

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441372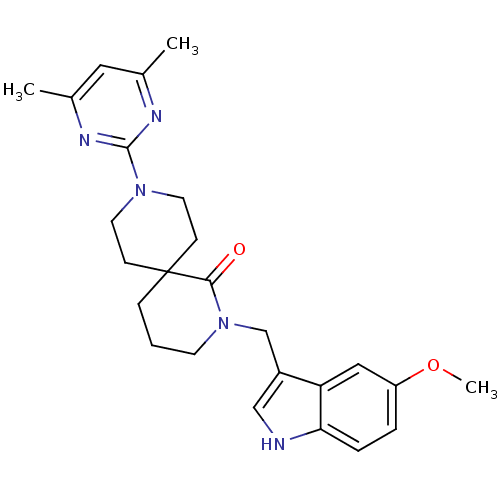 (CHEMBL2435400) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM102168 (US8530648, 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441378 (CHEMBL2435410) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441368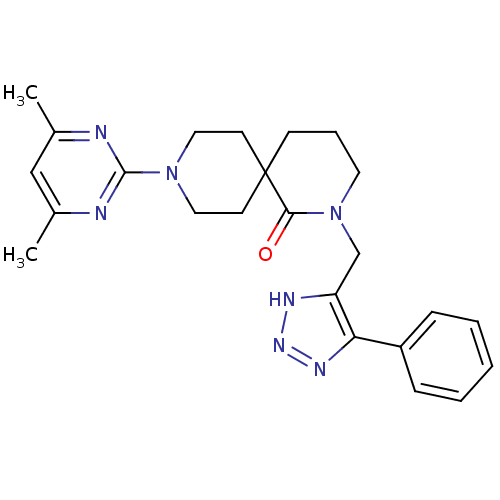 (CHEMBL2435396) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50198702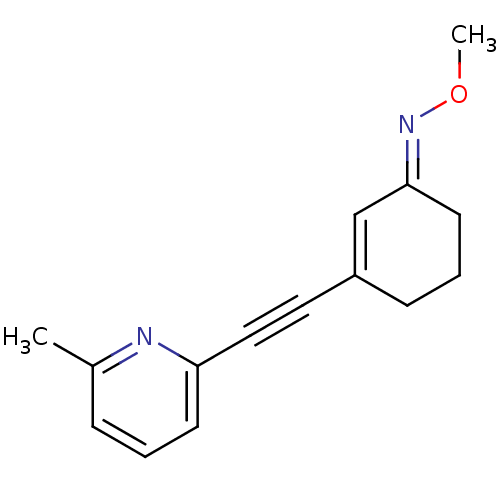 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Mus musculus) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse OX1 receptor expressed in CHO cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50198702 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from mGluR5 in rat brain membrane | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123325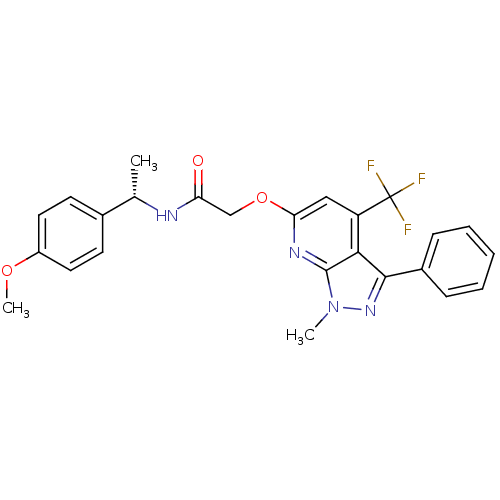 (US8742106, 1.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123327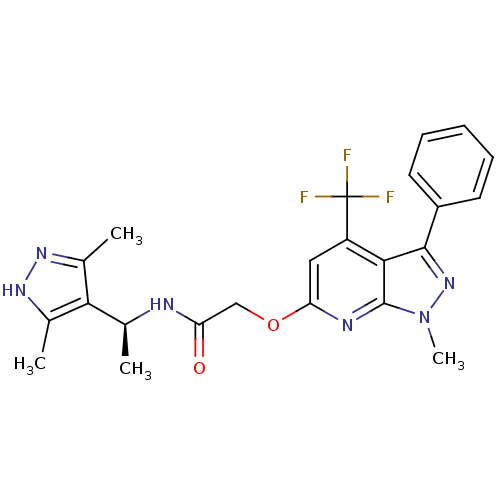 (US8742106, 1.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123341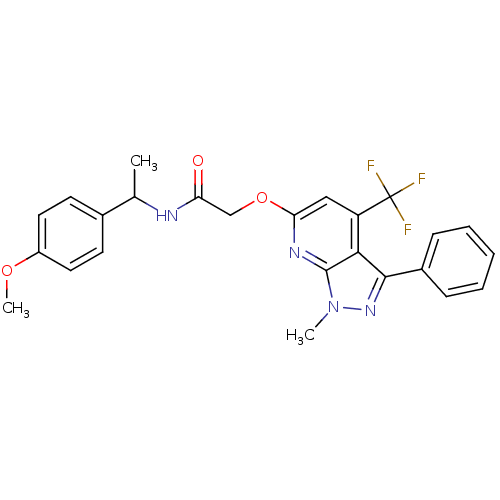 (US8742106, 1.45) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123338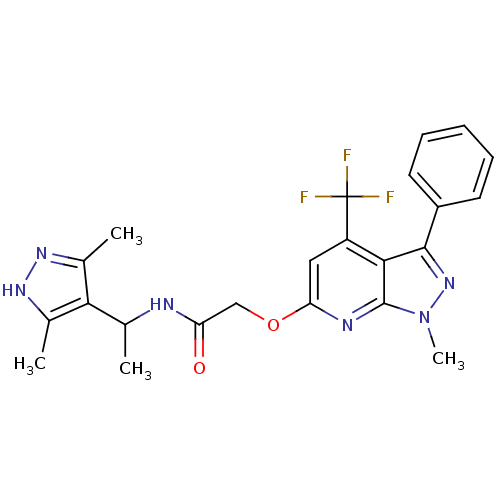 (US8742106, 1.38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123327 (US8742106, 1.