 Found 123 hits with Last Name = 'higaki' and Initial = 'm'
Found 123 hits with Last Name = 'higaki' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167890
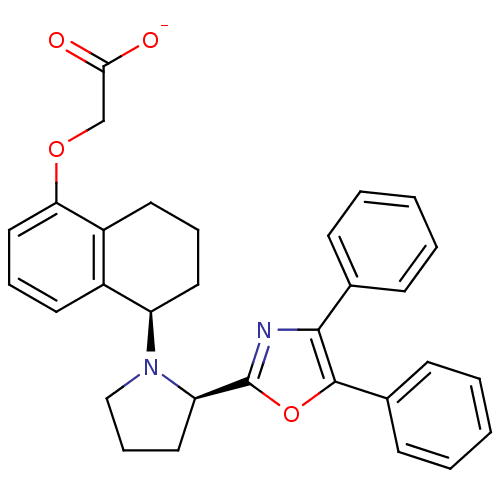
(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50370452
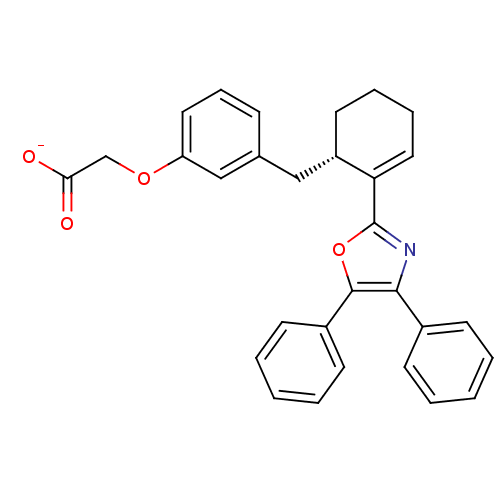
(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50167891
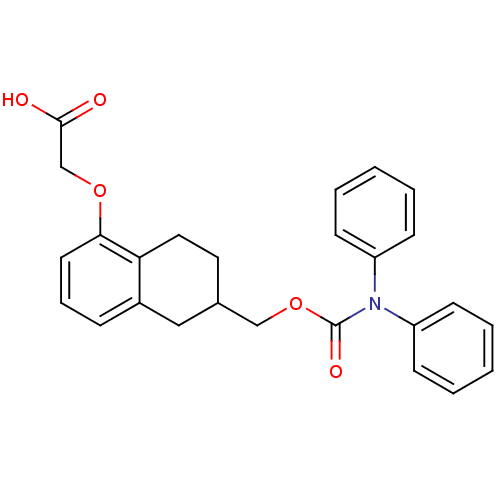
(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human Prostanoid IP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF-2 from human Prostanoid FP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ-29,548 from human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ-29,548 from human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD-2 from human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD-2 from human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF-2 from human Prostanoid FP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD-2 from human Prostanoid DP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50370452

(CHEMBL132589 | FR-181157)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP1 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50167891

(CHEMBL197319 | {6-[(Diphenylcarbamoyloxy)-methyl]-...)Show SMILES OC(=O)COc1cccc2CC(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO5/c28-25(29)18-31-24-13-7-8-20-16-19(14-15-23(20)24)17-32-26(30)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-13,19H,14-18H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGF-2 from human Prostanoid FP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50167890

(CHEMBL190497 | FR-193262 | Sodium; {(R)-5-[(R)-2-(...)Show SMILES [O-]C(=O)COc1cccc2[C@@H](CCCc12)N1CCC[C@@H]1c1nc(c(o1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H30N2O4/c34-28(35)20-36-27-18-8-14-23-24(27)15-7-16-25(23)33-19-9-17-26(33)31-32-29(21-10-3-1-4-11-21)30(37-31)22-12-5-2-6-13-22/h1-6,8,10-14,18,25-26H,7,9,15-17,19-20H2,(H,34,35)/p-1/t25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP3 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50167892

(CHEMBL195456 | {(S)-6-[(Diphenylcarbamoyloxy)-meth...)Show SMILES O[C@@H]1c2cccc(OCC(O)=O)c2CC[C@]1(O)COC(=O)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H25NO7/c28-23(29)16-33-22-13-7-12-21-20(22)14-15-26(32,24(21)30)17-34-25(31)27(18-8-3-1-4-9-18)19-10-5-2-6-11-19/h1-13,24,30,32H,14-17H2,(H,28,29)/t24-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SQ-29,548 from human Prostanoid TP receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50167887

