 Found 155 hits with Last Name = 'hibi' and Initial = 's'
Found 155 hits with Last Name = 'hibi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50116105

(3-(6-(dimethylamino)-4-methylpyridin-3-yl)-2,5-dim...)Show SMILES CCCN(CCC)c1cc(C)nc2c(c(C)nn12)-c1cnc(cc1C)N(C)C |(-1.91,-13.39,;-3.24,-14.16,;-4.58,-13.4,;-5.91,-14.18,;-7.24,-13.41,;-8.57,-14.19,;-9.91,-13.42,;-5.9,-15.72,;-7.22,-16.49,;-7.23,-18.03,;-8.56,-18.8,;-5.9,-18.8,;-4.55,-18.03,;-3.07,-18.5,;-2.16,-17.24,;-.62,-17.23,;-3.09,-15.99,;-4.56,-16.48,;-2.58,-19.96,;-3.61,-21.11,;-3.13,-22.57,;-1.62,-22.88,;-.59,-21.72,;-1.08,-20.27,;-.06,-19.11,;-1.13,-24.34,;.38,-24.65,;-2.15,-25.5,)| Show InChI InChI=1S/C22H32N6/c1-8-10-27(11-9-2)20-13-16(4)24-22-21(17(5)25-28(20)22)18-14-23-19(26(6)7)12-15(18)3/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF-stimulated cAMP accumulation |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420920
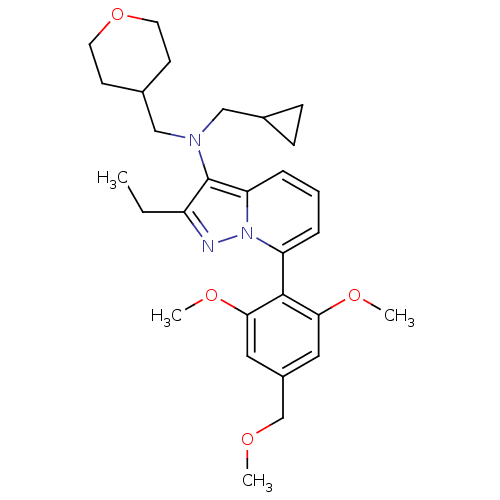
(CHEMBL2087549 | CHEMBL2087567)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(OC)cc(COC)cc1OC |(-4.48,2.67,;-3.74,1.32,;-2.2,1.29,;-1.32,.02,;.16,.47,;1.48,-.33,;2.82,.42,;2.86,1.96,;1.54,2.75,;.19,2.01,;-1.27,2.51,;-1.71,3.99,;-.66,5.11,;.86,4.84,;2.3,5.36,;2.03,3.85,;-3.21,4.34,;-3.58,5.83,;-5.06,6.26,;-5.43,7.76,;-4.32,8.82,;-2.84,8.39,;-2.47,6.9,;1.44,-1.87,;2.76,-2.66,;4.11,-1.92,;5.43,-2.72,;2.73,-4.2,;1.38,-4.95,;1.35,-6.49,;2.67,-7.28,;2.64,-8.82,;.07,-4.15,;.1,-2.61,;-1.22,-1.81,;-2.57,-2.56,)| Show InChI InChI=1S/C29H39N3O4/c1-5-23-29(31(17-20-9-10-20)18-21-11-13-36-14-12-21)25-8-6-7-24(32(25)30-23)28-26(34-3)15-22(19-33-2)16-27(28)35-4/h6-8,15-16,20-21H,5,9-14,17-19H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF-stimulated cAMP accumulation |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(Homo sapiens (Human)) | BDBM50420920

(CHEMBL2087549 | CHEMBL2087567)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(OC)cc(COC)cc1OC |(-4.48,2.67,;-3.74,1.32,;-2.2,1.29,;-1.32,.02,;.16,.47,;1.48,-.33,;2.82,.42,;2.86,1.96,;1.54,2.75,;.19,2.01,;-1.27,2.51,;-1.71,3.99,;-.66,5.11,;.86,4.84,;2.3,5.36,;2.03,3.85,;-3.21,4.34,;-3.58,5.83,;-5.06,6.26,;-5.43,7.76,;-4.32,8.82,;-2.84,8.39,;-2.47,6.9,;1.44,-1.87,;2.76,-2.66,;4.11,-1.92,;5.43,-2.72,;2.73,-4.2,;1.38,-4.95,;1.35,-6.49,;2.67,-7.28,;2.64,-8.82,;.07,-4.15,;.1,-2.61,;-1.22,-1.81,;-2.57,-2.56,)| Show InChI InChI=1S/C29H39N3O4/c1-5-23-29(31(17-20-9-10-20)18-21-11-13-36-14-12-21)25-8-6-7-24(32(25)30-23)28-26(34-3)15-22(19-33-2)16-27(28)35-4/h6-8,15-16,20-21H,5,9-14,17-19H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF2 receptor expressed in IMR32 cells assessed as inhibition of CRF-stimulated cAMP accumulation |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084833

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H27NO2/c1-24(2)13-14-25(3,4)20-15-18(9-10-19(20)24)22-12-11-21(26-22)16-5-7-17(8-6-16)23(27)28/h5-12,15,26H,13-14H2,1-4H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor gamma |
Bioorg Med Chem Lett 10: 619-22 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GFV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against Retinoic acid receptor gamma |
Bioorg Med Chem Lett 10: 623-5 (2000)
BindingDB Entry DOI: 10.7270/Q2028QSB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor alpha |
Bioorg Med Chem Lett 10: 619-22 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GFV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic acid receptor alpha |
Bioorg Med Chem Lett 10: 623-5 (2000)
BindingDB Entry DOI: 10.7270/Q2028QSB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor beta |
Bioorg Med Chem Lett 10: 619-22 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GFV |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic acid receptor beta |
Bioorg Med Chem Lett 10: 623-5 (2000)
BindingDB Entry DOI: 10.7270/Q2028QSB |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Rattus norvegicus) | BDBM50037039

(2-Amino-5,7-dimethyl-4-pyridin-3-ylmethyl-benzothi...)Show InChI InChI=1S/C15H15N3OS/c1-8-11(6-10-4-3-5-17-7-10)12-14(9(2)13(8)19)20-15(16)18-12/h3-5,7,19H,6H2,1-2H3,(H2,16,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against the production of thromboxane B2 (TXB2) in glycogen-induced peritoneal cells of rat |
J Med Chem 37: 3062-70 (1994)
BindingDB Entry DOI: 10.7270/Q2WM1F2J |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084832
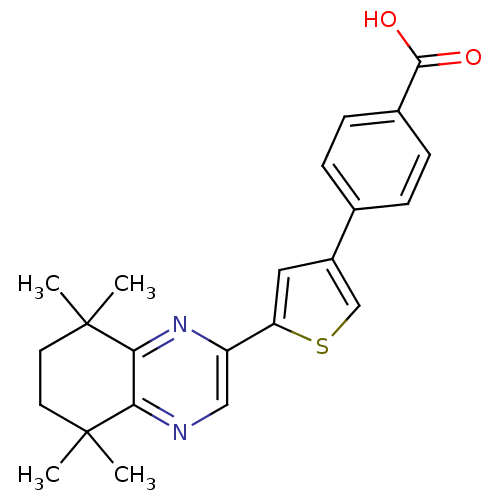
(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(cs1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor gamma (RAR gamma) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084835

