 Found 323 hits with Last Name = 'hernandez' and Initial = 'p'
Found 323 hits with Last Name = 'hernandez' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50032821
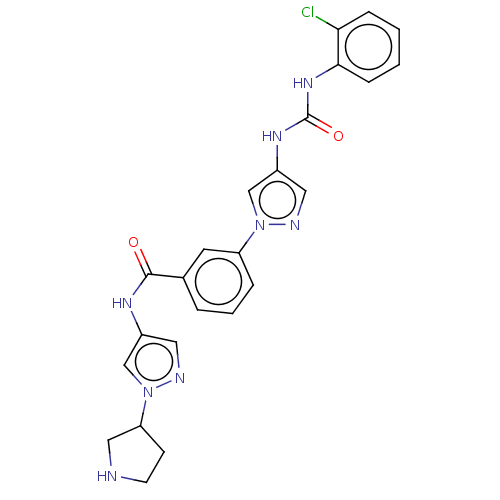
(CHEMBL3355178)Show SMILES Clc1ccccc1NC(=O)Nc1cnn(c1)-c1cccc(c1)C(=O)Nc1cnn(c1)C1CCNC1 Show InChI InChI=1S/C24H23ClN8O2/c25-21-6-1-2-7-22(21)31-24(35)30-18-12-27-32(15-18)19-5-3-4-16(10-19)23(34)29-17-11-28-33(14-17)20-8-9-26-13-20/h1-7,10-12,14-15,20,26H,8-9,13H2,(H,29,34)(H2,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha1 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050929

(2-((S)-2-{(S)-2-[3-(4-Methoxy-phenyl)-ureido]-4-me...)Show SMILES COc1ccc(NC(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(=O)NC(Cc2ccccc2)C(O)=O)cc1 Show InChI InChI=1S/C28H38N4O6S/c1-18(2)16-23(26(34)31-24(27(35)36)17-19-8-6-5-7-9-19)30-25(33)22(14-15-39-4)32-28(37)29-20-10-12-21(38-3)13-11-20/h5-13,18,22-24H,14-17H2,1-4H3,(H,30,33)(H,31,34)(H,35,36)(H2,29,32,37)/t22-,23-,24?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050933

((S)-2-((S)-2-{(S)-2-[3-(4-Chloro-phenyl)-ureido]-4...)Show SMILES CSCC[C@H](NC(=O)Nc1ccc(Cl)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C27H35ClN4O5S/c1-17(2)15-22(25(34)31-23(26(35)36)16-18-7-5-4-6-8-18)30-24(33)21(13-14-38-3)32-27(37)29-20-11-9-19(28)10-12-20/h4-12,17,21-23H,13-16H2,1-3H3,(H,30,33)(H,31,34)(H,35,36)(H2,29,32,37)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050935
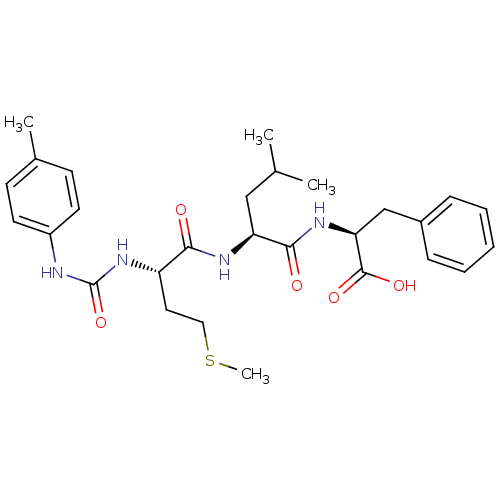
((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...)Show SMILES CSCC[C@H](NC(=O)Nc1ccc(C)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C28H38N4O5S/c1-18(2)16-23(26(34)31-24(27(35)36)17-20-8-6-5-7-9-20)30-25(33)22(14-15-38-4)32-28(37)29-21-12-10-19(3)11-13-21/h5-13,18,22-24H,14-17H2,1-4H3,(H,30,33)(H,31,34)(H,35,36)(H2,29,32,37)/t22-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50089225
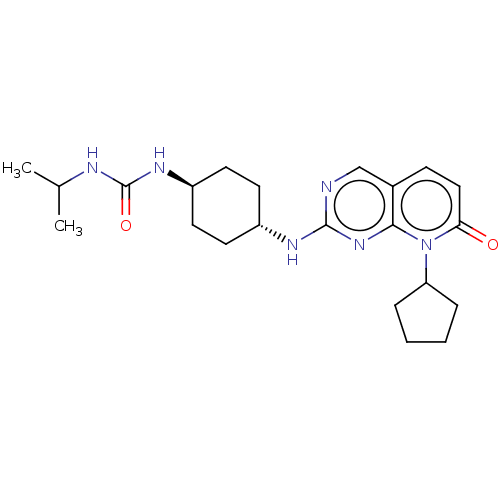
(CHEMBL3577877)Show SMILES CC(C)NC(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C3CCCC3)c2n1 |r,wU:10.13,wD:7.6,(-6.66,-12.02,;-6.66,-10.79,;-7.73,-10.18,;-5.33,-10.02,;-5.33,-8.48,;-6.4,-7.86,;-4,-7.7,;-4,-6.16,;-2.67,-5.39,;-2.67,-3.85,;-4.01,-3.08,;-5.34,-3.86,;-5.34,-5.4,;-4.01,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;1.33,-3.08,;2.58,-3.96,;2.1,-5.43,;.56,-5.42,;.08,-3.96,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C22H32N6O2/c1-14(2)24-22(30)26-17-10-8-16(9-11-17)25-21-23-13-15-7-12-19(29)28(20(15)27-21)18-5-3-4-6-18/h7,12-14,16-18H,3-6,8-11H2,1-2H3,(H,23,25,27)(H2,24,26,30)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072672
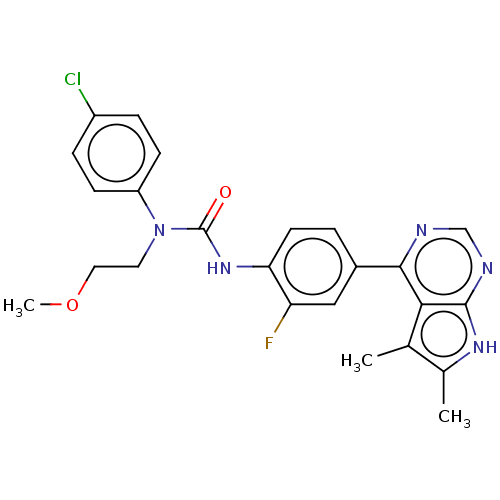
(CHEMBL3410056)Show SMILES COCCN(C(=O)Nc1ccc(cc1F)-c1ncnc2[nH]c(C)c(C)c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H23ClFN5O2/c1-14-15(2)29-23-21(14)22(27-13-28-23)16-4-9-20(19(26)12-16)30-24(32)31(10-11-33-3)18-7-5-17(25)6-8-18/h4-9,12-13H,10-11H2,1-3H3,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50089225

(CHEMBL3577877)Show SMILES CC(C)NC(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C3CCCC3)c2n1 |r,wU:10.13,wD:7.6,(-6.66,-12.02,;-6.66,-10.79,;-7.73,-10.18,;-5.33,-10.02,;-5.33,-8.48,;-6.4,-7.86,;-4,-7.7,;-4,-6.16,;-2.67,-5.39,;-2.67,-3.85,;-4.01,-3.08,;-5.34,-3.86,;-5.34,-5.4,;-4.01,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;1.33,-3.08,;2.58,-3.96,;2.1,-5.43,;.56,-5.42,;.08,-3.96,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C22H32N6O2/c1-14(2)24-22(30)26-17-10-8-16(9-11-17)25-21-23-13-15-7-12-19(29)28(20(15)27-21)18-5-3-4-6-18/h7,12-14,16-18H,3-6,8-11H2,1-2H3,(H,23,25,27)(H2,24,26,30)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha-1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM103909
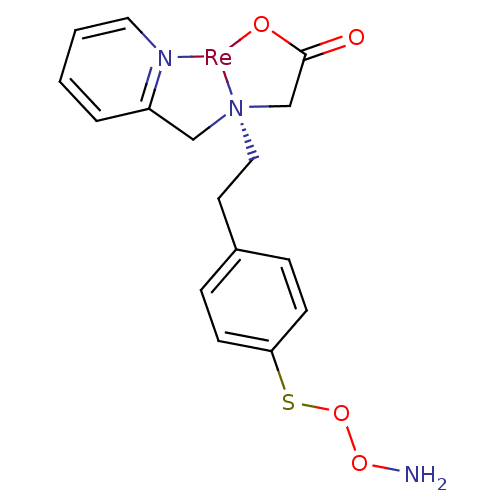
(JNK3 inhibitor 6 | US8562945, 242)Show SMILES NOOSc1ccc(CC[N@@]23CC(=O)O[Re]2[N]2=C(C3)C=CC=C2)cc1 |r,c:17,21,23| Show InChI InChI=1S/C16H19N3O4S.Re/c17-22-23-24-15-6-4-13(5-7-15)8-10-19(12-16(20)21)11-14-3-1-2-9-18-14;/h1-7,9H,8,10-12,17H2,(H,20,21);/q;+1/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
| |
ACS Chem Biol 8: 1747-54 (2013)
Article DOI: 10.1021/cb3006165
BindingDB Entry DOI: 10.7270/Q25719PN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50089225

(CHEMBL3577877)Show SMILES CC(C)NC(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C3CCCC3)c2n1 |r,wU:10.13,wD:7.6,(-6.66,-12.02,;-6.66,-10.79,;-7.73,-10.18,;-5.33,-10.02,;-5.33,-8.48,;-6.4,-7.86,;-4,-7.7,;-4,-6.16,;-2.67,-5.39,;-2.67,-3.85,;-4.01,-3.08,;-5.34,-3.86,;-5.34,-5.4,;-4.01,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;1.33,-3.08,;2.58,-3.96,;2.1,-5.43,;.56,-5.42,;.08,-3.96,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C22H32N6O2/c1-14(2)24-22(30)26-17-10-8-16(9-11-17)25-21-23-13-15-7-12-19(29)28(20(15)27-21)18-5-3-4-6-18/h7,12-14,16-18H,3-6,8-11H2,1-2H3,(H,23,25,27)(H2,24,26,30)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50089233
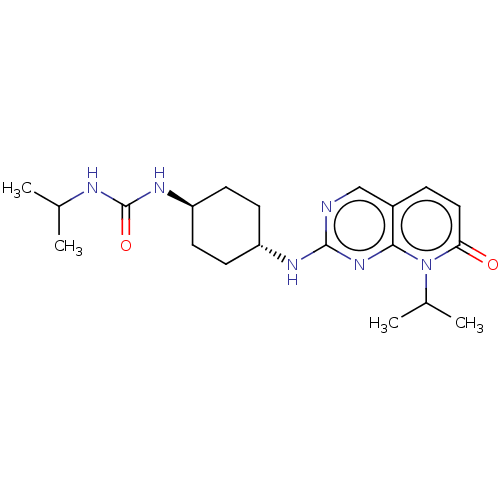
(CHEMBL3577868)Show SMILES CC(C)NC(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 |r,wU:10.13,wD:7.6,(-6.67,-12.05,;-6.67,-10.81,;-7.74,-10.2,;-5.34,-10.04,;-5.34,-8.49,;-6.41,-7.88,;-4,-7.72,;-4.01,-6.17,;-2.67,-5.4,;-2.68,-3.86,;-4.02,-3.09,;-5.35,-3.86,;-5.35,-5.41,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;1.34,-3.08,;2.41,-3.7,;.27,-3.7,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C20H30N6O2/c1-12(2)22-20(28)24-16-8-6-15(7-9-16)23-19-21-11-14-5-10-17(27)26(13(3)4)18(14)25-19/h5,10-13,15-16H,6-9H2,1-4H3,(H,21,23,25)(H2,22,24,28)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha-1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50089223
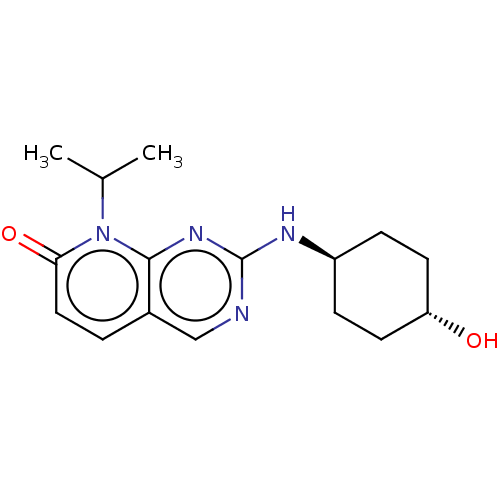
(CHEMBL3577855)Show SMILES CC(C)n1c2nc(N[C@H]3CC[C@H](O)CC3)ncc2ccc1=O |r,wU:8.7,wD:11.11,(2.41,-3.7,;1.34,-3.08,;.27,-3.7,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-4.02,-3.09,;-2.68,-3.86,;-2.67,-5.4,;-4.01,-6.17,;-4,-7.41,;-5.35,-5.41,;-5.35,-3.86,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,)| Show InChI InChI=1S/C16H22N4O2/c1-10(2)20-14(22)8-3-11-9-17-16(19-15(11)20)18-12-4-6-13(21)7-5-12/h3,8-10,12-13,21H,4-7H2,1-2H3,(H,17,18,19)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50089223