((R)-2-(6-((diphenylcarbamoyloxy)methyl)-6-hydroxy-...)Show SMILES OC(=O)COc1cccc2C[C@@](O)(COC(=O)N(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H25NO6/c28-24(29)17-32-23-13-7-8-19-16-26(31,15-14-22(19)23)18-33-25(30)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-13,31H,14-18H2,(H,28,29)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE-2 from human Prostanoid EP4 receptor |
Bioorg Med Chem Lett 15: 3091-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.047
BindingDB Entry DOI: 10.7270/Q2SF2WXV |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072905
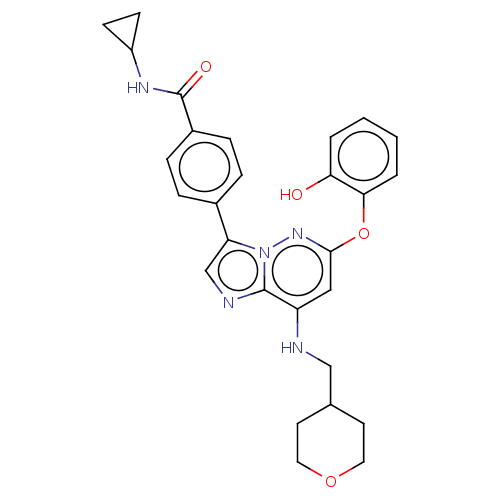
(CHEMBL3410078)Show SMILES Oc1ccccc1Oc1cc(NCC2CCOCC2)c2ncc(-c3ccc(cc3)C(=O)NC3CC3)n2n1 Show InChI InChI=1S/C28H29N5O4/c34-24-3-1-2-4-25(24)37-26-15-22(29-16-18-11-13-36-14-12-18)27-30-17-23(33(27)32-26)19-5-7-20(8-6-19)28(35)31-21-9-10-21/h1-8,15,17-18,21,29,34H,9-14,16H2,(H,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072911
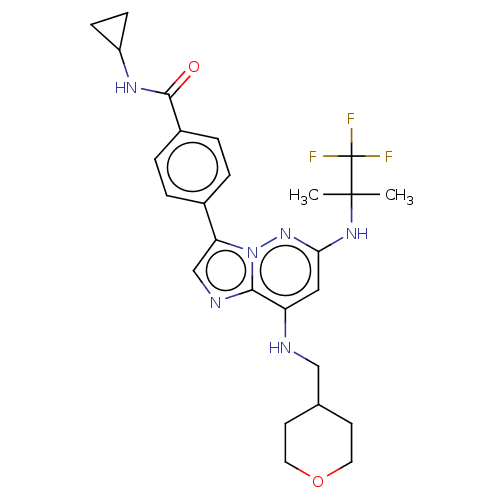
(CHEMBL3410084)Show SMILES CC(C)(Nc1cc(NCC2CCOCC2)c2ncc(-c3ccc(cc3)C(=O)NC3CC3)n2n1)C(F)(F)F Show InChI InChI=1S/C26H31F3N6O2/c1-25(2,26(27,28)29)33-22-13-20(30-14-16-9-11-37-12-10-16)23-31-15-21(35(23)34-22)17-3-5-18(6-4-17)24(36)32-19-7-8-19/h3-6,13,15-16,19,30H,7-12,14H2,1-2H3,(H,32,36)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072900