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25N3O2/c1-22(2)11-12-23(3,4)20-19(22)24-13-18(26-20)17-10-9-16(25-17)14-5-7-15(8-6-14)21(27)28/h5-10,13,25H,11-12H2,1-4H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084832

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(cs1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50084834
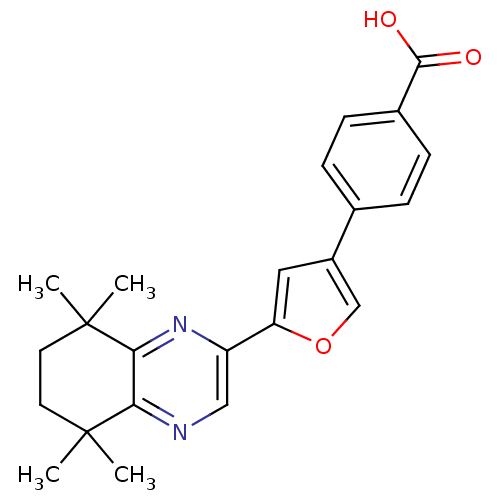
(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(co1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor beta (RAR beta) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084830
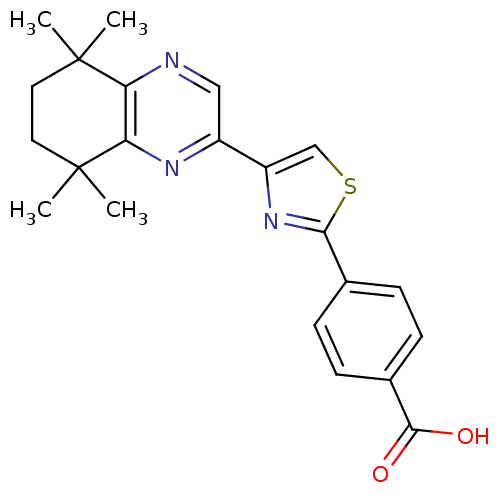
(4-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1csc(n1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H23N3O2S/c1-21(2)9-10-22(3,4)18-17(21)23-11-15(24-18)16-12-28-19(25-16)13-5-7-14(8-6-13)20(26)27/h5-8,11-12H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor gamma (RAR gamma) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50116105

(3-(6-(dimethylamino)-4-methylpyridin-3-yl)-2,5-dim...)Show SMILES CCCN(CCC)c1cc(C)nc2c(c(C)nn12)-c1cnc(cc1C)N(C)C |(-1.91,-13.39,;-3.24,-14.16,;-4.58,-13.4,;-5.91,-14.18,;-7.24,-13.41,;-8.57,-14.19,;-9.91,-13.42,;-5.9,-15.72,;-7.22,-16.49,;-7.23,-18.03,;-8.56,-18.8,;-5.9,-18.8,;-4.55,-18.03,;-3.07,-18.5,;-2.16,-17.24,;-.62,-17.23,;-3.09,-15.99,;-4.56,-16.48,;-2.58,-19.96,;-3.61,-21.11,;-3.13,-22.57,;-1.62,-22.88,;-.59,-21.72,;-1.08,-20.27,;-.06,-19.11,;-1.13,-24.34,;.38,-24.65,;-2.15,-25.5,)| Show InChI InChI=1S/C22H32N6/c1-8-10-27(11-9-2)20-13-16(4)24-22-21(17(5)25-28(20)22)18-14-23-19(26(6)7)12-15(18)3/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at CRF1 receptor in human IMR32 cells assessed as inhibition of CRF-induced intracellular cAMP accumulation after 30 mins by immu... |
J Med Chem 55: 8450-63 (2012)
Article DOI: 10.1021/jm300864p
BindingDB Entry DOI: 10.7270/Q2KD202P |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50084832

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(cs1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor beta (RAR beta) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084831