(CHEMBL3577855)Show SMILES CC(C)n1c2nc(N[C@H]3CC[C@H](O)CC3)ncc2ccc1=O |r,wU:8.7,wD:11.11,(2.41,-3.7,;1.34,-3.08,;.27,-3.7,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-4.02,-3.09,;-2.68,-3.86,;-2.67,-5.4,;-4.01,-6.17,;-4,-7.41,;-5.35,-5.41,;-5.35,-3.86,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,)| Show InChI InChI=1S/C16H22N4O2/c1-10(2)20-14(22)8-3-11-9-17-16(19-15(11)20)18-12-4-6-13(21)7-5-12/h3,8-10,12-13,21H,4-7H2,1-2H3,(H,17,18,19)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072662
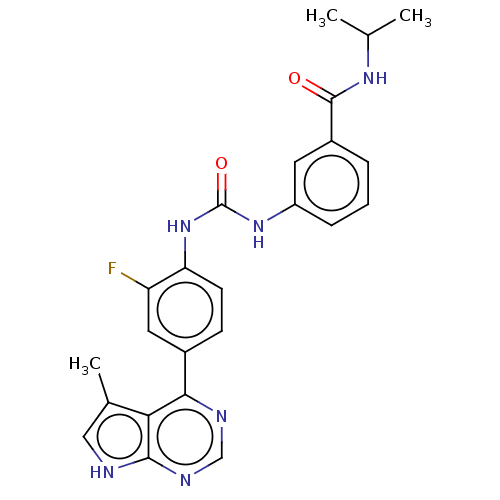
(CHEMBL3407526)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2F)-c2ncnc3[nH]cc(C)c23)c1 Show InChI InChI=1S/C24H23FN6O2/c1-13(2)29-23(32)16-5-4-6-17(9-16)30-24(33)31-19-8-7-15(10-18(19)25)21-20-14(3)11-26-22(20)28-12-27-21/h4-13H,1-3H3,(H,29,32)(H,26,27,28)(H2,30,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072671
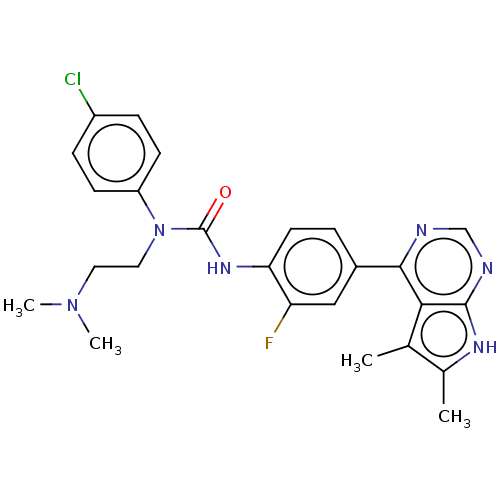
(CHEMBL3410055)Show SMILES CN(C)CCN(C(=O)Nc1ccc(cc1F)-c1ncnc2[nH]c(C)c(C)c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C25H26ClFN6O/c1-15-16(2)30-24-22(15)23(28-14-29-24)17-5-10-21(20(27)13-17)31-25(34)33(12-11-32(3)4)19-8-6-18(26)7-9-19/h5-10,13-14H,11-12H2,1-4H3,(H,31,34)(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050928
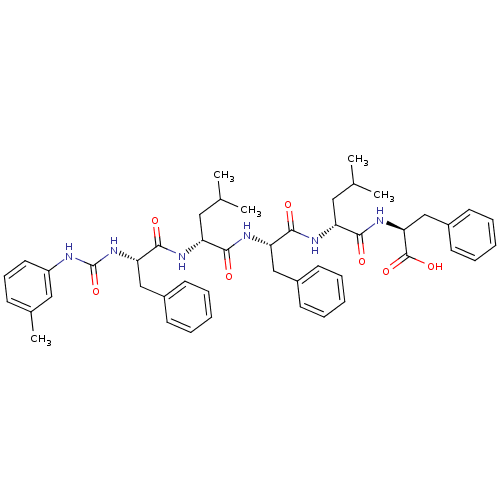
((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)Nc1cccc(C)c1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H58N6O7/c1-30(2)24-37(50-45(57)40(28-34-19-11-7-12-20-34)53-47(60)48-36-23-15-16-32(5)26-36)42(54)51-39(27-33-17-9-6-10-18-33)44(56)49-38(25-31(3)4)43(55)52-41(46(58)59)29-35-21-13-8-14-22-35/h6-23,26,30-31,37-41H,24-25,27-29H2,1-5H3,(H,49,56)(H,50,57)(H,51,54)(H,52,55)(H,58,59)(H2,48,53,60)/t37-,38-,39+,40+,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072728
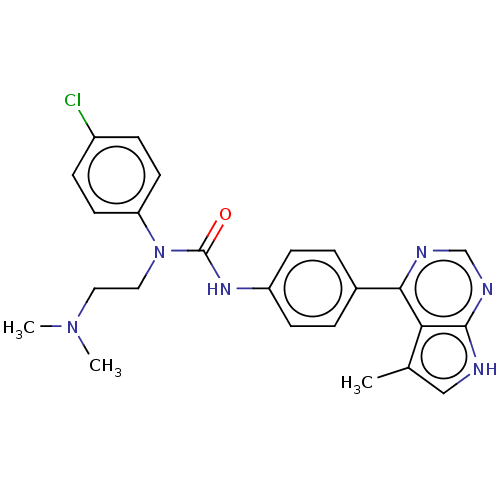
(CHEMBL3410052)Show SMILES CN(C)CCN(C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12)c1ccc(Cl)cc1 Show InChI InChI=1S/C24H25ClN6O/c1-16-14-26-23-21(16)22(27-15-28-23)17-4-8-19(9-5-17)29-24(32)31(13-12-30(2)3)20-10-6-18(25)7-11-20/h4-11,14-15H,12-13H2,1-3H3,(H,29,32)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050945

((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Adamantan-1-y...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)NC12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |TLB:42:43:47:41.40.46,46:41:48:45.47.44,46:45:48:41.40.42,THB:42:41:47:43.48.44| Show InChI InChI=1S/C50H66N6O7/c1-31(2)20-39(52-47(60)42(26-34-16-10-6-11-17-34)55-49(63)56-50-28-36-22-37(29-50)24-38(23-36)30-50)44(57)53-41(25-33-14-8-5-9-15-33)46(59)51-40(21-32(3)4)45(58)54-43(48(61)62)27-35-18-12-7-13-19-35/h5-19,31-32,36-43H,20-30H2,1-4H3,(H,51,59)(H,52,60)(H,53,57)(H,54,58)(H,61,62)(H2,55,56,63)/t36?,37?,38?,39-,40-,41+,42+,43+,50?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050937
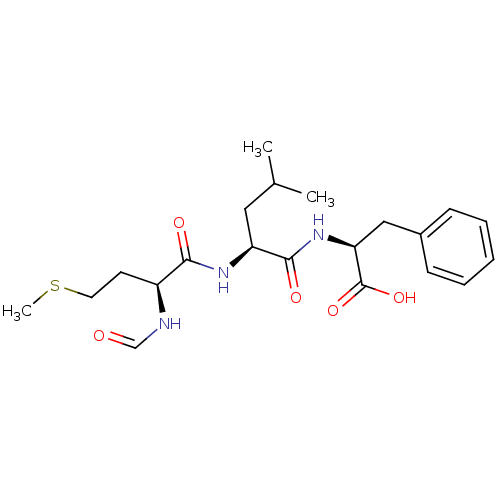
((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...)Show SMILES CSCC[C@H](NC=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C21H31N3O5S/c1-14(2)11-17(23-19(26)16(22-13-25)9-10-30-3)20(27)24-18(21(28)29)12-15-7-5-4-6-8-15/h4-8,13-14,16-18H,9-12H2,1-3H3,(H,22,25)(H,23,26)(H,24,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072670
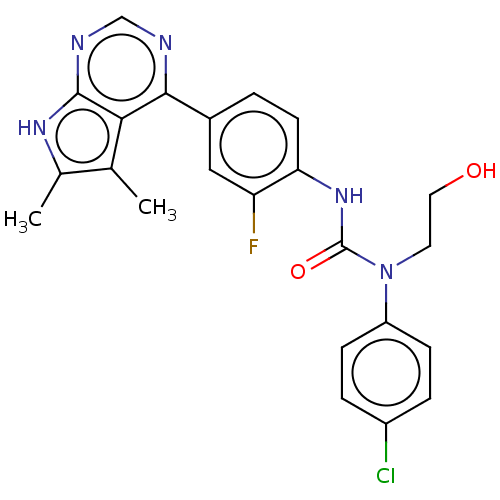
(CHEMBL3410053)Show SMILES Cc1[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(Cl)cc4)c(F)c3)c2c1C Show InChI InChI=1S/C23H21ClFN5O2/c1-13-14(2)28-22-20(13)21(26-12-27-22)15-3-8-19(18(25)11-15)29-23(32)30(9-10-31)17-6-4-16(24)5-7-17/h3-8,11-12,31H,9-10H2,1-2H3,(H,29,32)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072727
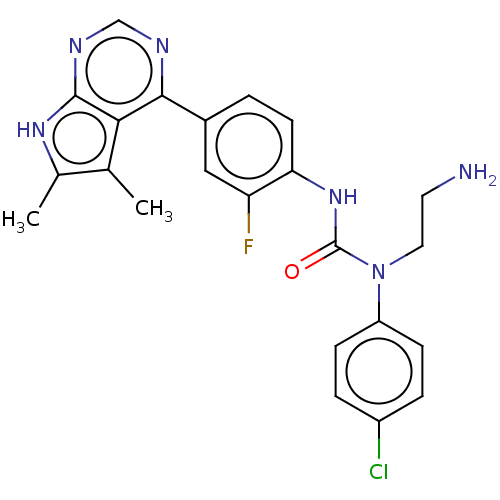
(CHEMBL3410054)Show SMILES Cc1[nH]c2ncnc(-c3ccc(NC(=O)N(CCN)c4ccc(Cl)cc4)c(F)c3)c2c1C Show InChI InChI=1S/C23H22ClFN6O/c1-13-14(2)29-22-20(13)21(27-12-28-22)15-3-8-19(18(25)11-15)30-23(32)31(10-9-26)17-6-4-16(24)5-7-17/h3-8,11-12H,9-10,26H2,1-2H3,(H,30,32)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50089233

(CHEMBL3577868)Show SMILES CC(C)NC(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 |r,wU:10.13,wD:7.6,(-6.67,-12.05,;-6.67,-10.81,;-7.74,-10.2,;-5.34,-10.04,;-5.34,-8.49,;-6.41,-7.88,;-4,-7.72,;-4.01,-6.17,;-2.67,-5.4,;-2.68,-3.86,;-4.02,-3.09,;-5.35,-3.86,;-5.35,-5.41,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;1.34,-3.08,;2.41,-3.7,;.27,-3.7,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C20H30N6O2/c1-12(2)22-20(28)24-16-8-6-15(7-9-16)23-19-21-11-14-5-10-17(27)26(13(3)4)18(14)25-19/h5,10-13,15-16H,6-9H2,1-4H3,(H,21,23,25)(H2,22,24,28)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072730
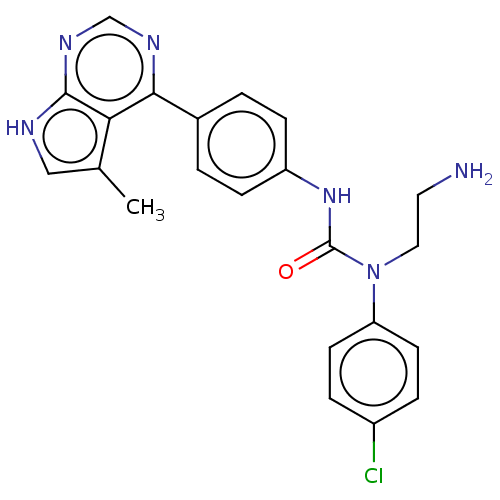
(CHEMBL3410050)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCN)c4ccc(Cl)cc4)cc3)c12 Show InChI InChI=1S/C22H21ClN6O/c1-14-12-25-21-19(14)20(26-13-27-21)15-2-6-17(7-3-15)28-22(30)29(11-10-24)18-8-4-16(23)5-9-18/h2-9,12-13H,10-11,24H2,1H3,(H,28,30)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50032819
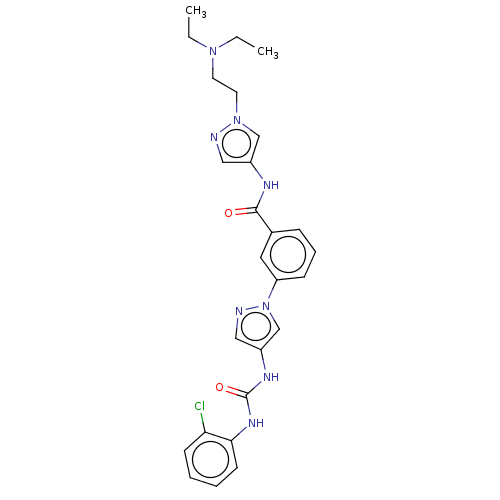
(CHEMBL3355175)Show SMILES CCN(CC)CCn1cc(NC(=O)c2cccc(c2)-n2cc(NC(=O)Nc3ccccc3Cl)cn2)cn1 Show InChI InChI=1S/C26H29ClN8O2/c1-3-33(4-2)12-13-34-17-20(15-28-34)30-25(36)19-8-7-9-22(14-19)35-18-21(16-29-35)31-26(37)32-24-11-6-5-10-23(24)27/h5-11,14-18H,3-4,12-13H2,1-2H3,(H,30,36)(H2,31,32,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha1 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50032820