(CHEMBL3410073)Show SMILES Nc1cc(ccc1-c1cc(NCC2CCOCC2)c2ncc(-c3ccc(cc3)C(=O)NC3CC3)n2n1)C#N Show InChI InChI=1S/C29H29N7O2/c30-15-19-1-8-23(24(31)13-19)25-14-26(32-16-18-9-11-38-12-10-18)28-33-17-27(36(28)35-25)20-2-4-21(5-3-20)29(37)34-22-6-7-22/h1-5,8,13-14,17-18,22,32H,6-7,9-12,16,31H2,(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50386816
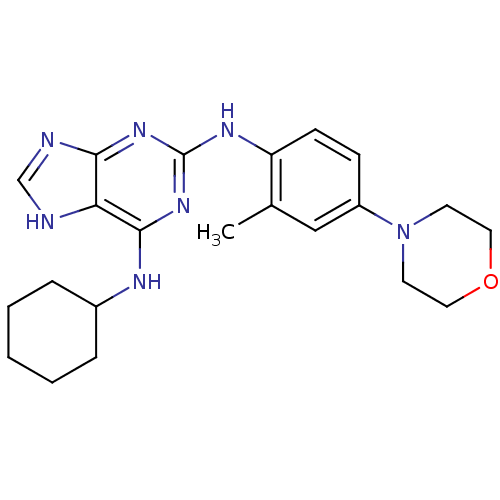
(CHEMBL2047943)Show SMILES Cc1cc(ccc1Nc1nc(NC2CCCCC2)c2[nH]cnc2n1)N1CCOCC1 Show InChI InChI=1S/C22H29N7O/c1-15-13-17(29-9-11-30-12-10-29)7-8-18(15)26-22-27-20-19(23-14-24-20)21(28-22)25-16-5-3-2-4-6-16/h7-8,13-14,16H,2-6,9-12H2,1H3,(H3,23,24,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human MPS1 expressed in Escherichia coli |
J Med Chem 56: 4343-56 (2013)
Article DOI: 10.1021/jm4000215
BindingDB Entry DOI: 10.7270/Q2SF2XJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072900

(CHEMBL3410073)Show SMILES Nc1cc(ccc1-c1cc(NCC2CCOCC2)c2ncc(-c3ccc(cc3)C(=O)NC3CC3)n2n1)C#N Show InChI InChI=1S/C29H29N7O2/c30-15-19-1-8-23(24(31)13-19)25-14-26(32-16-18-9-11-38-12-10-18)28-33-17-27(36(28)35-25)20-2-4-21(5-3-20)29(37)34-22-6-7-22/h1-5,8,13-14,17-18,22,32H,6-7,9-12,16,31H2,(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assay |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072905

(CHEMBL3410078)Show SMILES Oc1ccccc1Oc1cc(NCC2CCOCC2)c2ncc(-c3ccc(cc3)C(=O)NC3CC3)n2n1 Show InChI InChI=1S/C28H29N5O4/c34-24-3-1-2-4-25(24)37-26-15-22(29-16-18-11-13-36-14-12-18)27-30-17-23(33(27)32-26)19-5-7-20(8-6-19)28(35)31-21-9-10-21/h1-8,15,17-18,21,29,34H,9-14,16H2,(H,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assay |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072911