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc(o1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)11-12-23(3,4)20-19(22)24-13-16(25-20)18-10-9-17(28-18)14-5-7-15(8-6-14)21(26)27/h5-10,13H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor gamma (RAR gamma) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50398565
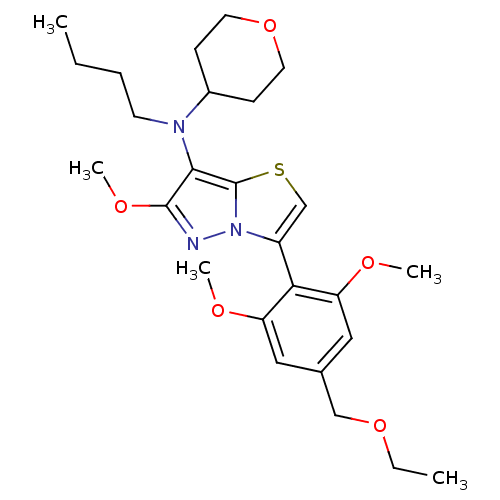
(CHEMBL2179195)Show SMILES CCCCN(C1CCOCC1)c1c(OC)nn2c(csc12)-c1c(OC)cc(COCC)cc1OC |(2.51,-41.6,;3.29,-42.93,;4.83,-42.92,;5.59,-41.58,;7.13,-41.58,;7.85,-40.22,;9.39,-40.17,;10.11,-38.82,;9.3,-37.51,;7.76,-37.56,;7.03,-38.92,;7.67,-43.03,;6.79,-44.29,;5.26,-44.32,;4.52,-45.67,;7.72,-45.51,;9.18,-45.01,;10.66,-45.45,;11.53,-44.19,;10.6,-42.96,;9.15,-43.47,;11.4,-46.8,;10.62,-48.12,;9.08,-48.09,;8.28,-49.41,;11.36,-49.46,;12.9,-49.49,;13.65,-50.84,;15.19,-50.87,;15.93,-52.21,;17.47,-52.24,;13.69,-48.17,;12.94,-46.82,;13.74,-45.5,;12.99,-44.16,)| Show InChI InChI=1S/C26H37N3O5S/c1-6-8-11-28(19-9-12-34-13-10-19)24-25(32-5)27-29-20(17-35-26(24)29)23-21(30-3)14-18(16-33-7-2)15-22(23)31-4/h14-15,17,19H,6-13,16H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at CRF1 receptor in human IMR32 cells assessed as inhibition of CRF-induced intracellular cAMP accumulation after 30 mins by immu... |
J Med Chem 55: 8450-63 (2012)
Article DOI: 10.1021/jm300864p
BindingDB Entry DOI: 10.7270/Q2KD202P |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50037037
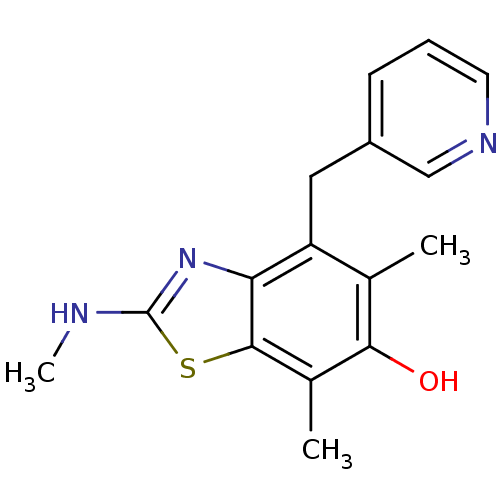
(5,7-Dimethyl-2-methylamino-4-pyridin-3-ylmethyl-be...)Show InChI InChI=1S/C16H17N3OS/c1-9-12(7-11-5-4-6-18-8-11)13-15(10(2)14(9)20)21-16(17-3)19-13/h4-6,8,20H,7H2,1-3H3,(H,17,19) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thromboxane A2 (TXA2) synthetase in microsome of human platelets |
J Med Chem 37: 3062-70 (1994)
BindingDB Entry DOI: 10.7270/Q2WM1F2J |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420904
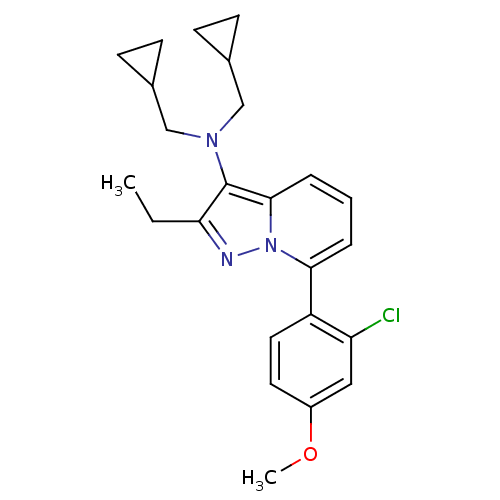
(CHEMBL2087550)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CC1)-c1ccc(OC)cc1Cl Show InChI InChI=1S/C24H28ClN3O/c1-3-21-24(27(14-16-7-8-16)15-17-9-10-17)23-6-4-5-22(28(23)26-21)19-12-11-18(29-2)13-20(19)25/h4-6,11-13,16-17H,3,7-10,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50037040
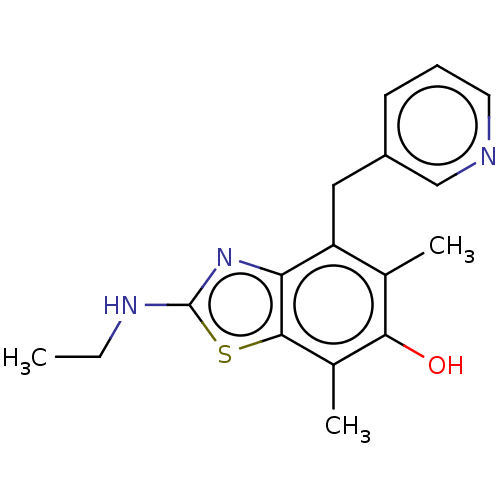
(2-Ethylamino-5,7-dimethyl-4-pyridin-3-ylmethyl-ben...)Show InChI InChI=1S/C17H19N3OS/c1-4-19-17-20-14-13(8-12-6-5-7-18-9-12)10(2)15(21)11(3)16(14)22-17/h5-7,9,21H,4,8H2,1-3H3,(H,19,20) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against thromboxane A2 (TXA2) synthetase in microsome of human platelets |
J Med Chem 37: 3062-70 (1994)
BindingDB Entry DOI: 10.7270/Q2WM1F2J |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420906

(CHEMBL2087552)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CC1)-c1ccc(C)cc1OC Show InChI InChI=1S/C25H31N3O/c1-4-21-25(27(15-18-9-10-18)16-19-11-12-19)23-7-5-6-22(28(23)26-21)20-13-8-17(2)14-24(20)29-3/h5-8,13-14,18-19H,4,9-12,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420908
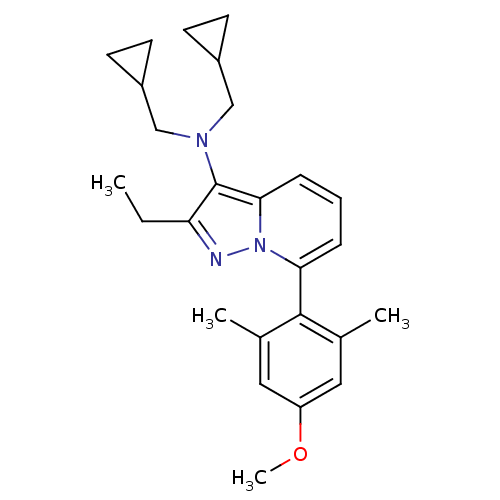
(CHEMBL2087554)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CC1)-c1c(C)cc(OC)cc1C |(-4.21,2.14,;-3.44,.81,;-1.9,.81,;-.99,-.43,;.47,.05,;1.81,-.72,;3.14,.05,;3.13,1.59,;1.8,2.36,;.47,1.59,;-1,2.06,;-1.48,3.53,;-2.98,3.84,;-3.38,5.33,;-2.99,6.82,;-4.48,6.42,;-.45,4.67,;-.85,6.16,;-.45,7.65,;-1.94,7.25,;1.81,-2.26,;3.14,-3.03,;4.48,-2.25,;3.15,-4.57,;1.81,-5.34,;1.82,-6.88,;3.15,-7.65,;.48,-4.57,;.48,-3.03,;-.86,-2.26,)| Show InChI InChI=1S/C26H33N3O/c1-5-22-26(28(15-19-9-10-19)16-20-11-12-20)24-8-6-7-23(29(24)27-22)25-17(2)13-21(30-4)14-18(25)3/h6-8,13-14,19-20H,5,9-12,15-16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420909
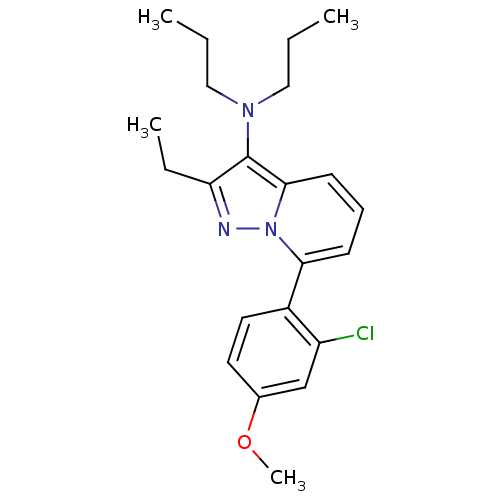
(CHEMBL2087555)Show SMILES CCCN(CCC)c1c(CC)nn2c(cccc12)-c1ccc(OC)cc1Cl Show InChI InChI=1S/C22H28ClN3O/c1-5-13-25(14-6-2)22-19(7-3)24-26-20(9-8-10-21(22)26)17-12-11-16(27-4)15-18(17)23/h8-12,15H,5-7,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
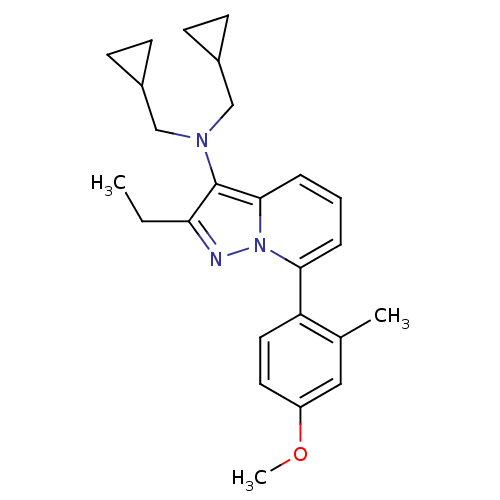
(Homo sapiens (Human)) | BDBM50420905
(CHEMBL2087551)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CC1)-c1ccc(OC)cc1C Show InChI InChI=1S/C25H31N3O/c1-4-22-25(27(15-18-8-9-18)16-19-10-11-19)24-7-5-6-23(28(24)26-22)21-13-12-20(29-3)14-17(21)2/h5-7,12-14,18-19H,4,8-11,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084829
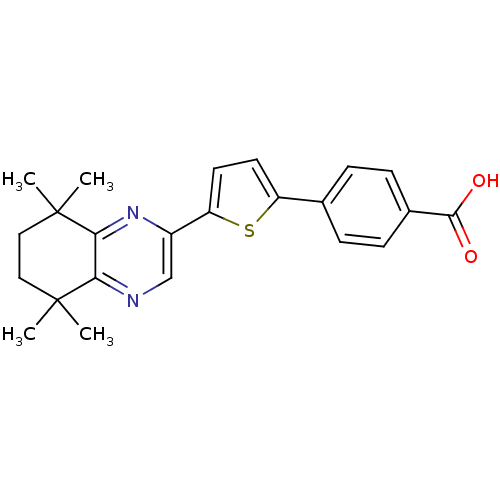
(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc(s1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)11-12-23(3,4)20-19(22)24-13-16(25-20)18-10-9-17(28-18)14-5-7-15(8-6-14)21(26)27/h5-10,13H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor gamma (RAR gamma) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084834