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125l]orexin A from human orexin 1 receptor expressed in CHO cells after 1 hr | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM102172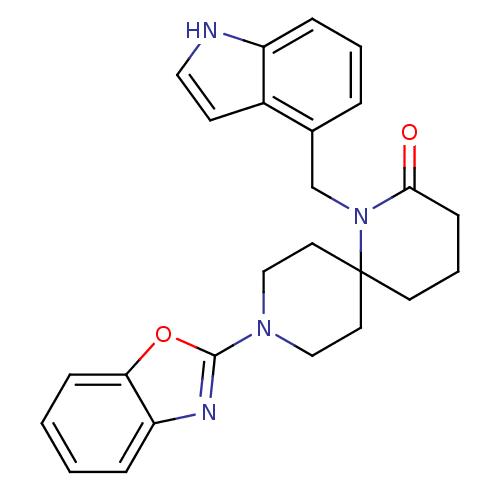 (US8530648, 94) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50198702 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441369 (CHEMBL2435397) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123338 (US8742106, 1.38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123357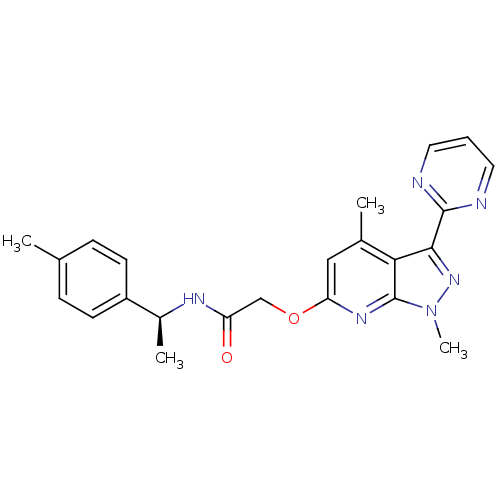 (US8742106, 4.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123325 (US8742106, 1.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125l]orexin A from human orexin 2 receptor expressed in CHO cells after 1 hr | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441380 (CHEMBL2435395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123327 (US8742106, 1.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123357 (US8742106, 4.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441371 (CHEMBL2435398) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441374 (CHEMBL2435403) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50133874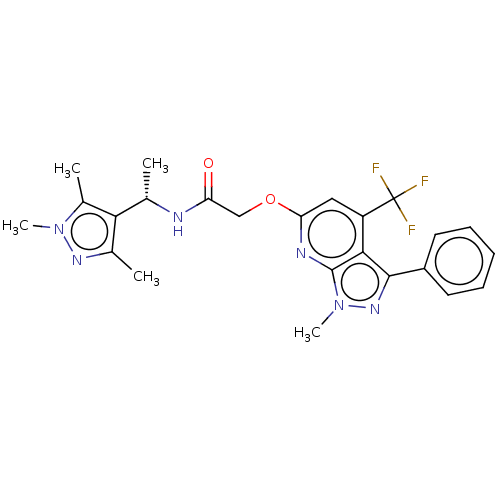 (CHEMBL3634021) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123327 (US8742106, 1.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125l]orexin A from human orexin 2 receptor expressed in CHO cells after 1 hr | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123325 (US8742106, 1.