(CHEMBL3355176)Show SMILES Clc1ccccc1NC(=O)Nc1cnn(c1)-c1cccc(c1)C(=O)Nc1cnn(CCN2CCCC2)c1 Show InChI InChI=1S/C26H27ClN8O2/c27-23-8-1-2-9-24(23)32-26(37)31-21-16-29-35(18-21)22-7-5-6-19(14-22)25(36)30-20-15-28-34(17-20)13-12-33-10-3-4-11-33/h1-2,5-9,14-18H,3-4,10-13H2,(H,30,36)(H2,31,32,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha1 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50032749

(CHEMBL3355177)Show SMILES Clc1ccccc1NC(=O)Nc1cnn(c1)-c1cccc(c1)C(=O)Nc1cnn(c1)C1CCNCC1 Show InChI InChI=1S/C25H25ClN8O2/c26-22-6-1-2-7-23(22)32-25(36)31-19-14-29-34(16-19)21-5-3-4-17(12-21)24(35)30-18-13-28-33(15-18)20-8-10-27-11-9-20/h1-7,12-16,20,27H,8-11H2,(H,30,35)(H2,31,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha1 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50089234
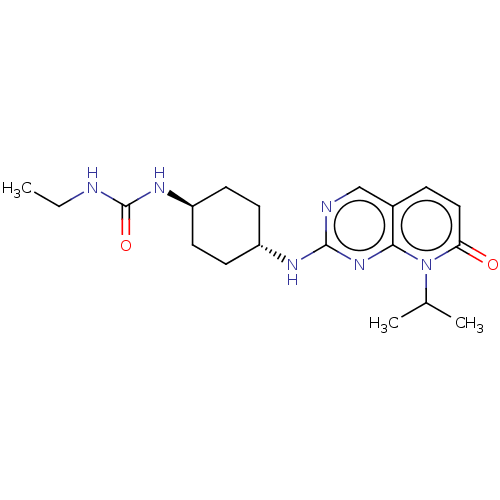
(CHEMBL3577867)Show SMILES CCNC(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 |r,wU:9.12,wD:6.5,(-6.67,-12.05,;-6.67,-10.81,;-5.34,-10.04,;-5.34,-8.49,;-6.41,-7.88,;-4,-7.72,;-4.01,-6.17,;-2.67,-5.4,;-2.68,-3.86,;-4.02,-3.09,;-5.35,-3.86,;-5.35,-5.41,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;1.34,-3.08,;2.41,-3.7,;.27,-3.7,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C19H28N6O2/c1-4-20-19(27)23-15-8-6-14(7-9-15)22-18-21-11-13-5-10-16(26)25(12(2)3)17(13)24-18/h5,10-12,14-15H,4,6-9H2,1-3H3,(H2,20,23,27)(H,21,22,24)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50032749

(CHEMBL3355177)Show SMILES Clc1ccccc1NC(=O)Nc1cnn(c1)-c1cccc(c1)C(=O)Nc1cnn(c1)C1CCNCC1 Show InChI InChI=1S/C25H25ClN8O2/c26-22-6-1-2-7-23(22)32-25(36)31-19-14-29-34(16-19)21-5-3-4-17(12-21)24(35)30-18-13-28-33(15-18)20-8-10-27-11-9-20/h1-7,12-16,20,27H,8-11H2,(H,30,35)(H2,31,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072667
(CHEMBL3410042)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(Cl)cc4)cc3)c12 Show InChI InChI=1S/C22H20ClN5O2/c1-14-12-24-21-19(14)20(25-13-26-21)15-2-6-17(7-3-15)27-22(30)28(10-11-29)18-8-4-16(23)5-9-18/h2-9,12-13,29H,10-11H2,1H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50089234