(CHEMBL3410084)Show SMILES CC(C)(Nc1cc(NCC2CCOCC2)c2ncc(-c3ccc(cc3)C(=O)NC3CC3)n2n1)C(F)(F)F Show InChI InChI=1S/C26H31F3N6O2/c1-25(2,26(27,28)29)33-22-13-20(30-14-16-9-11-37-12-10-16)23-31-15-21(35(23)34-22)17-3-5-18(6-4-17)24(36)32-19-7-8-19/h3-6,13,15-16,19,30H,7-12,14H2,1-2H3,(H,32,36)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assay |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50433907
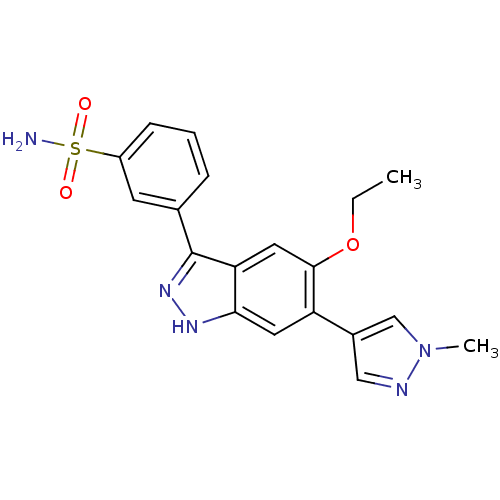
(CHEMBL2380582)Show SMILES CCOc1cc2c(n[nH]c2cc1-c1cnn(C)c1)-c1cccc(c1)S(N)(=O)=O Show InChI InChI=1S/C19H19N5O3S/c1-3-27-18-9-16-17(8-15(18)13-10-21-24(2)11-13)22-23-19(16)12-5-4-6-14(7-12)28(20,25)26/h4-11H,3H2,1-2H3,(H,22,23)(H2,20,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA |
J Med Chem 56: 4343-56 (2013)
Article DOI: 10.1021/jm4000215
BindingDB Entry DOI: 10.7270/Q2SF2XJB |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072910
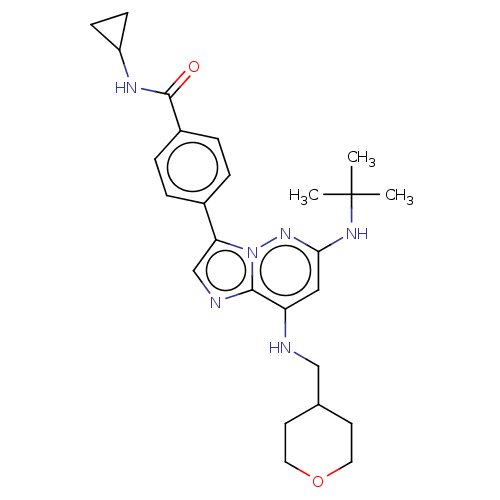
(CHEMBL3410083)Show SMILES CC(C)(C)Nc1cc(NCC2CCOCC2)c2ncc(-c3ccc(cc3)C(=O)NC3CC3)n2n1 Show InChI InChI=1S/C26H34N6O2/c1-26(2,3)30-23-14-21(27-15-17-10-12-34-13-11-17)24-28-16-22(32(24)31-23)18-4-6-19(7-5-18)25(33)29-20-8-9-20/h4-7,14,16-17,20,27H,8-13,15H2,1-3H3,(H,29,33)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072902

(CHEMBL3410075)Show SMILES O=C(NC1CC1)c1ccc(cc1)-c1cnc2c(NCC3CCOCC3)cc(Oc3ccccc3)nn12 Show InChI InChI=1S/C28H29N5O3/c34-28(31-22-10-11-22)21-8-6-20(7-9-21)25-18-30-27-24(29-17-19-12-14-35-15-13-19)16-26(32-33(25)27)36-23-4-2-1-3-5-23/h1-9,16,18-19,22,29H,10-15,17H2,(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072904

(CHEMBL3410077)Show SMILES Fc1ccccc1Oc1cc(NCC2CCOCC2)c2ncc(-c3ccc(cc3)C(=O)NC3CC3)n2n1 Show InChI InChI=1S/C28H28FN5O3/c29-22-3-1-2-4-25(22)37-26-15-23(30-16-18-11-13-36-14-12-18)27-31-17-24(34(27)33-26)19-5-7-20(8-6-19)28(35)32-21-9-10-21/h1-8,15,17-18,21,30H,9-14,16H2,(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
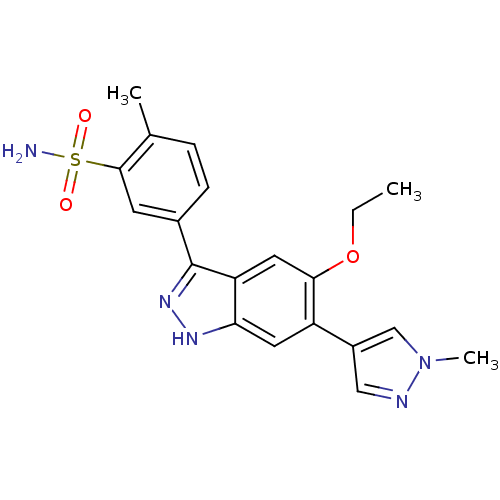
(Homo sapiens (Human)) | BDBM50433906
(CHEMBL2380583)Show SMILES CCOc1cc2c(n[nH]c2cc1-c1cnn(C)c1)-c1ccc(C)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H21N5O3S/c1-4-28-18-9-16-17(8-15(18)14-10-22-25(3)11-14)23-24-20(16)13-6-5-12(2)19(7-13)29(21,26)27/h5-11H,4H2,1-3H3,(H,23,24)(H2,21,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA |
J Med Chem 56: 4343-56 (2013)
Article DOI: 10.1021/jm4000215
BindingDB Entry DOI: 10.7270/Q2SF2XJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase TTK
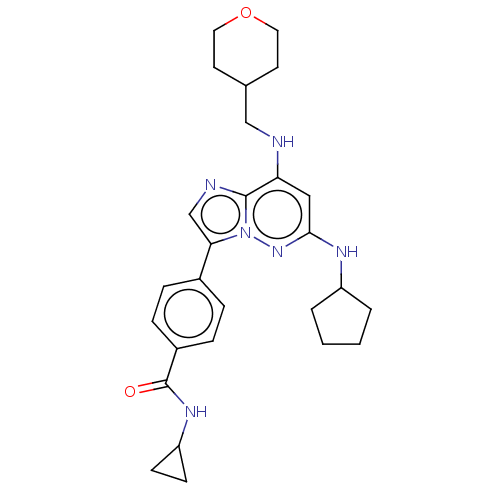
(Homo sapiens (Human)) | BDBM50072908
(CHEMBL3410081)Show SMILES O=C(NC1CC1)c1ccc(cc1)-c1cnc2c(NCC3CCOCC3)cc(NC3CCCC3)nn12 Show InChI InChI=1S/C27H34N6O2/c34-27(31-22-9-10-22)20-7-5-19(6-8-20)24-17-29-26-23(28-16-18-11-13-35-14-12-18)15-25(32-33(24)26)30-21-3-1-2-4-21/h5-8,15,17-18,21-22,28H,1-4,9-14,16H2,(H,30,32)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072899