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(co1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420916
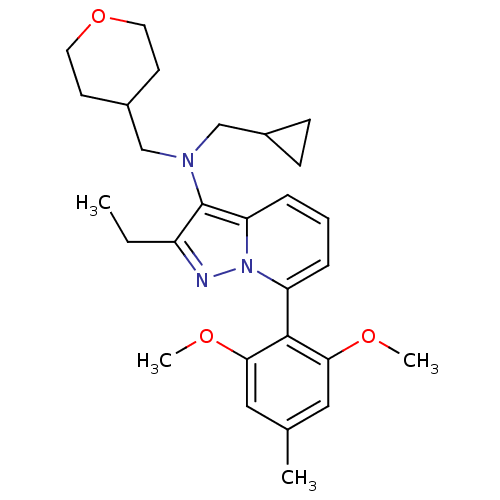
(CHEMBL2087562)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(OC)cc(C)cc1OC |(-4.48,1.41,;-3.7,.09,;-2.16,.1,;-1.25,-1.14,;.21,-.65,;1.55,-1.41,;2.88,-.63,;2.87,.91,;1.53,1.67,;.2,.89,;-1.27,1.35,;-1.75,2.81,;-.73,3.96,;.79,3.73,;2.22,4.3,;2,2.77,;-3.26,3.12,;-3.67,4.6,;-5.16,4.99,;-5.57,6.48,;-4.49,7.57,;-3,7.19,;-2.59,5.7,;1.57,-2.95,;2.91,-3.71,;4.23,-2.93,;5.57,-3.69,;2.92,-5.25,;1.59,-6.03,;1.6,-7.57,;.25,-5.27,;.24,-3.73,;-1.1,-2.97,;-2.43,-3.75,)| Show InChI InChI=1S/C28H37N3O3/c1-5-22-28(30(17-20-9-10-20)18-21-11-13-34-14-12-21)24-8-6-7-23(31(24)29-22)27-25(32-3)15-19(2)16-26(27)33-4/h6-8,15-16,20-21H,5,9-14,17-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF1 receptor expressed in HEK293 cells assessed as inhibition of CRF-stimulated cAMP accumulation after 30 mins by fluo... |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420912
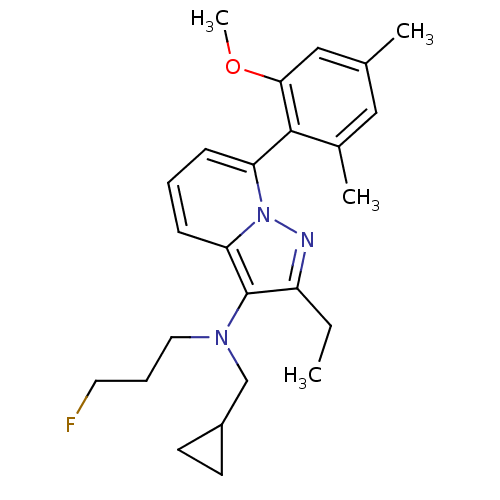
(CHEMBL2087558)Show SMILES CCc1nn2c(cccc2c1N(CCCF)CC1CC1)-c1c(C)cc(C)cc1OC |(-4.75,1.19,;-3.97,-.14,;-2.43,-.13,;-1.52,-1.37,;-.06,-.88,;1.28,-1.65,;2.61,-.87,;2.6,.67,;1.26,1.43,;-.07,.66,;-1.54,1.12,;-2.02,2.58,;-1,3.74,;-1.56,5.17,;-.59,6.37,;-1.16,7.81,;-3.53,2.9,;-4.09,4.33,;-5.29,5.29,;-3.86,5.85,;1.29,-3.19,;-.04,-3.96,;-1.38,-3.2,;-.03,-5.5,;1.3,-6.27,;1.31,-7.81,;2.63,-5.49,;2.62,-3.95,;3.95,-3.17,;5.29,-3.93,)| Show InChI InChI=1S/C25H32FN3O/c1-5-20-25(28(13-7-12-26)16-19-10-11-19)22-9-6-8-21(29(22)27-20)24-18(3)14-17(2)15-23(24)30-4/h6,8-9,14-15,19H,5,7,10-13,16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50084830

(4-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1csc(n1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H23N3O2S/c1-21(2)9-10-22(3,4)18-17(21)23-11-15(24-18)16-12-28-19(25-16)13-5-7-14(8-6-13)20(26)27/h5-8,11-12H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor beta (RAR beta) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084833

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H27NO2/c1-24(2)13-14-25(3,4)20-15-18(9-10-19(20)24)22-12-11-21(26-22)16-5-7-17(8-6-16)23(27)28/h5-12,15,26H,13-14H2,1-4H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic acid receptor gamma |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420915
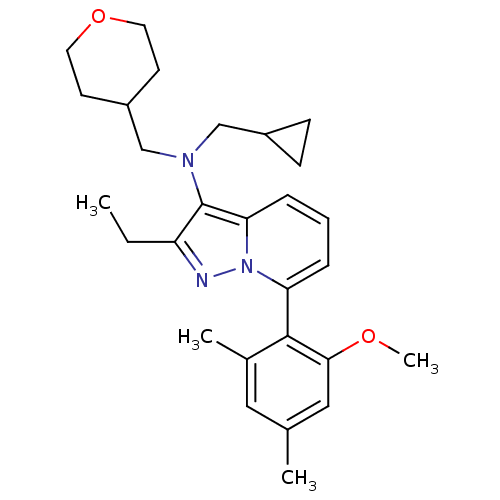
(CHEMBL2087561)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(C)cc(C)cc1OC |(-4.15,-1.62,;-3.38,-.29,;-2.03,-.24,;-1.12,-1.48,;.34,-1,;1.68,-1.76,;3.01,-.99,;3,.55,;1.66,1.32,;.33,.54,;-1.13,1.01,;-1.62,2.47,;-3.13,2.78,;-4.21,1.69,;-5.69,1.28,;-4.6,.2,;-.59,3.62,;-1.15,5.05,;-.19,6.26,;-.75,7.69,;-2.27,7.92,;-3.23,6.72,;-2.67,5.29,;1.69,-3.3,;.36,-4.08,;-.98,-3.32,;.37,-5.62,;1.71,-6.38,;1.71,-7.92,;3.03,-5.61,;3.03,-4.07,;4.36,-3.29,;5.69,-4.05,)| Show InChI InChI=1S/C28H37N3O2/c1-5-23-28(30(17-21-9-10-21)18-22-11-13-33-14-12-22)25-8-6-7-24(31(25)29-23)27-20(3)15-19(2)16-26(27)32-4/h6-8,15-16,21-22H,5,9-14,17-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420916

(CHEMBL2087562)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(OC)cc(C)cc1OC |(-4.48,1.41,;-3.7,.09,;-2.16,.1,;-1.25,-1.14,;.21,-.65,;1.55,-1.41,;2.88,-.63,;2.87,.91,;1.53,1.67,;.2,.89,;-1.27,1.35,;-1.75,2.81,;-.73,3.96,;.79,3.73,;2.22,4.3,;2,2.77,;-3.26,3.12,;-3.67,4.6,;-5.16,4.99,;-5.57,6.48,;-4.49,7.57,;-3,7.19,;-2.59,5.7,;1.57,-2.95,;2.91,-3.71,;4.23,-2.93,;5.57,-3.69,;2.92,-5.25,;1.59,-6.03,;1.6,-7.57,;.25,-5.27,;.24,-3.73,;-1.1,-2.97,;-2.43,-3.75,)| Show InChI InChI=1S/C28H37N3O3/c1-5-22-28(30(17-20-9-10-20)18-21-11-13-34-14-12-21)24-8-6-7-23(31(24)29-22)27-25(32-3)15-19(2)16-26(27)33-4/h6-8,15-16,20-21H,5,9-14,17-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420920

(CHEMBL2087549 | CHEMBL2087567)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(OC)cc(COC)cc1OC |(-4.48,2.67,;-3.74,1.32,;-2.2,1.29,;-1.32,.02,;.16,.47,;1.48,-.33,;2.82,.42,;2.86,1.96,;1.54,2.75,;.19,2.01,;-1.27,2.51,;-1.71,3.99,;-.66,5.11,;.86,4.84,;2.3,5.36,;2.03,3.85,;-3.21,4.34,;-3.58,5.83,;-5.06,6.26,;-5.43,7.76,;-4.32,8.82,;-2.84,8.39,;-2.47,6.9,;1.44,-1.87,;2.76,-2.66,;4.11,-1.92,;5.43,-2.72,;2.73,-4.2,;1.38,-4.95,;1.35,-6.49,;2.67,-7.28,;2.64,-8.82,;.07,-4.15,;.1,-2.61,;-1.22,-1.81,;-2.57,-2.56,)| Show InChI InChI=1S/C29H39N3O4/c1-5-23-29(31(17-20-9-10-20)18-21-11-13-36-14-12-21)25-8-6-7-24(32(25)30-23)28-26(34-3)15-22(19-33-2)16-27(28)35-4/h6-8,15-16,20-21H,5,9-14,17-19H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF1 receptor expressed in HEK293 cells assessed as inhibition of CRF-stimulated cAMP accumulation after 30 mins by fluo... |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420907
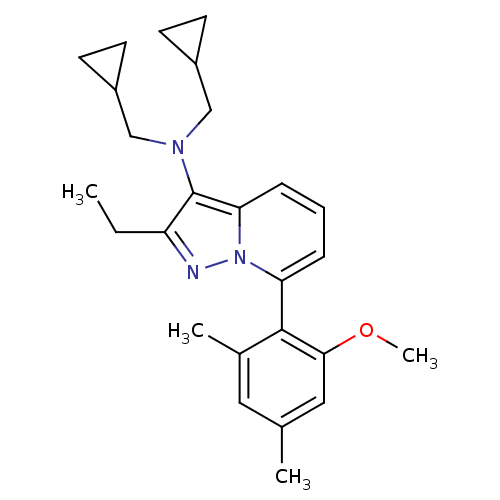
(CHEMBL2087553)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CC1)-c1c(C)cc(C)cc1OC |(-4.21,1.75,;-3.44,.42,;-1.9,.42,;-.99,-.82,;.48,-.34,;1.81,-1.1,;3.14,-.32,;3.14,1.22,;1.8,1.98,;.47,1.2,;-1,1.67,;-1.48,3.14,;-2.99,3.45,;-3.39,4.94,;-3,6.43,;-4.49,6.02,;-.45,4.29,;-.86,5.77,;-.47,7.26,;-1.95,6.86,;1.82,-2.64,;3.16,-3.4,;4.49,-2.63,;3.16,-4.94,;1.83,-5.72,;1.84,-7.26,;.5,-4.96,;.49,-3.42,;-.85,-2.65,;-2.18,-3.43,)| Show InChI InChI=1S/C26H33N3O/c1-5-21-26(28(15-19-9-10-19)16-20-11-12-20)23-8-6-7-22(29(23)27-21)25-18(3)13-17(2)14-24(25)30-4/h6-8,13-14,19-20H,5,9-12,15-16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420930
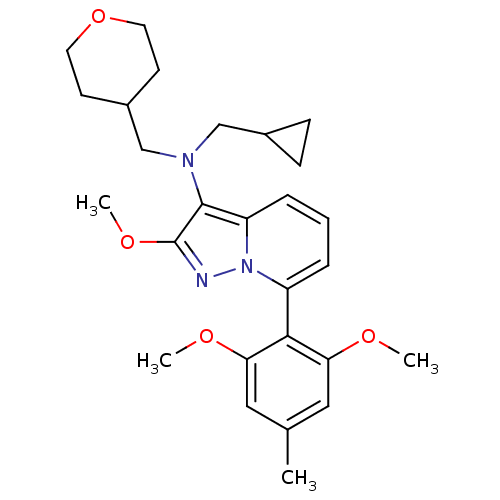
(CHEMBL2087795)Show SMILES COc1nn2c(cccc2c1N(CC1CC1)CC1CCOCC1)-c1c(OC)cc(C)cc1OC |(-4.48,1.44,;-3.71,.1,;-2.17,.1,;-1.27,-1.14,;.2,-.67,;1.53,-1.44,;2.87,-.67,;2.87,.87,;1.53,1.64,;.2,.87,;-1.27,1.35,;-1.74,2.81,;-.71,3.96,;.81,3.72,;2.25,4.27,;2.01,2.75,;-3.25,3.13,;-3.65,4.62,;-5.13,5.02,;-5.53,6.51,;-4.44,7.6,;-2.96,7.2,;-2.56,5.71,;1.53,-2.98,;2.86,-3.75,;4.2,-2.98,;5.53,-3.75,;2.86,-5.29,;1.53,-6.06,;1.53,-7.6,;.2,-5.29,;.2,-3.75,;-1.14,-2.98,;-2.47,-3.75,)| Show InChI InChI=1S/C27H35N3O4/c1-18-14-23(31-2)25(24(15-18)32-3)21-6-5-7-22-26(27(33-4)28-30(21)22)29(16-19-8-9-19)17-20-10-12-34-13-11-20/h5-7,14-15,19-20H,8-13,16-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50084834

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1cc(co1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)9-10-23(3,4)20-19(22)24-12-17(25-20)18-11-16(13-28-18)14-5-7-15(8-6-14)21(26)27/h5-8,11-13H,9-10H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor gamma (RAR gamma) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420935
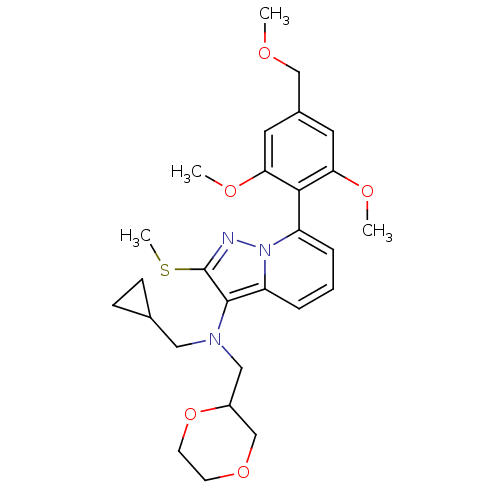
(CHEMBL2087799)Show SMILES COCc1cc(OC)c(c(OC)c1)-c1cccc2c(N(CC3CC3)CC3COCCO3)c(SC)nn12 |(2.79,-8.78,;2.8,-7.24,;1.47,-6.46,;1.48,-4.92,;2.82,-4.16,;2.83,-2.62,;4.17,-1.86,;5.5,-2.64,;1.5,-1.84,;.16,-2.6,;-1.17,-1.82,;-2.5,-2.58,;.15,-4.14,;1.51,-.3,;2.85,.46,;2.86,2,;1.53,2.78,;.19,2.02,;-1.27,2.51,;-1.73,3.98,;-.69,5.11,;.83,4.86,;2.27,5.4,;2.02,3.88,;-3.24,4.31,;-3.62,5.8,;-2.53,6.88,;-2.92,8.37,;-4.4,8.78,;-5.5,7.7,;-5.11,6.21,;-2.18,1.27,;-3.72,1.28,;-4.48,2.62,;-1.28,.02,;.18,.48,)| Show InChI InChI=1S/C27H35N3O5S/c1-31-16-19-12-23(32-2)25(24(13-19)33-3)21-6-5-7-22-26(27(36-4)28-30(21)22)29(14-18-8-9-18)15-20-17-34-10-11-35-20/h5-7,12-13,18,20H,8-11,14-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420927
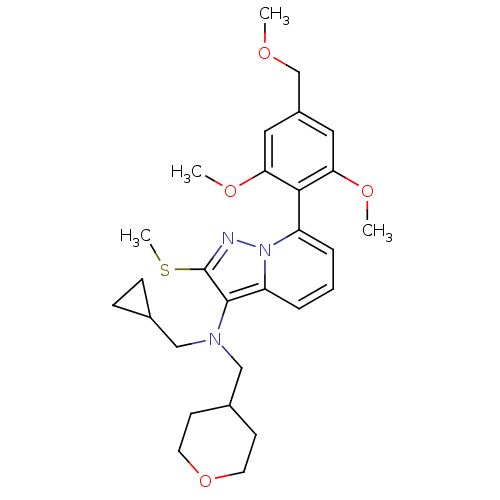
(CHEMBL2087574)Show SMILES COCc1cc(OC)c(c(OC)c1)-c1cccc2c(N(CC3CC3)CC3CCOCC3)c(SC)nn12 |(2.74,-8.79,;2.75,-7.25,;1.43,-6.47,;1.45,-4.93,;2.79,-4.17,;2.81,-2.63,;4.15,-1.88,;5.47,-2.66,;1.48,-1.85,;.14,-2.6,;-1.18,-1.82,;-2.53,-2.57,;.12,-4.14,;1.5,-.31,;2.84,.45,;2.86,1.99,;1.53,2.77,;.19,2.02,;-1.27,2.51,;-1.73,3.98,;-.68,5.11,;.84,4.85,;2.28,5.39,;2.02,3.87,;-3.23,4.32,;-3.61,5.81,;-5.09,6.22,;-5.47,7.72,;-4.37,8.79,;-2.89,8.38,;-2.51,6.89,;-2.19,1.27,;-3.73,1.29,;-4.48,2.63,;-1.29,.02,;.18,.48,)| Show InChI InChI=1S/C28H37N3O4S/c1-32-18-21-14-24(33-2)26(25(15-21)34-3)22-6-5-7-23-27(28(36-4)29-31(22)23)30(16-19-8-9-19)17-20-10-12-35-13-11-20/h5-7,14-15,19-20H,8-13,16-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF1 receptor expressed in HEK293 cells assessed as inhibition of CRF-stimulated cAMP accumulation after 30 mins by fluo... |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50037044
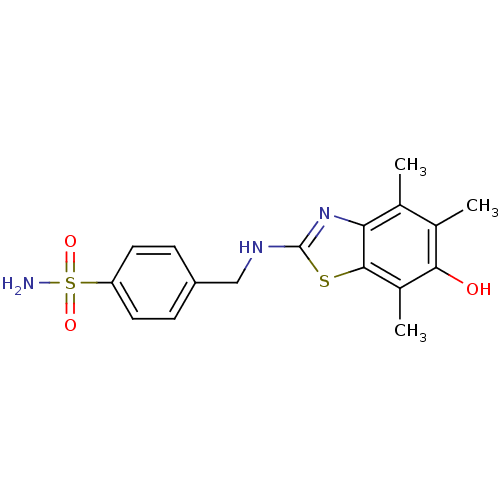
(4-[(6-Hydroxy-4,5,7-trimethyl-benzothiazol-2-ylami...)Show SMILES Cc1c(C)c2nc(NCc3ccc(cc3)S(N)(=O)=O)sc2c(C)c1O Show InChI InChI=1S/C17H19N3O3S2/c1-9-10(2)15(21)11(3)16-14(9)20-17(24-16)19-8-12-4-6-13(7-5-12)25(18,22)23/h4-7,21H,8H2,1-3H3,(H,19,20)(H2,18,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against leukotriene B4 (LTB4) receptor in human neutrophils |
J Med Chem 37: 3062-70 (1994)
BindingDB Entry DOI: 10.7270/Q2WM1F2J |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50099474
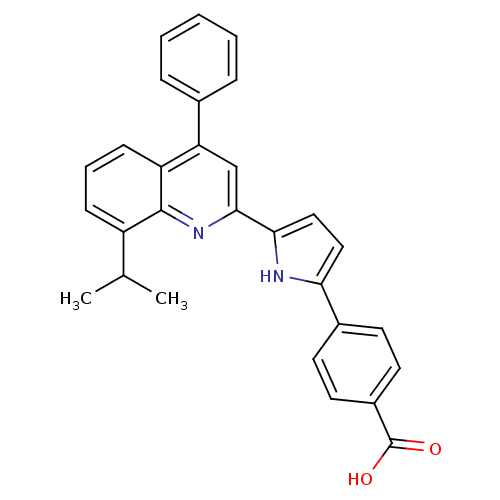
(4-[5-(8-Isopropyl-4-phenyl-quinolin-2-yl)-1H-pyrro...)Show SMILES CC(C)c1cccc2c(cc(nc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C29H24N2O2/c1-18(2)22-9-6-10-23-24(19-7-4-3-5-8-19)17-27(31-28(22)23)26-16-15-25(30-26)20-11-13-21(14-12-20)29(32)33/h3-18,30H,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound was evaluated in terms of inhibition of Retinoic acid receptor alpha transactivation by ATRA (50 nM) |
Bioorg Med Chem Lett 11: 1215-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M3643 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50084831

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc(o1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-22(2)11-12-23(3,4)20-19(22)24-13-16(25-20)18-10-9-17(28-18)14-5-7-15(8-6-14)21(26)27/h5-10,13H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420914
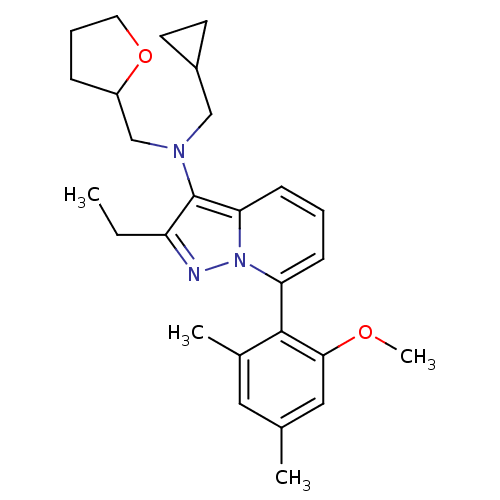
(CHEMBL2087560)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCCO1)-c1c(C)cc(C)cc1OC |(-4.19,-1.4,;-3.42,-.06,;-1.88,-.05,;-.97,-1.29,;.49,-.81,;1.83,-1.57,;3.16,-.8,;3.15,.74,;1.81,1.51,;.48,.73,;-.98,1.2,;-1.47,2.66,;-2.98,2.97,;-4.3,2.19,;-5.07,.86,;-5.84,2.18,;-.44,3.81,;-1,5.24,;-2.49,5.64,;-2.58,7.17,;-1.15,7.73,;-.17,6.54,;1.84,-3.11,;.51,-3.89,;-.83,-3.13,;.52,-5.43,;1.86,-6.19,;1.86,-7.73,;3.19,-5.42,;3.18,-3.88,;4.51,-3.1,;5.84,-3.86,)| Show InChI InChI=1S/C27H35N3O2/c1-5-22-27(29(16-20-11-12-20)17-21-8-7-13-32-21)24-10-6-9-23(30(24)28-22)26-19(3)14-18(2)15-25(26)31-4/h6,9-10,14-15,20-21H,5,7-8,11-13,16-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420937
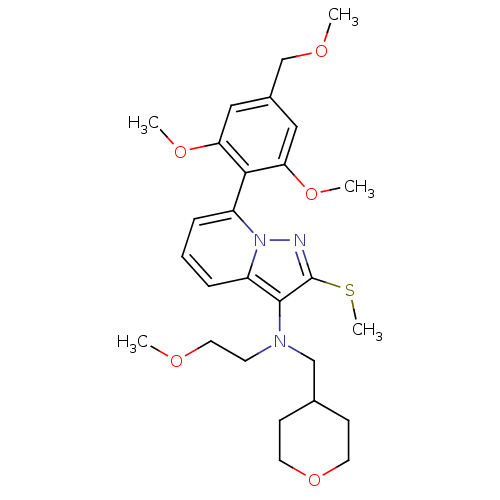
(CHEMBL2087801)Show SMILES COCCN(CC1CCOCC1)c1c(SC)nn2c(cccc12)-c1c(OC)cc(COC)cc1OC |(-3.55,8.8,;-3.16,7.31,;-4.26,6.23,;-3.88,4.74,;-2.37,4.4,;-1.02,5.88,;.5,5.63,;1.04,4.18,;2.56,3.93,;3.54,5.12,;3,6.56,;1.48,6.81,;-1.77,2.49,;-2.69,1.26,;-4.23,1.27,;-4.99,2.61,;-1.79,0,;-.32,.47,;1,-.32,;2.34,.44,;2.36,1.98,;1.03,2.76,;-.31,2.01,;.99,-1.86,;2.32,-2.64,;3.66,-1.88,;4.99,-2.66,;2.3,-4.18,;.96,-4.94,;.95,-6.48,;2.28,-7.26,;2.27,-8.8,;-.36,-4.15,;-.35,-2.61,;-1.68,-1.83,;-3.02,-2.59,)| Show InChI InChI=1S/C27H37N3O5S/c1-31-14-11-29(17-19-9-12-35-13-10-19)26-22-8-6-7-21(30(22)28-27(26)36-5)25-23(33-3)15-20(18-32-2)16-24(25)34-4/h6-8,15-16,19H,9-14,17-18H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420927

(CHEMBL2087574)Show SMILES COCc1cc(OC)c(c(OC)c1)-c1cccc2c(N(CC3CC3)CC3CCOCC3)c(SC)nn12 |(2.74,-8.79,;2.75,-7.25,;1.43,-6.47,;1.45,-4.93,;2.79,-4.17,;2.81,-2.63,;4.15,-1.88,;5.47,-2.66,;1.48,-1.85,;.14,-2.6,;-1.18,-1.82,;-2.53,-2.57,;.12,-4.14,;1.5,-.31,;2.84,.45,;2.86,1.99,;1.53,2.77,;.19,2.02,;-1.27,2.51,;-1.73,3.98,;-.68,5.11,;.84,4.85,;2.28,5.39,;2.02,3.87,;-3.23,4.32,;-3.61,5.81,;-5.09,6.22,;-5.47,7.72,;-4.37,8.79,;-2.89,8.38,;-2.51,6.89,;-2.19,1.27,;-3.73,1.29,;-4.48,2.63,;-1.29,.02,;.18,.48,)| Show InChI InChI=1S/C28H37N3O4S/c1-32-18-21-14-24(33-2)26(25(15-21)34-3)22-6-5-7-23-27(28(36-4)29-31(22)23)30(16-19-8-9-19)17-20-10-12-35-13-11-20/h5-7,14-15,19-20H,8-13,16-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50084829

(4-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-quino...)Show SMILES CC1(C)CCC(C)(C)c2nc(cnc12)-c1ccc(s1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N2O2S/c1-22(2)11-12-23(3,4)20-19(22)24-13-16(25-20)18-10-9-17(28-18)14-5-7-15(8-6-14)21(26)27/h5-10,13H,11-12H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor beta (RAR beta) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50420913
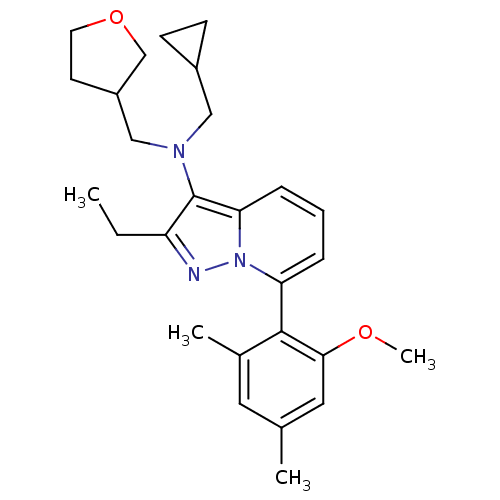
(CHEMBL2087559)Show SMILES CCc1nn2c(cccc2c1N(CC1CC1)CC1CCOC1)-c1c(C)cc(C)cc1OC |(-4.51,-1.39,;-3.74,-.05,;-2.2,-.05,;-1.3,-1.28,;.17,-.81,;1.5,-1.58,;2.83,-.81,;2.83,.73,;1.5,1.5,;.17,.73,;-1.3,1.21,;-1.78,2.67,;-3.5,2.95,;-4.3,4.26,;-5.81,4.72,;-4.41,5.79,;-.69,3.76,;-1.09,5.25,;-2.52,5.8,;-2.44,7.34,;-.96,7.74,;-.12,6.44,;1.51,-3.12,;.17,-3.89,;-1.16,-3.12,;.17,-5.43,;1.51,-6.2,;1.51,-7.74,;2.84,-5.42,;2.85,-3.89,;4.18,-3.12,;5.51,-3.89,)| Show InChI InChI=1S/C27H35N3O2/c1-5-22-27(29(15-20-9-10-20)16-21-11-12-32-17-21)24-8-6-7-23(30(24)28-22)26-19(3)13-18(2)14-25(26)31-4/h6-8,13-14,20-21H,5,9-12,15-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CRF from human CRF1 receptor expressed in HEK293 cells after 2 hrs by liquid scintillation counter |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
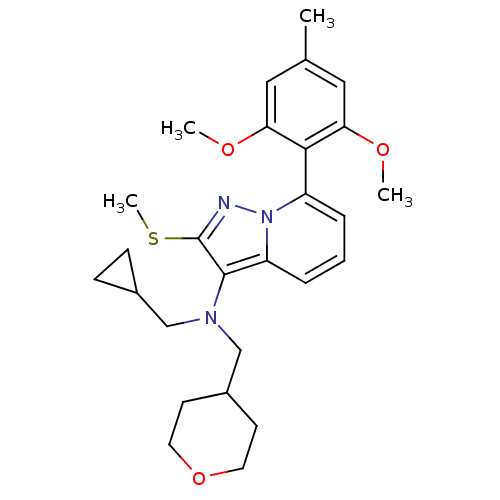
(Homo sapiens (Human)) | BDBM50420931
(CHEMBL2087796)Show SMILES COc1cc(C)cc(OC)c1-c1cccc2c(N(CC3CC3)CC3CCOCC3)c(SC)nn12 |(5.46,-3.84,;4.14,-3.05,;2.8,-3.8,;2.78,-5.34,;1.43,-6.1,;1.41,-7.64,;.11,-5.31,;.13,-3.77,;-1.19,-2.98,;-2.54,-3.73,;1.47,-3.02,;1.49,-1.48,;2.84,-.72,;2.86,.82,;1.54,1.6,;.19,.85,;-1.27,1.35,;-1.72,2.82,;-.68,3.95,;.84,3.69,;2.29,4.22,;2.02,2.7,;-3.23,3.16,;-3.6,4.65,;-5.09,5.07,;-5.46,6.56,;-4.36,7.64,;-2.88,7.22,;-2.5,5.72,;-2.19,.11,;-3.73,.13,;-4.48,1.48,;-1.3,-1.15,;.17,-.69,)| Show InChI InChI=1S/C27H35N3O3S/c1-18-14-23(31-2)25(24(15-18)32-3)21-6-5-7-22-26(27(34-4)28-30(21)22)29(16-19-8-9-19)17-20-10-12-33-13-11-20/h5-7,14-15,19-20H,8-13,16-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CRF1 receptor expressed in HEK293 cells assessed as inhibition of CRF-stimulated cAMP accumulation after 30 mins by fluo... |
J Med Chem 55: 5255-69 (2012)
Article DOI: 10.1021/jm300259r
BindingDB Entry DOI: 10.7270/Q29P32XC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data