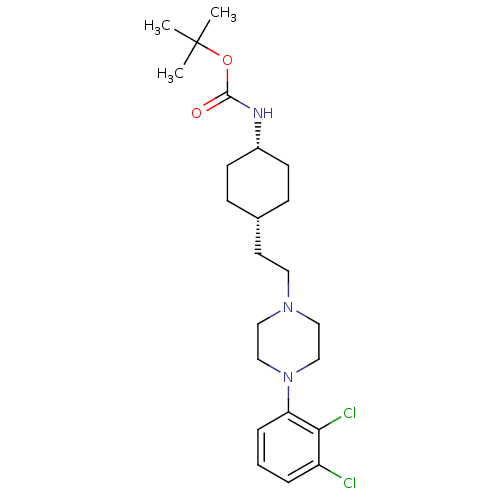 (CHEMBL3085824) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443099 (CHEMBL3085821) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233627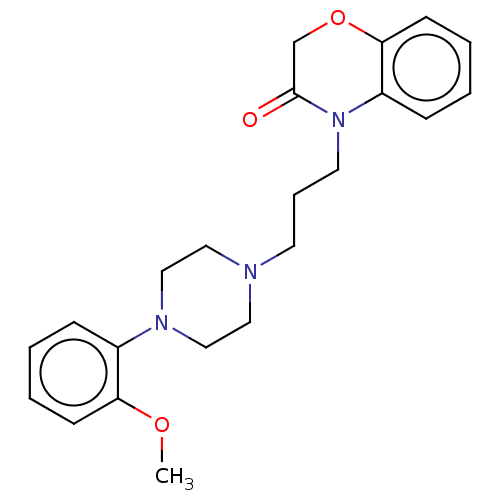 (CHEMBL4066435) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233627 (CHEMBL4066435) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443095 (CHEMBL3085825) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233624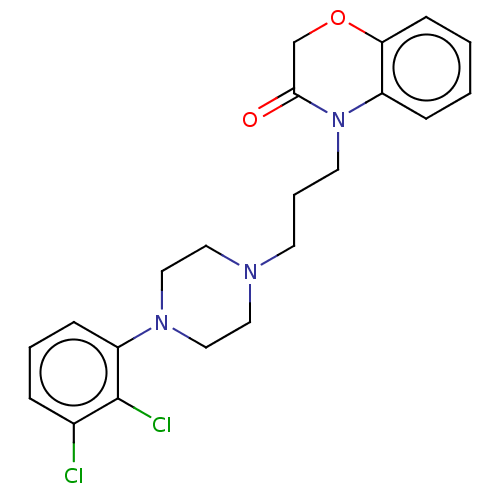 (CHEMBL4093980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233624 (CHEMBL4093980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443094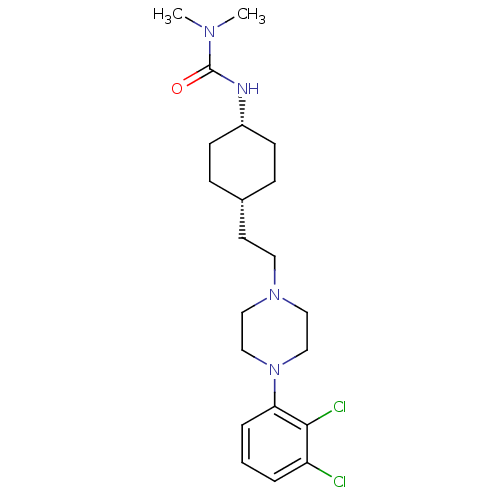 (CHEMBL3085826) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443097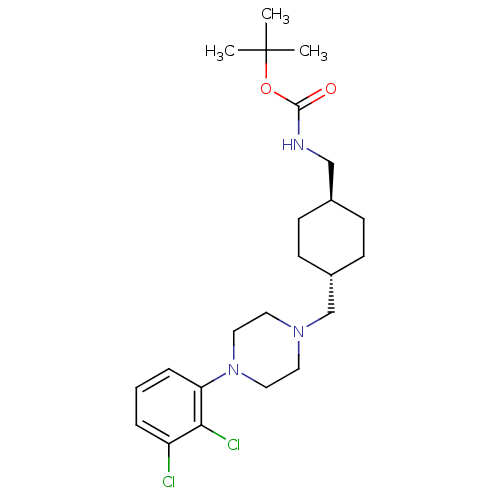 (CHEMBL3085823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443110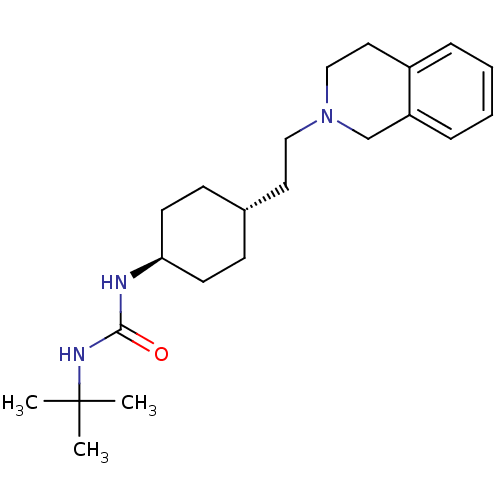 (CHEMBL3088215) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233624 (CHEMBL4093980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233627 (CHEMBL4066435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50233624 (CHEMBL4093980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233627 (CHEMBL4066435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443098 (CHEMBL3085822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443109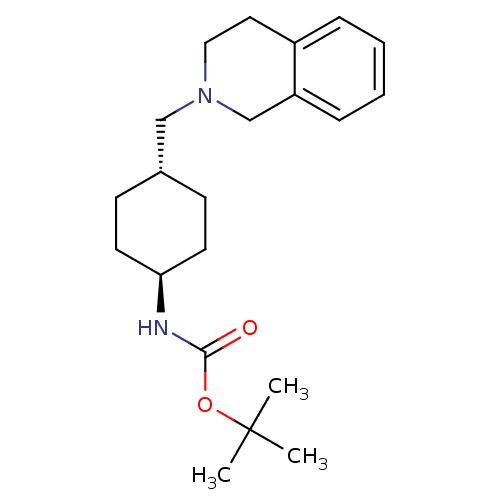 (CHEMBL3085812) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443104 (CHEMBL3085817) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443106 (CHEMBL3085815) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233623 (CHEMBL4104013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50233623 (CHEMBL4104013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 568 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443115 (CHEMBL3088209) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50326218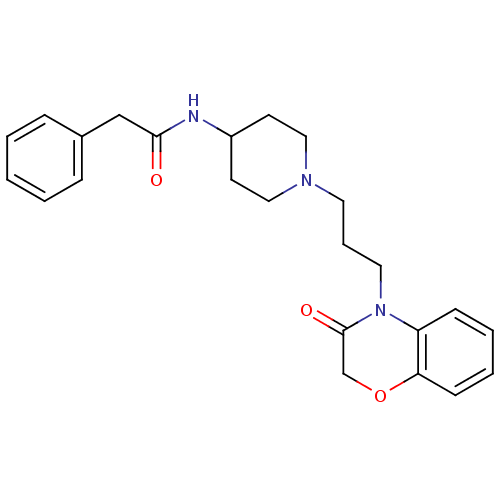 (CHEMBL1242923 | N-{1-[3-(3-Oxo-2,3-dihydrobenzo[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50326218 (CHEMBL1242923 | N-{1-[3-(3-Oxo-2,3-dihydrobenzo[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 834 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443112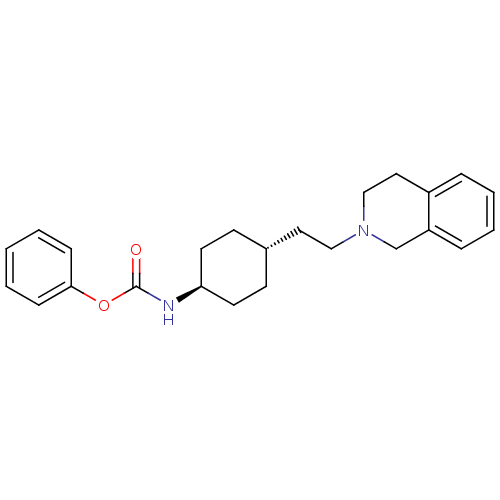 (CHEMBL3088214) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443111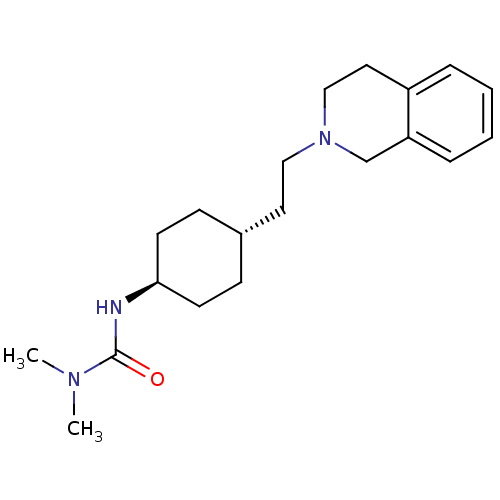 (CHEMBL3085802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50326218 (CHEMBL1242923 | N-{1-[3-(3-Oxo-2,3-dihydrobenzo[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50326218 (CHEMBL1242923 | N-{1-[3-(3-Oxo-2,3-dihydrobenzo[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor expressed in F1pIn CHO cell membranes after 1 hr by scintillation counting method | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443107 (CHEMBL3085814) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443108 (CHEMBL3085813) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443114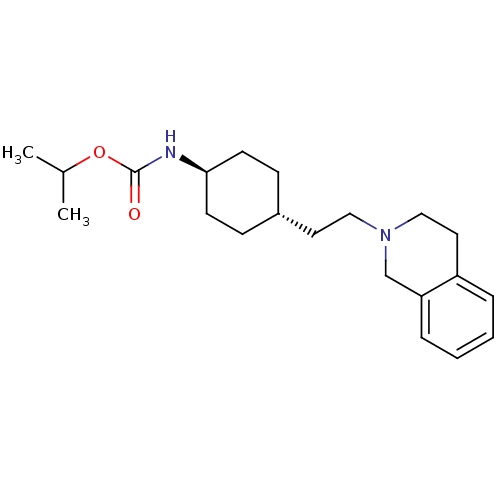 (CHEMBL3088212) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443103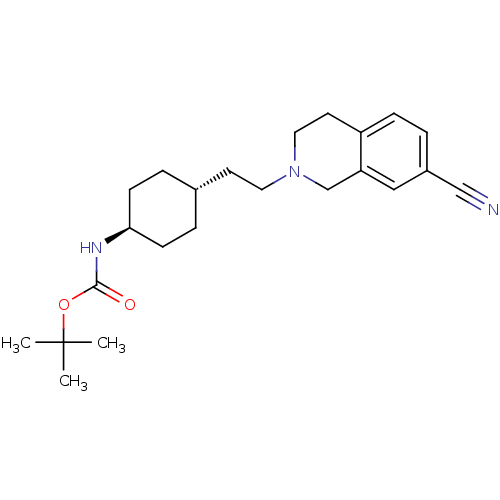 (CHEMBL3085818) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443116 (CHEMBL3088211) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443113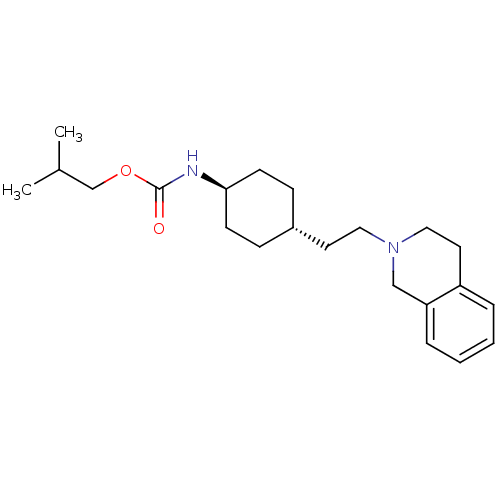 (CHEMBL3088213) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443117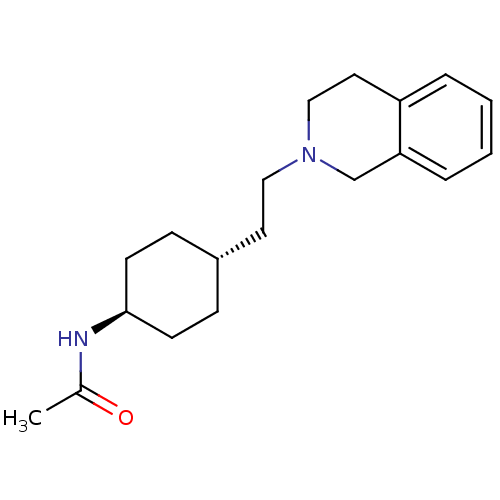 (CHEMBL3088210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443105 (CHEMBL3085816) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D2L receptor expressed in CHO cell membranes after 3 hrs by liquid scintillation counting analysis | J Med Chem 56: 9199-221 (2013) Article DOI: 10.1021/jm401318w BindingDB Entry DOI: 10.7270/Q2RF5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50233624 (CHEMBL4093980) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human mAChR1 receptor expressed in F1pIn CHO cells after 60 mins by scintillation counting analysis | J Med Chem 58: 1550-5 (2015) Article DOI: 10.1021/jm5013243 BindingDB Entry DOI: 10.7270/Q2F76FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |