

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402386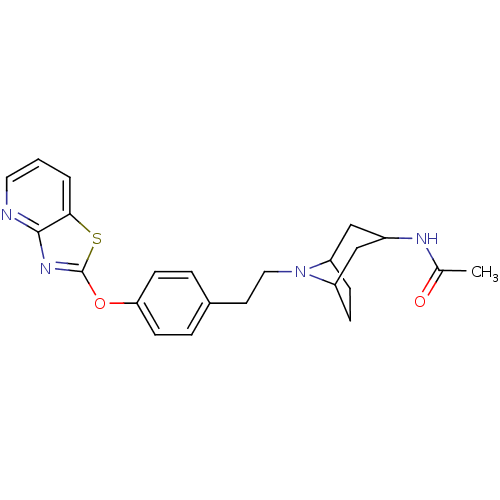 (CHEMBL2207747) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402382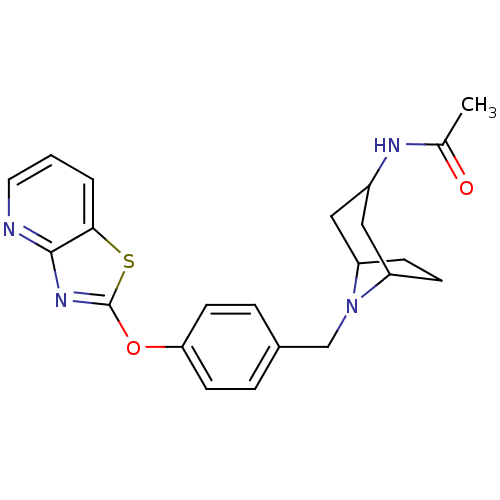 (CHEMBL2207751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM67690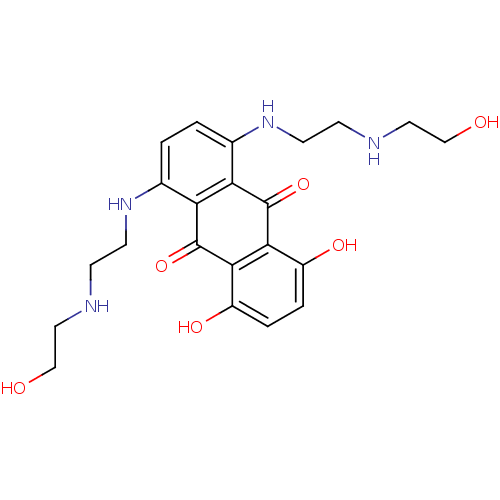 (1,4-bis[2-(2-hydroxyethylamino)ethylamino]-5,8-bis...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: cell accumulation in MCF7/MRP1-M24 cells | Mol Pharmacol 69: 1499-505 (2006) Article DOI: 10.1124/mol.105.017988 BindingDB Entry DOI: 10.7270/Q2GT5PDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM67690 (1,4-bis[2-(2-hydroxyethylamino)ethylamino]-5,8-bis...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: cell accumulation in MCF7/MRP1-M6 cells | Mol Pharmacol 69: 1499-505 (2006) Article DOI: 10.1124/mol.105.017988 BindingDB Entry DOI: 10.7270/Q2GT5PDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425166 (CHEMBL2313573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM67690 (1,4-bis[2-(2-hydroxyethylamino)ethylamino]-5,8-bis...