

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of specific [3H]propionyl-CCK-8 binding to rat pancreas membrane(CCK-A) | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The kinase activity was determined by incubation of enzyme and its substrate, and test compound, in the presence ATP/[gamma-33P] ATP. After incubatio... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of specific [3H]propionyl-CCK-8 binding to rat cerebral cortex membrane(CCK-B) | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50450461 (CHEMBL2367718) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat cerebral cortex membrane Cholecystokinin type B receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50291421 ((S)-3-{(S)-2-[(1S,2S)-2-Benzyloxycarbonylamino-1-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat cerebral cortex membrane Cholecystokinin type B receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of specific [3H]propionyl-CCK-8 binding to guinea pig pancreatic Cholecystokinin type B receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | 6.8 | 25 |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [V123M] (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | 6.8 | 25 |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I,Q181K,T183A] (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50291428 (2-{2-[(S)-2-((S)-Benzyloxycarbonylamino)-1-cyano-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat cerebral cortex membrane Cholecystokinin type B receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [T183A] (Bos taurus (bovine)) | BDBM14030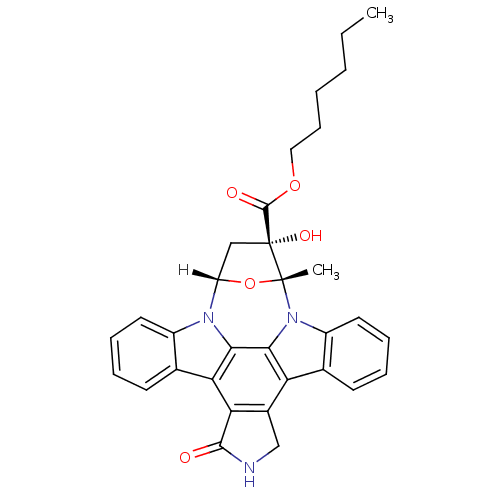 (KT5720 | hexyl (15R,16S,18S)-16-hydroxy-15-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [T183A] (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 2 (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [V123M,Q181K,T183A] (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [E127D] (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I,V123M,E127D,Q181K,T183A] (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [Q181K,T183A] (Bos taurus (bovine)) | BDBM14030 (KT5720 | hexyl (15R,16S,18S)-16-hydroxy-15-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [Q181K,T183A] (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <45 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I] (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 6.8 | 25 |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I,V123M,Q181K,T183A] (Bos taurus (bovine)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM14030 (KT5720 | hexyl (15R,16S,18S)-16-hydroxy-15-methyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | 6.8 | 25 |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I,Q181K,T183A] (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50011197 (3-{2-[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the inhibition of specific [3H]propionyl-CCK-8 binding to guinea pig pancreatic Cholecystokinin type B receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [V123M,Q181K,T183A] (Bos taurus (bovine)) | BDBM14030 (KT5720 | hexyl (15R,16S,18S)-16-hydroxy-15-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I,V123M,Q181K,T183A] (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [Q181K,T183A] (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [T183A] (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I,V123M,E127D,Q181K,T183A] (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50291423 ((S)-3-{(1R,2S)-2-[(S)-2-tert-Butoxycarbonylamino-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat cerebral cortex membrane Cholecystokinin type B receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50291425 (2-{2-[(S)-2-((S)-Benzyloxycarbonylamino)-1-cyano-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat pancreas membrane Cholecystokinin type A receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [V123M] (Bos taurus (bovine)) | BDBM14030 (KT5720 | hexyl (15R,16S,18S)-16-hydroxy-15-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 222 | n/a | n/a | n/a | n/a | 6.8 | 25 |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [V123M,Q181K,T183A] (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50291426 ((R)-2-((R)-2-Benzyloxycarbonylamino-1-cyano-3-(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 272 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat pancreas membrane Cholecystokinin type A receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [V123M,T183A] (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50291429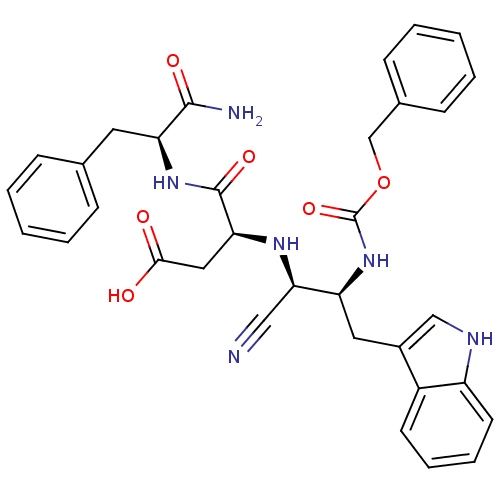 ((S)-3-[(1S,2S)-2-Benzyloxycarbonylamino-1-cyano-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 344 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat pancreas membrane Cholecystokinin type A receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 394 | n/a | n/a | n/a | n/a | 6.8 | 25 |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I] (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 426 | n/a | n/a | n/a | n/a | 6.8 | 25 |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50291428 (2-{2-[(S)-2-((S)-Benzyloxycarbonylamino)-1-cyano-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 483 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat pancreas membrane Cholecystokinin type A receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Bos taurus (bovine)) | BDBM14027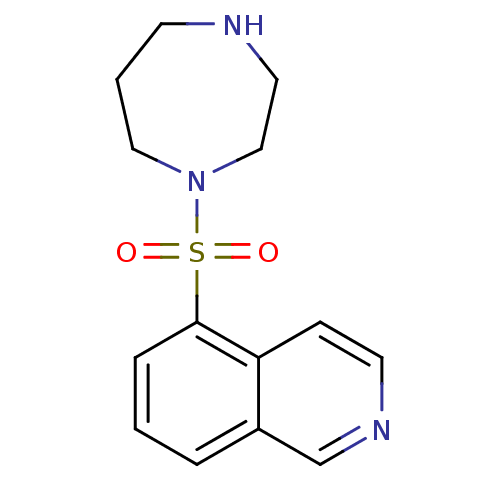 (5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [E127D] (Bos taurus (bovine)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 493 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 2 (Bos taurus (bovine)) | BDBM14029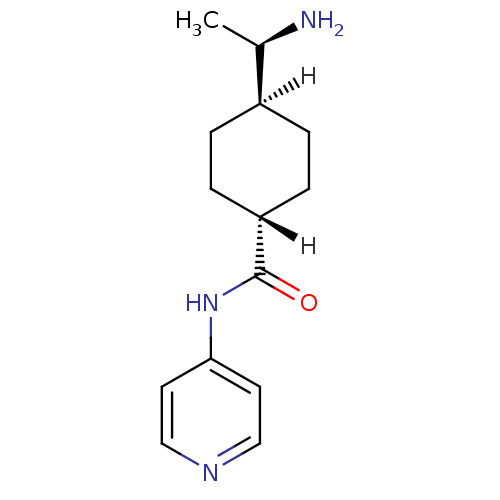 ((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [Q181K,T183A] (Bos taurus (bovine)) | BDBM14027 (5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 541 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [T183A] (Bos taurus (bovine)) | BDBM14027 (5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 607 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50291427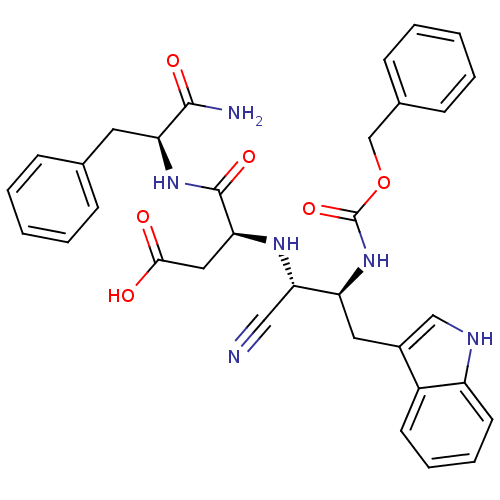 ((S)-3-[(1R,2S)-2-Benzyloxycarbonylamino-1-cyano-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 719 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat pancreas membrane Cholecystokinin type A receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I,Q181K,T183A] (Bos taurus (bovine)) | BDBM14027 (5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I] (Bos taurus (bovine)) | BDBM14027 (5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 893 | n/a | n/a | n/a | n/a | 6.8 | 25 |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50291431 ((S)-3-{(1S,2S)-2-[(S)-2-tert-Butoxycarbonylamino-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat cerebral cortex membrane Cholecystokinin type B receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50291425 (2-{2-[(S)-2-((S)-Benzyloxycarbonylamino)-1-cyano-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 938 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat cerebral cortex membrane Cholecystokinin type B receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50291424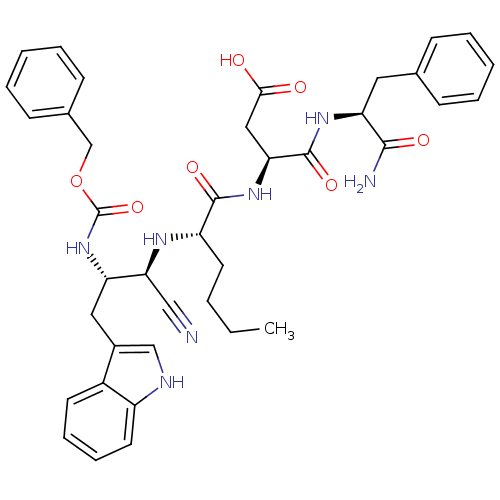 ((S)-3-{(S)-2-[(1R,2S)-2-Benzyloxycarbonylamino-1-c...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 953 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific [3H]propionyl-CCK-8 binding to rat cerebral cortex membrane Cholecystokinin type B receptor | Bioorg Med Chem Lett 7: 855-860 (1997) Article DOI: 10.1016/S0960-894X(97)00107-8 BindingDB Entry DOI: 10.7270/Q218370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha [L49I,V123M,Q181K,T183A] (Bos taurus (bovine)) | BDBM14027 (5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German Cancer Research Center | Assay Description The enzyme activity was assayed by using an ATP regenerative NADH consuming system. The reaction was started with adding ATP to the mixture containin... | J Biol Chem 281: 24818-30 (2006) Article DOI: 10.1074/jbc.M512374200 BindingDB Entry DOI: 10.7270/Q2PG1PZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 91 total ) | Next | Last >> |