(CHEMBL3577867)Show SMILES CCNC(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 |r,wU:9.12,wD:6.5,(-6.67,-12.05,;-6.67,-10.81,;-5.34,-10.04,;-5.34,-8.49,;-6.41,-7.88,;-4,-7.72,;-4.01,-6.17,;-2.67,-5.4,;-2.68,-3.86,;-4.02,-3.09,;-5.35,-3.86,;-5.35,-5.41,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;1.34,-3.08,;2.41,-3.7,;.27,-3.7,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C19H28N6O2/c1-4-20-19(27)23-15-8-6-14(7-9-15)22-18-21-11-13-5-10-16(26)25(12(2)3)17(13)24-18/h5,10-12,14-15H,4,6-9H2,1-3H3,(H2,20,23,27)(H,21,22,24)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072731
(CHEMBL3410049)Show SMILES COc1ccc(cc1)N(CCN)C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12 Show InChI InChI=1S/C23H24N6O2/c1-15-13-25-22-20(15)21(26-14-27-22)16-3-5-17(6-4-16)28-23(30)29(12-11-24)18-7-9-19(31-2)10-8-18/h3-10,13-14H,11-12,24H2,1-2H3,(H,28,30)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50089237
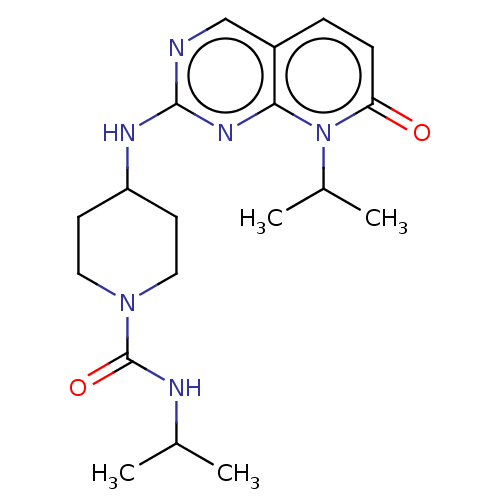
(CHEMBL3577874)Show SMILES CC(C)NC(=O)N1CCC(CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 Show InChI InChI=1S/C19H28N6O2/c1-12(2)21-19(27)24-9-7-15(8-10-24)22-18-20-11-14-5-6-16(26)25(13(3)4)17(14)23-18/h5-6,11-13,15H,7-10H2,1-4H3,(H,21,27)(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha-1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50089235
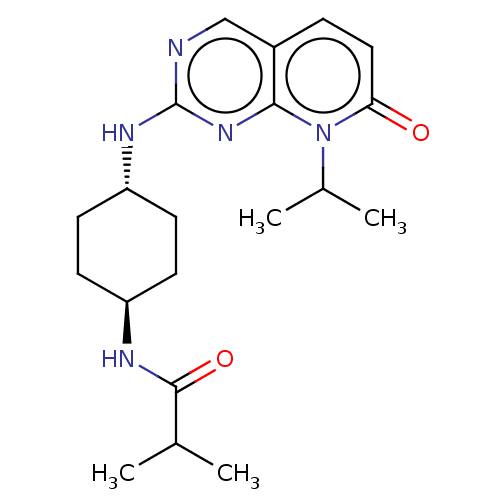
(CHEMBL3577866)Show SMILES CC(C)C(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 |r,wU:9.12,wD:6.5,(-6.41,-10.66,;-5.34,-10.04,;-4.27,-10.65,;-5.34,-8.49,;-6.41,-7.88,;-4,-7.72,;-4.01,-6.17,;-2.67,-5.4,;-2.68,-3.86,;-4.02,-3.09,;-5.35,-3.86,;-5.35,-5.41,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;1.34,-3.08,;2.41,-3.7,;.27,-3.7,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C20H29N5O2/c1-12(2)19(27)22-15-6-8-16(9-7-15)23-20-21-11-14-5-10-17(26)25(13(3)4)18(14)24-20/h5,10-13,15-16H,6-9H2,1-4H3,(H,22,27)(H,21,23,24)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50032826
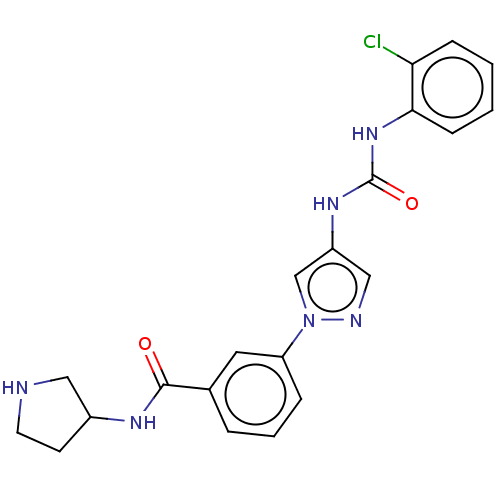
(CHEMBL3355184)Show SMILES Clc1ccccc1NC(=O)Nc1cnn(c1)-c1cccc(c1)C(=O)NC1CCNC1 Show InChI InChI=1S/C21H21ClN6O2/c22-18-6-1-2-7-19(18)27-21(30)26-16-12-24-28(13-16)17-5-3-4-14(10-17)20(29)25-15-8-9-23-11-15/h1-7,10,12-13,15,23H,8-9,11H2,(H,25,29)(H2,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha1 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050946
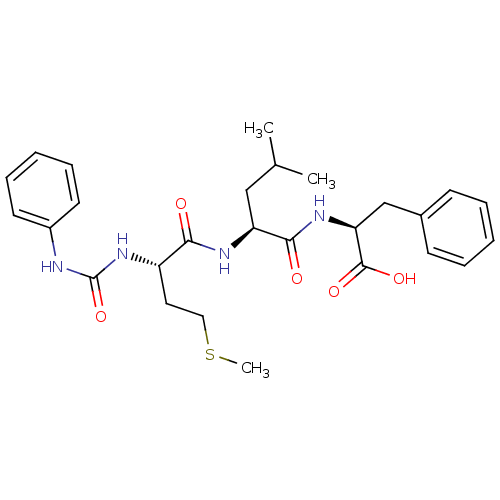
((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...)Show SMILES CSCC[C@H](NC(=O)Nc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C27H36N4O5S/c1-18(2)16-22(25(33)30-23(26(34)35)17-19-10-6-4-7-11-19)29-24(32)21(14-15-37-3)31-27(36)28-20-12-8-5-9-13-20/h4-13,18,21-23H,14-17H2,1-3H3,(H,29,32)(H,30,33)(H,34,35)(H2,28,31,36)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50089235

(CHEMBL3577866)Show SMILES CC(C)C(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 |r,wU:9.12,wD:6.5,(-6.41,-10.66,;-5.34,-10.04,;-4.27,-10.65,;-5.34,-8.49,;-6.41,-7.88,;-4,-7.72,;-4.01,-6.17,;-2.67,-5.4,;-2.68,-3.86,;-4.02,-3.09,;-5.35,-3.86,;-5.35,-5.41,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;1.34,-3.08,;2.41,-3.7,;.27,-3.7,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C20H29N5O2/c1-12(2)19(27)22-15-6-8-16(9-7-15)23-20-21-11-14-5-10-17(26)25(13(3)4)18(14)24-20/h5,10-13,15-16H,6-9H2,1-4H3,(H,22,27)(H,21,23,24)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha-1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50089230

(CHEMBL3577871)Show SMILES CCNC(=O)N[C@H]1CC[C@H](CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 |r,wU:9.12,6.5,(-6.67,-12.05,;-6.67,-10.81,;-5.34,-10.04,;-5.34,-8.49,;-6.41,-7.88,;-4,-7.72,;-4.01,-6.17,;-5.35,-5.41,;-5.35,-3.86,;-4.02,-3.09,;-2.68,-3.86,;-2.67,-5.4,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;1.34,-3.08,;2.41,-3.7,;.27,-3.7,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C19H28N6O2/c1-4-20-19(27)23-15-8-6-14(7-9-15)22-18-21-11-13-5-10-16(26)25(12(2)3)17(13)24-18/h5,10-12,14-15H,4,6-9H2,1-3H3,(H2,20,23,27)(H,21,22,24)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50032825

(CHEMBL3355183)Show SMILES Clc1ccccc1NC(=O)Nc1cnn(c1)-c1cccc(c1)C(=O)NC1CNC1 Show InChI InChI=1S/C20H19ClN6O2/c21-17-6-1-2-7-18(17)26-20(29)25-15-11-23-27(12-15)16-5-3-4-13(8-16)19(28)24-14-9-22-10-14/h1-8,11-12,14,22H,9-10H2,(H,24,28)(H2,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha1 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50032818
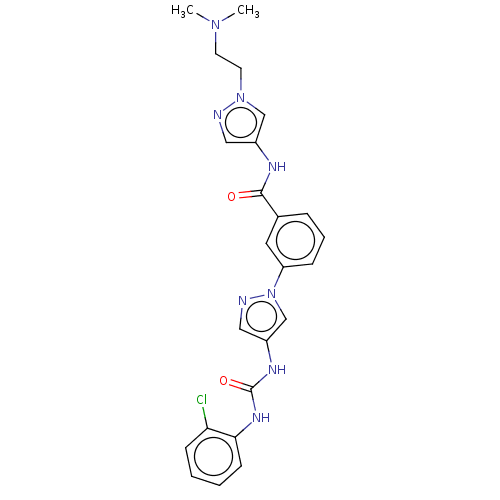
(CHEMBL3352845)Show SMILES CN(C)CCn1cc(NC(=O)c2cccc(c2)-n2cc(NC(=O)Nc3ccccc3Cl)cn2)cn1 Show InChI InChI=1S/C24H25ClN8O2/c1-31(2)10-11-32-15-18(13-26-32)28-23(34)17-6-5-7-20(12-17)33-16-19(14-27-33)29-24(35)30-22-9-4-3-8-21(22)25/h3-9,12-16H,10-11H2,1-2H3,(H,28,34)(H2,29,30,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha1 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072723
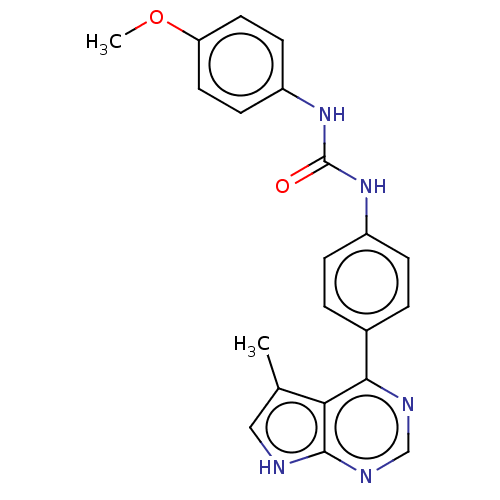
(CHEMBL3410032)Show SMILES COc1ccc(NC(=O)Nc2ccc(cc2)-c2ncnc3[nH]cc(C)c23)cc1 Show InChI InChI=1S/C21H19N5O2/c1-13-11-22-20-18(13)19(23-12-24-20)14-3-5-15(6-4-14)25-21(27)26-16-7-9-17(28-2)10-8-16/h3-12H,1-2H3,(H,22,23,24)(H2,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50089237