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: cell accumulation in MCF7/MRP1-10 cells | Mol Pharmacol 69: 1499-505 (2006) Article DOI: 10.1124/mol.105.017988 BindingDB Entry DOI: 10.7270/Q2GT5PDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402384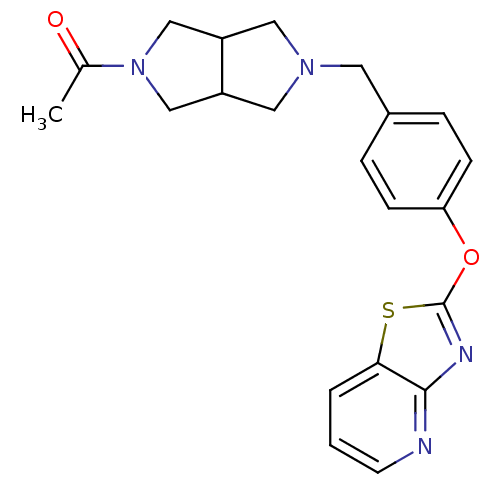 (CHEMBL2207749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402403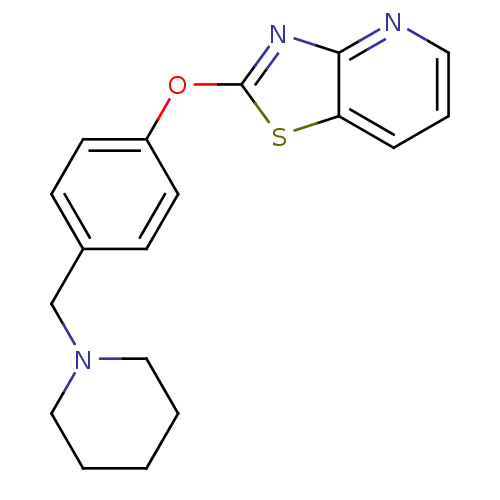 (CHEMBL2207730) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402391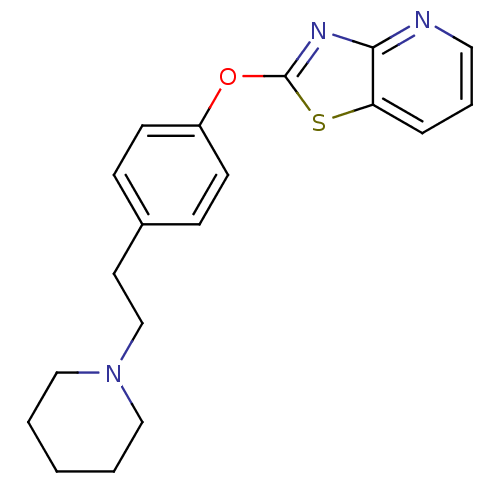 (CHEMBL2207742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425168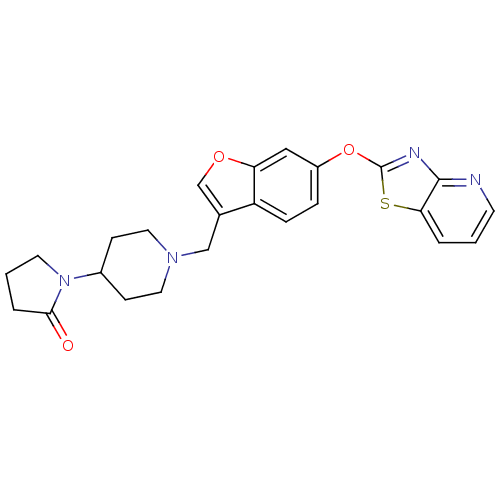 (CHEMBL2313571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM220233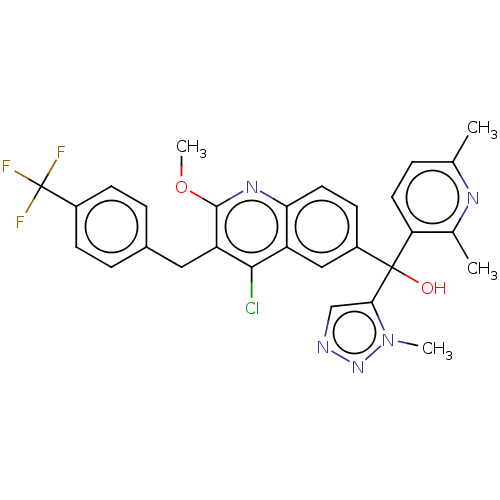 (US9290476, 77A | US9290476, 77B | US9290476, 77C) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in human PBMC derived CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by m... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425170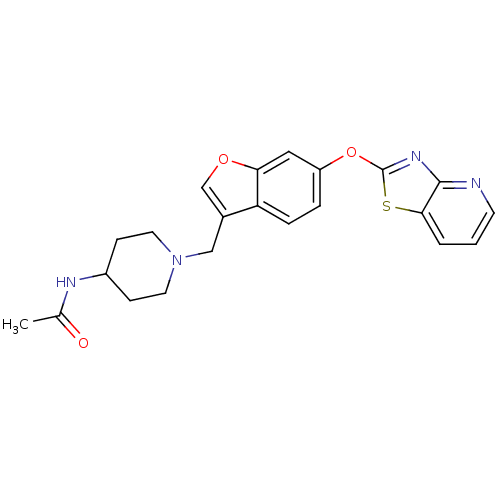 (CHEMBL2313569) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM220233 (US9290476, 77A | US9290476, 77B | US9290476, 77C) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as inhibition o... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402392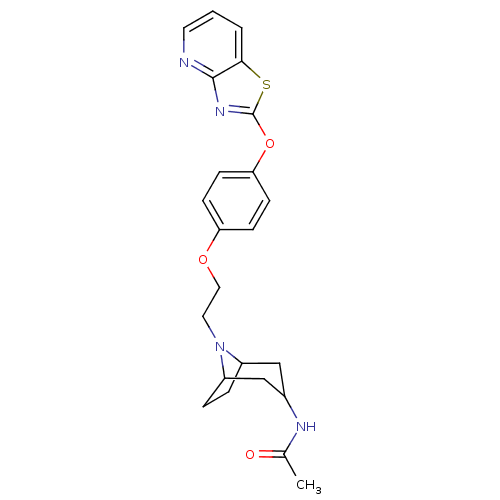 (CHEMBL2207741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425151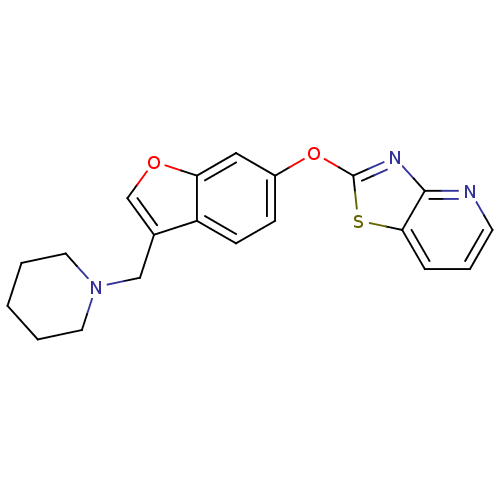 (CHEMBL2313563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402383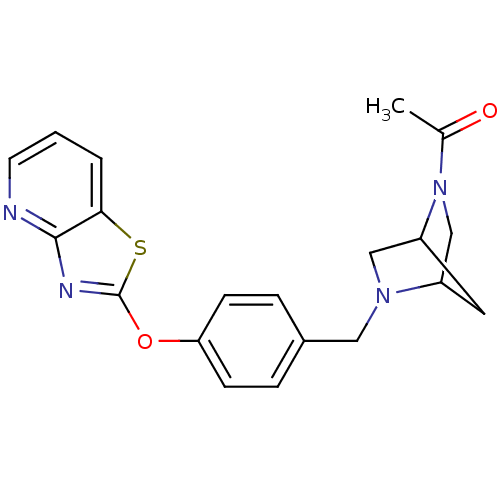 (CHEMBL2207750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50510354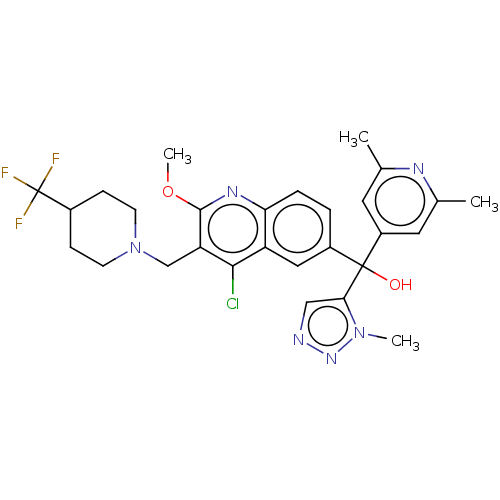 (CHEMBL4441287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human full length RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as ... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402385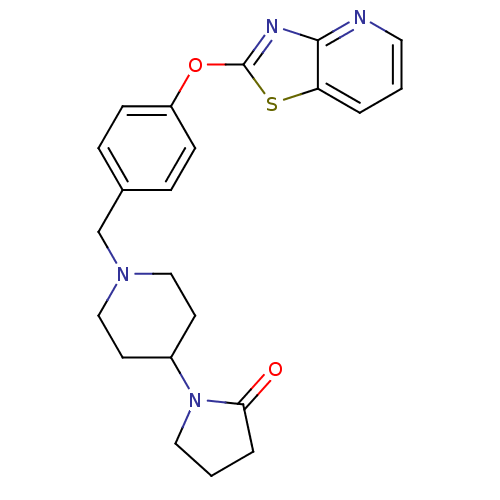 (CHEMBL2207748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402405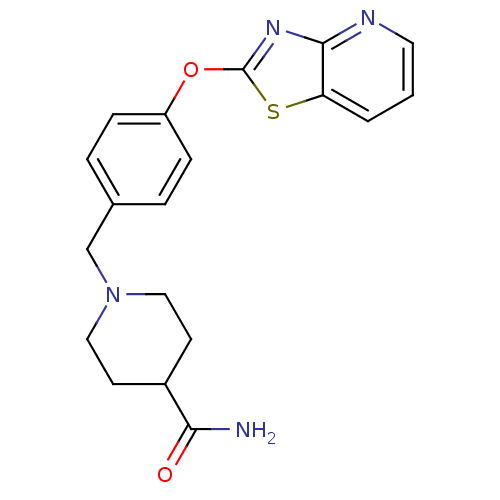 (CHEMBL2207752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402395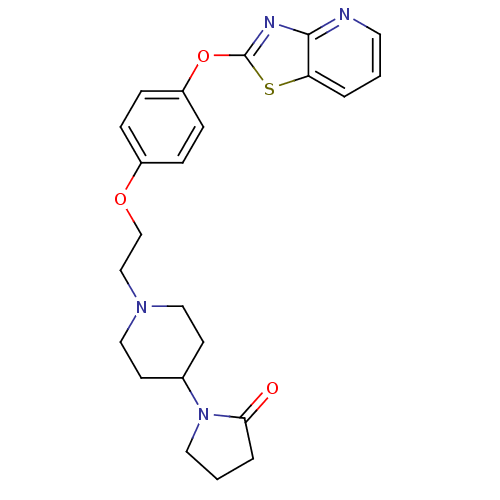 (CHEMBL2207738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402388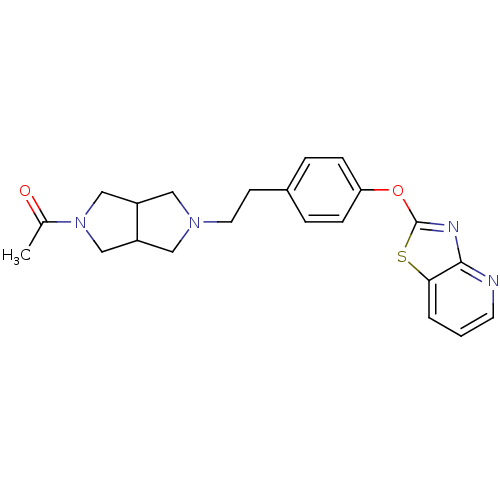 (CHEMBL2207745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402394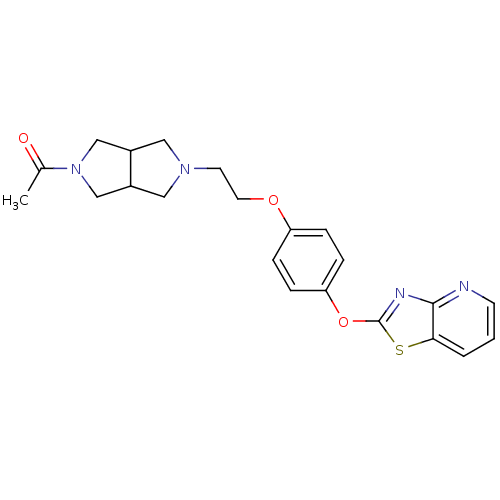 (CHEMBL2207739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425163 (CHEMBL2313266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402393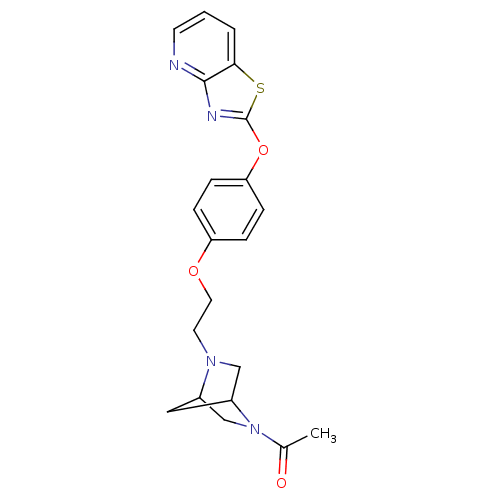 (CHEMBL2207740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425164 (CHEMBL2313265) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425167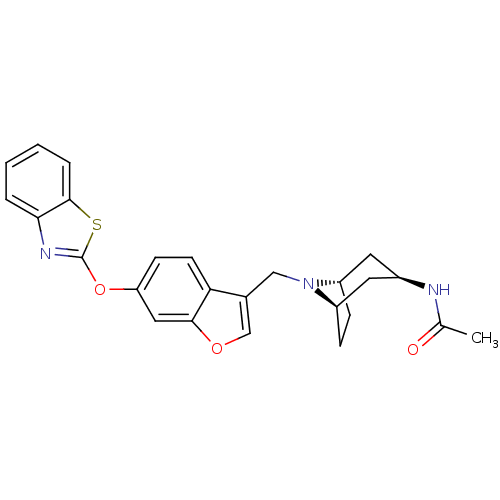 (CHEMBL2313572) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425157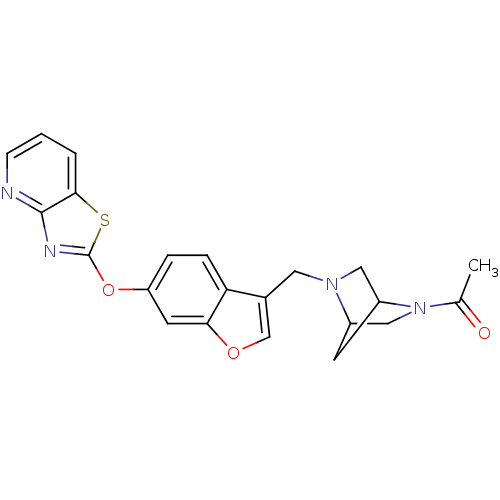 (CHEMBL2313272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50510357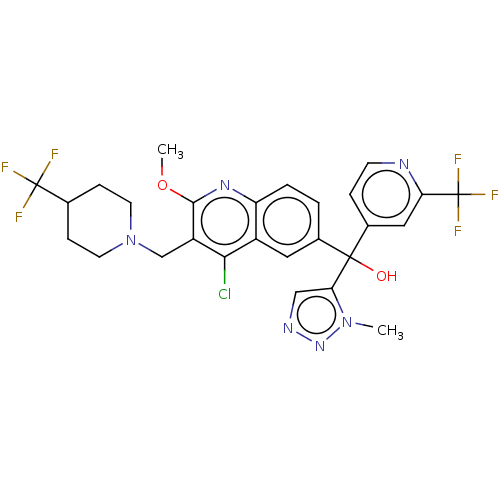 (CHEMBL4543938) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in human PBMC derived CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by m... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402397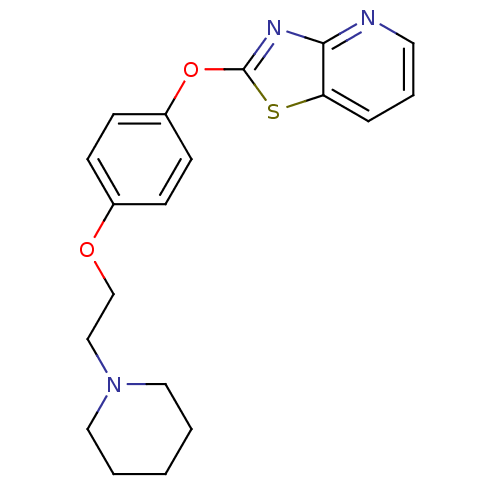 (CHEMBL2207736) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425147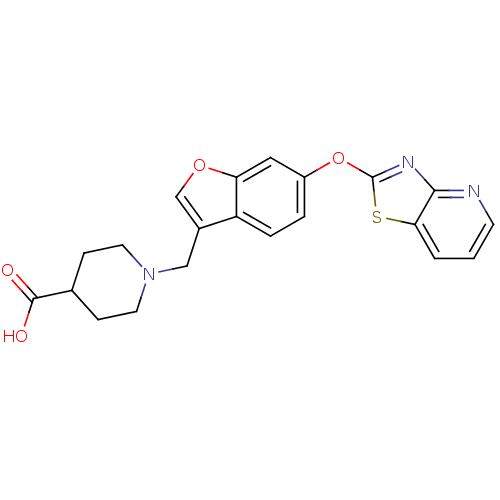 (CHEMBL2313567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24220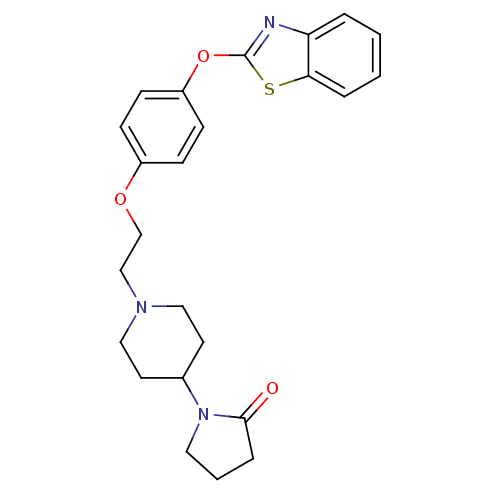 (1-(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Mus musculus) | BDBM50425166 (CHEMBL2313573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of LTA4H in CD1 mouse whole blood assessed as decrease in calcium ionophore-stimulated LTB4 production incubated for 15 mins prior to calc... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50510349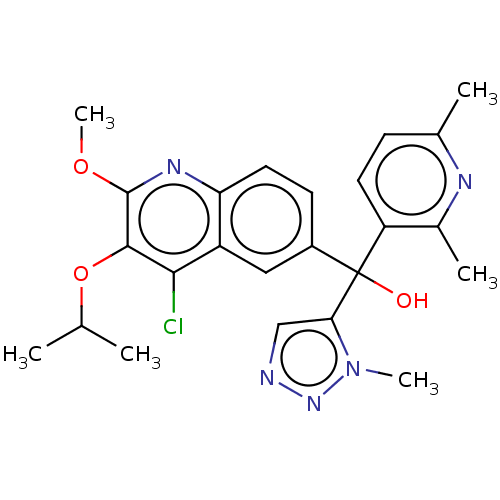 (CHEMBL4434891) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in human PBMC derived CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by m... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402390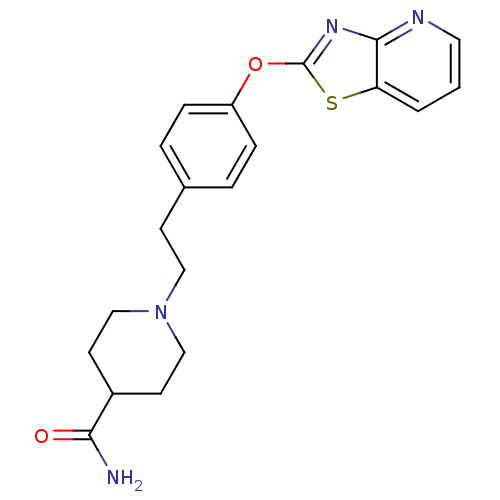 (CHEMBL2207743) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50510357 (CHEMBL4543938) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human full length RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as ... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50510355 (CHEMBL4552142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as inhibition o... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50510352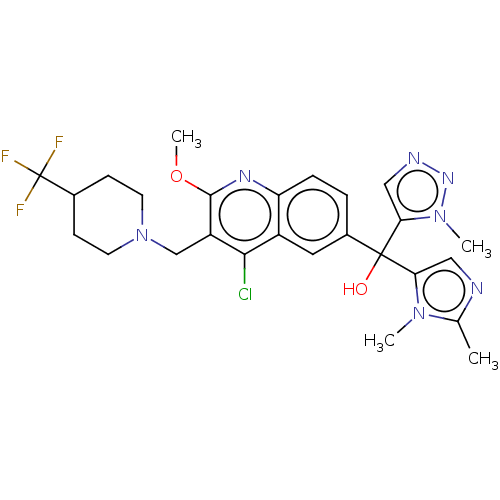 (CHEMBL4468588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in human PBMC derived CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by m... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339252 (US10201546, Example 126b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human full length RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as ... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402387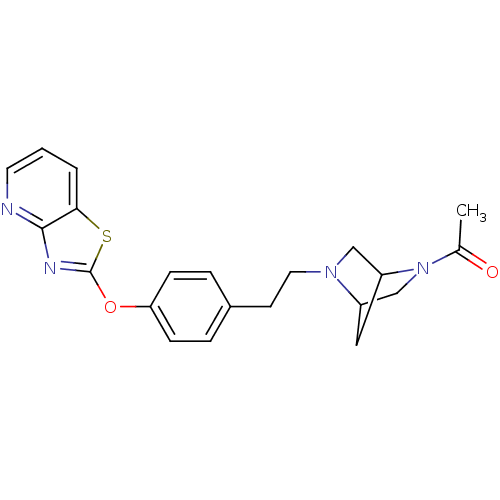 (CHEMBL2207746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339276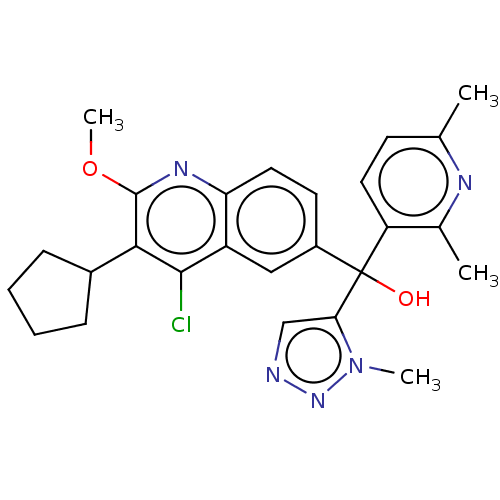 (US10201546, Example 136a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as inhibition o... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425148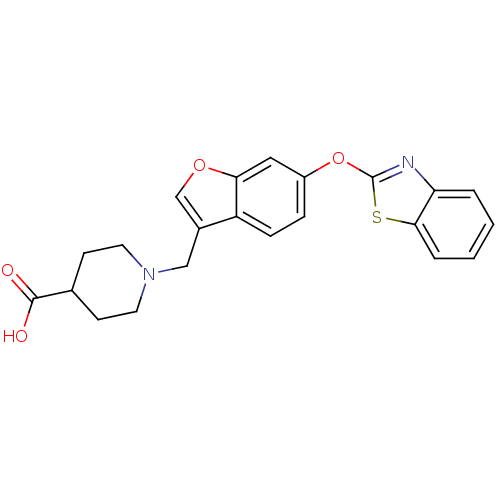 (CHEMBL2313566) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402389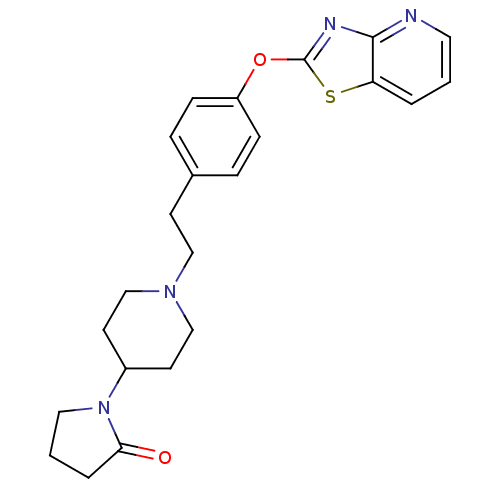 (CHEMBL2207744) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402396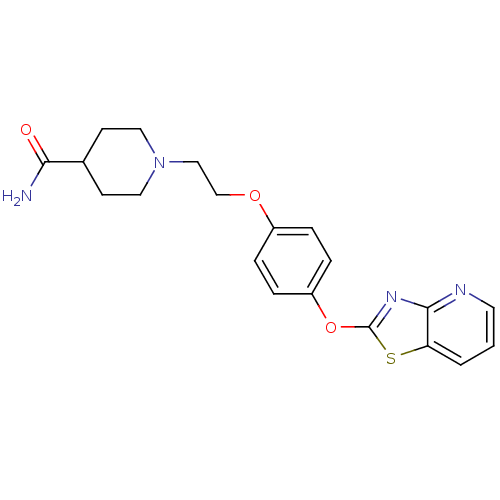 (CHEMBL2207737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Mus musculus) | BDBM50425170 (CHEMBL2313569) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of LTA4H in CD1 mouse whole blood assessed as decrease in calcium ionophore-stimulated LTB4 production incubated for 15 mins prior to calc... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339227 (US10201546, Example 112c | US10201546, Example 191...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in human PBMC derived CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by m... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50510352 (CHEMBL4468588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as inhibition o... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339216 ((4-Chloro-3-((4,4-difluoropiperidin-1-yl)methyl)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as inhibition o... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Mus musculus) | BDBM50425148 (CHEMBL2313566) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of LTA4H in CD1 mouse whole blood assessed as decrease in calcium ionophore-stimulated LTB4 production incubated for 15 mins prior to calc... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339134 (US10201546, Example 68a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-DNA binding domain-fused human RORgammat LBD expressed in pGL4.31 transfected HEK293T cells assessed as inhibition o... | Bioorg Med Chem Lett 29: 1463-1470 (2019) Article DOI: 10.1016/j.bmcl.2019.04.021 BindingDB Entry DOI: 10.7270/Q2862KRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Mus musculus) | BDBM50425151 (CHEMBL2313563) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of LTA4H in CD1 mouse whole blood assessed as decrease in calcium ionophore-stimulated LTB4 production incubated for 15 mins prior to calc... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 204 total ) | Next | Last >> |