(CHEMBL3410072)Show SMILES O=C(NC1CC1)c1ccc(cc1)-c1cnc2c(NCC3CCOCC3)cc(nn12)-c1ccc(cc1)C#N Show InChI InChI=1S/C29H28N6O2/c30-16-19-1-3-21(4-2-19)25-15-26(31-17-20-11-13-37-14-12-20)28-32-18-27(35(28)34-25)22-5-7-23(8-6-22)29(36)33-24-9-10-24/h1-8,15,18,20,24,31H,9-14,17H2,(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assay |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
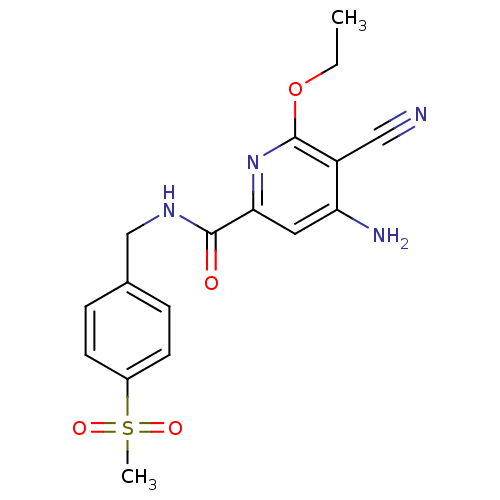
(Homo sapiens (Human)) | BDBM15913
(2-pyridinecarboxamide deriv. 8c | 4-Amino-5-cyano-...)Show SMILES CCOc1nc(cc(N)c1C#N)C(=O)NCc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C17H18N4O4S/c1-3-25-17-13(9-18)14(19)8-15(21-17)16(22)20-10-11-4-6-12(7-5-11)26(2,23)24/h4-8H,3,10H2,1-2H3,(H2,19,21)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1-mediated ATF2 phosphorylation after 1 hr by ELISA |
ACS Med Chem Lett 3: 560-564 (2012)
Article DOI: 10.1021/ml3000879
BindingDB Entry DOI: 10.7270/Q2RV0PZW |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50072908

(CHEMBL3410081)Show SMILES O=C(NC1CC1)c1ccc(cc1)-c1cnc2c(NCC3CCOCC3)cc(NC3CCCC3)nn12 Show InChI InChI=1S/C27H34N6O2/c34-27(31-22-9-10-22)20-7-5-19(6-8-20)24-17-29-26-23(28-16-18-11-13-35-14-12-18)15-25(32-33(24)26)30-21-3-1-2-4-21/h5-8,15,17-18,21-22,28H,1-4,9-14,16H2,(H,30,32)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assay |
J Med Chem 58: 1760-75 (2015)
Article DOI: 10.1021/jm501599u
BindingDB Entry DOI: 10.7270/Q2FF3V2H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data