(CHEMBL3577874)Show SMILES CC(C)NC(=O)N1CCC(CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 Show InChI InChI=1S/C19H28N6O2/c1-12(2)21-19(27)24-9-7-15(8-10-24)22-18-20-11-14-5-6-16(26)25(13(3)4)17(14)23-18/h5-6,11-13,15H,7-10H2,1-4H3,(H,21,27)(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50089230

(CHEMBL3577871)Show SMILES CCNC(=O)N[C@H]1CC[C@H](CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 |r,wU:9.12,6.5,(-6.67,-12.05,;-6.67,-10.81,;-5.34,-10.04,;-5.34,-8.49,;-6.41,-7.88,;-4,-7.72,;-4.01,-6.17,;-5.35,-5.41,;-5.35,-3.86,;-4.02,-3.09,;-2.68,-3.86,;-2.67,-5.4,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;1.34,-3.08,;2.41,-3.7,;.27,-3.7,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C19H28N6O2/c1-4-20-19(27)23-15-8-6-14(7-9-15)22-18-21-11-13-5-10-16(26)25(12(2)3)17(13)24-18/h5,10-12,14-15H,4,6-9H2,1-3H3,(H2,20,23,27)(H,21,22,24)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha-1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072668
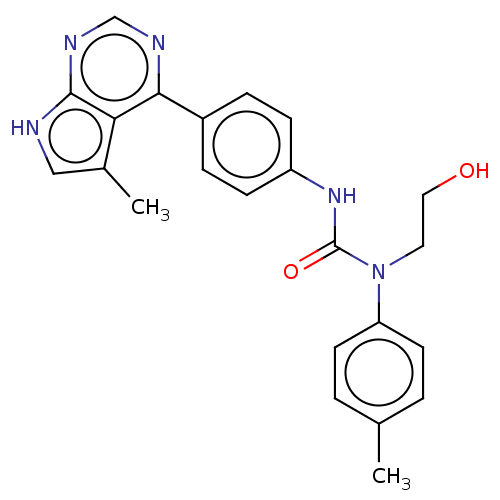
(CHEMBL3410045)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccc(C)cc4)cc3)c12 Show InChI InChI=1S/C23H23N5O2/c1-15-3-9-19(10-4-15)28(11-12-29)23(30)27-18-7-5-17(6-8-18)21-20-16(2)13-24-22(20)26-14-25-21/h3-10,13-14,29H,11-12H2,1-2H3,(H,27,30)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM103904
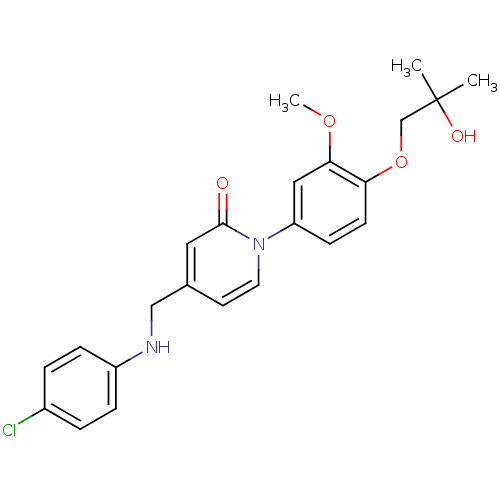
(JNK3 inhibitor 1 | US8563583, A-33)Show SMILES COc1cc(ccc1OCC(C)(C)O)-n1ccc(CNc2ccc(Cl)cc2)cc1=O Show InChI InChI=1S/C23H25ClN2O4/c1-23(2,28)15-30-20-9-8-19(13-21(20)29-3)26-11-10-16(12-22(26)27)14-25-18-6-4-17(24)5-7-18/h4-13,25,28H,14-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37.8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
| |
ACS Chem Biol 8: 1747-54 (2013)
Article DOI: 10.1021/cb3006165
BindingDB Entry DOI: 10.7270/Q25719PN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50032816
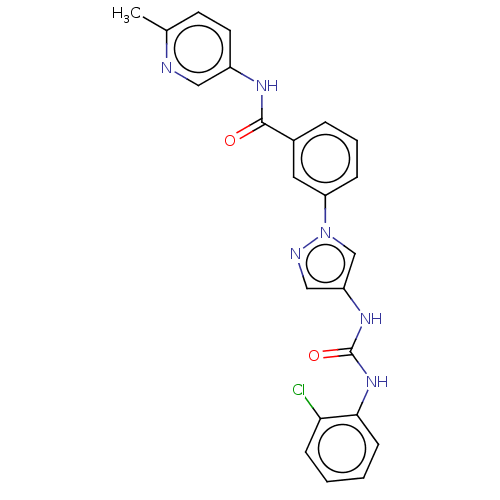
(CHEMBL3355146)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)-n2cc(NC(=O)Nc3ccccc3Cl)cn2)cn1 Show InChI InChI=1S/C23H19ClN6O2/c1-15-9-10-17(12-25-15)27-22(31)16-5-4-6-19(11-16)30-14-18(13-26-30)28-23(32)29-21-8-3-2-7-20(21)24/h2-14H,1H3,(H,27,31)(H2,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK3alpha1 (unknown origin) after 1 hr by homogeneous time-resolved fluorescence assay |
J Med Chem 57: 10013-30 (2014)
Article DOI: 10.1021/jm501256y
BindingDB Entry DOI: 10.7270/Q25140TS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50089232

(CHEMBL3577869)Show SMILES CC(C)n1c2nc(N[C@H]3CC[C@@H](CC3)NC(=O)NC3CCCC3)ncc2ccc1=O |r,wU:8.7,wD:11.14,(2.4,-3.69,;1.33,-3.08,;.27,-3.7,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-4.01,-1.54,;-4.01,-3.08,;-2.67,-3.85,;-2.67,-5.39,;-4,-6.16,;-5.34,-5.4,;-5.34,-3.86,;-4,-7.7,;-5.33,-8.48,;-6.4,-7.86,;-5.33,-10.02,;-6.66,-10.79,;-6.8,-12.31,;-8.31,-12.63,;-9.08,-11.3,;-8.05,-10.15,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C22H32N6O2/c1-14(2)28-19(29)12-7-15-13-23-21(27-20(15)28)24-17-8-10-18(11-9-17)26-22(30)25-16-5-3-4-6-16/h7,12-14,16-18H,3-6,8-11H2,1-2H3,(H,23,24,27)(H2,25,26,30)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072664
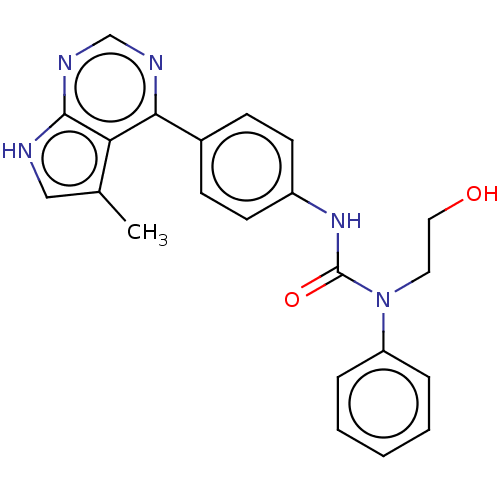
(CHEMBL3410036)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccccc4)cc3)c12 Show InChI InChI=1S/C22H21N5O2/c1-15-13-23-21-19(15)20(24-14-25-21)16-7-9-17(10-8-16)26-22(29)27(11-12-28)18-5-3-2-4-6-18/h2-10,13-14,28H,11-12H2,1H3,(H,26,29)(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50072675
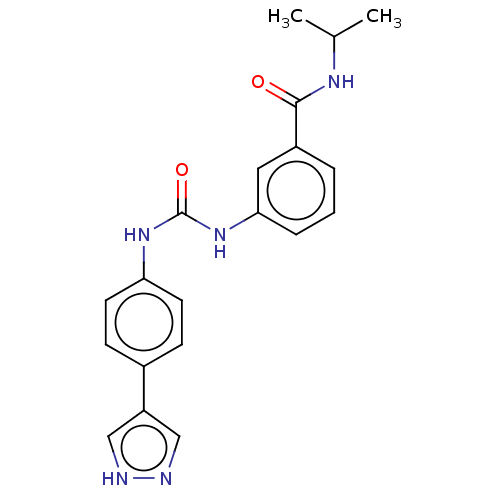
(CHEMBL3410022)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)Nc2ccc(cc2)-c2cn[nH]c2)c1 Show InChI InChI=1S/C20H21N5O2/c1-13(2)23-19(26)15-4-3-5-18(10-15)25-20(27)24-17-8-6-14(7-9-17)16-11-21-22-12-16/h3-13H,1-2H3,(H,21,22)(H,23,26)(H2,24,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK2 using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK peptide substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50072729
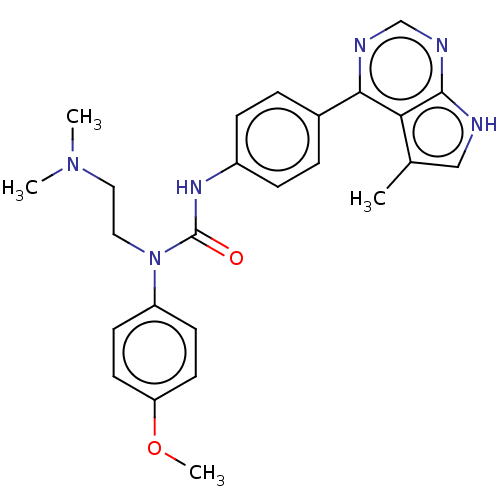
(CHEMBL3410051)Show SMILES COc1ccc(cc1)N(CCN(C)C)C(=O)Nc1ccc(cc1)-c1ncnc2[nH]cc(C)c12 Show InChI InChI=1S/C25H28N6O2/c1-17-15-26-24-22(17)23(27-16-28-24)18-5-7-19(8-6-18)29-25(32)31(14-13-30(2)3)20-9-11-21(33-4)12-10-20/h5-12,15-16H,13-14H2,1-4H3,(H,29,32)(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Limk1 using cofilin substrate, ATP and [gamma33P]ATP |
J Med Chem 58: 1846-61 (2015)
Article DOI: 10.1021/jm501680m
BindingDB Entry DOI: 10.7270/Q2K64KRC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50089235

(CHEMBL3577866)Show SMILES CC(C)C(=O)N[C@H]1CC[C@@H](CC1)Nc1ncc2ccc(=O)n(C(C)C)c2n1 |r,wU:9.12,wD:6.5,(-6.41,-10.66,;-5.34,-10.04,;-4.27,-10.65,;-5.34,-8.49,;-6.41,-7.88,;-4,-7.72,;-4.01,-6.17,;-2.67,-5.4,;-2.68,-3.86,;-4.02,-3.09,;-5.35,-3.86,;-5.35,-5.41,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;1.34,-3.08,;2.41,-3.7,;.27,-3.7,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C20H29N5O2/c1-12(2)19(27)22-15-6-8-16(9-7-15)23-20-21-11-14-5-10-17(26)25(13(3)4)18(14)24-20/h5,10-13,15-16H,6-9H2,1-4H3,(H,22,27)(H,21,23,24)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) using biotinylated FL-ATF-2 as substrate after 1 hr by homogeneous time-resolved fluorescence assay |
ACS Med Chem Lett 6: 413-8 (2015)
Article DOI: 10.1021/ml500474d
BindingDB Entry DOI: 10.7270/Q2KH0Q2N |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50050942

((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)Nc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C46H56N6O7/c1-30(2)25-36(49-44(56)39(28-33-19-11-6-12-20-33)52-46(59)47-35-23-15-8-16-24-35)41(53)50-38(27-32-17-9-5-10-18-32)43(55)48-37(26-31(3)4)42(54)51-40(45(57)58)29-34-21-13-7-14-22-34/h5-24,30-31,36-40H,25-29H2,1-4H3,(H,48,55)(H,49,56)(H,50,53)(H,51,54)(H,57,58)(H2,47,52,59)/t36-,37-,38+,39+,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards fMLF receptor using human neutrophils |
J Med Chem 39: 1013-5 (1996)
Article DOI: 10.1021/jm950908d
BindingDB Entry DOI: 10.7270/Q2TD9WF6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data