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125l]orexin A from human orexin 1 receptor expressed in CHO cells after 1 hr | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Mus musculus) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM102168 (US8530648, 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX1 receptor expressed in CHO cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50133874 (CHEMBL3634021) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50123005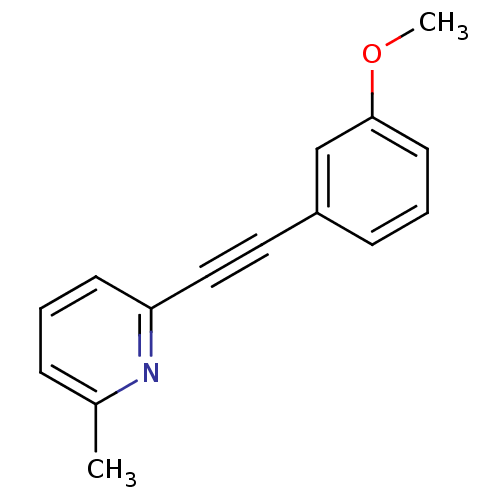 (2-(3-Methoxy-phenylethynyl)-6-methyl-pyridine | 2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50123005 (2-(3-Methoxy-phenylethynyl)-6-methyl-pyridine | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from mGluR5 in rat brain membrane | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM102171 (US8530648, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123325 (US8742106, 1.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441375 (CHEMBL2435402) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123358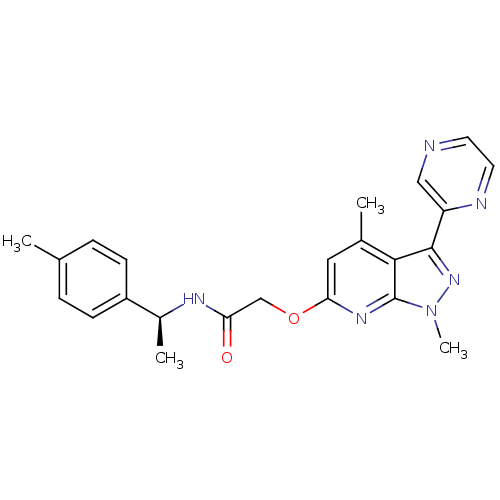 (US8742106, 4.8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441370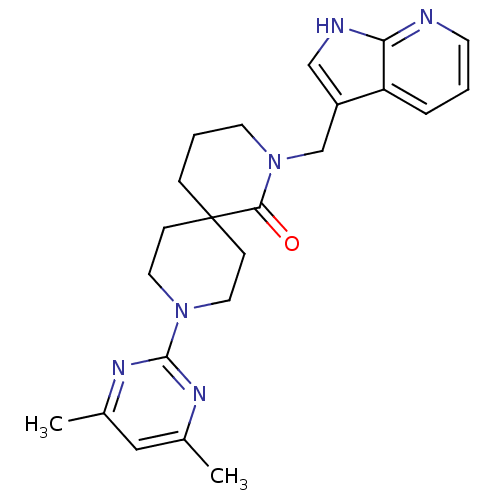 (CHEMBL2435399) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441381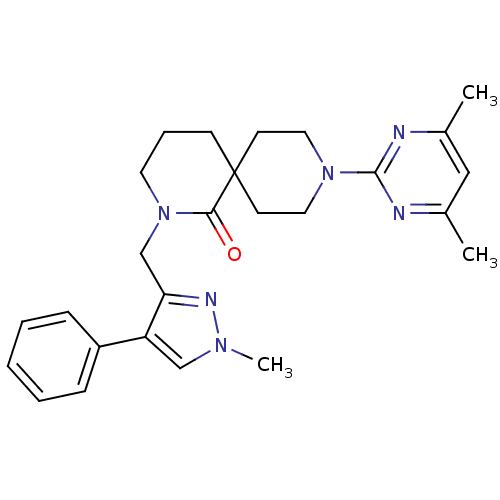 (CHEMBL2435394) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM102172 (US8530648, 94) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX1 receptor expressed in CHO cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50084137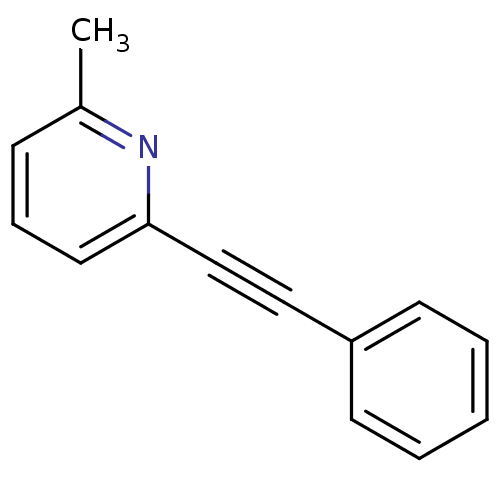 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from mGluR5 in rat brain membrane | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50084137 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cells | Bioorg Med Chem 15: 903-14 (2006) Article DOI: 10.1016/j.bmc.2006.10.038 BindingDB Entry DOI: 10.7270/Q25D8SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123342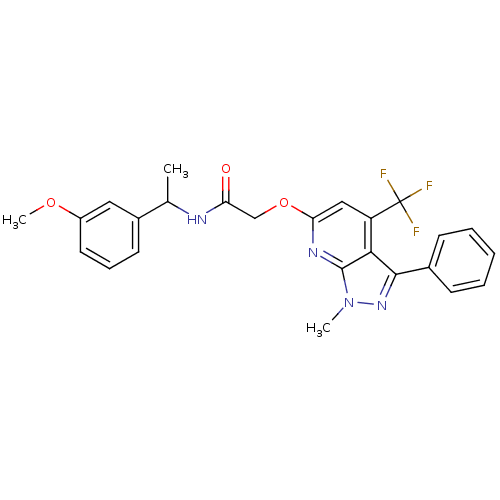 (US8742106, 1.46) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50441372 (CHEMBL2435400) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX1 receptor expressed in CHO cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441373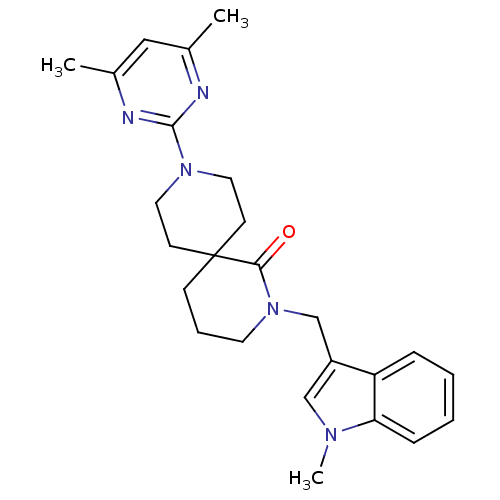 (CHEMBL2435401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123398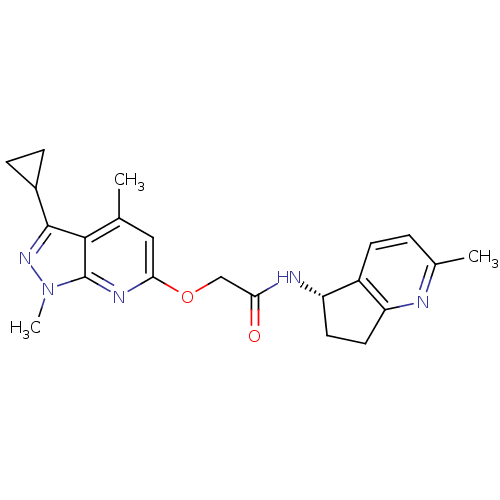 (US8742106, 6.63) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123342 (US8742106, 1.46) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50441376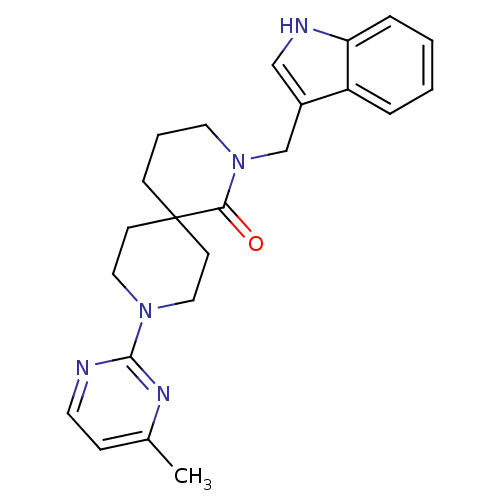 (CHEMBL2435404) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123341 (US8742106, 1.45) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123323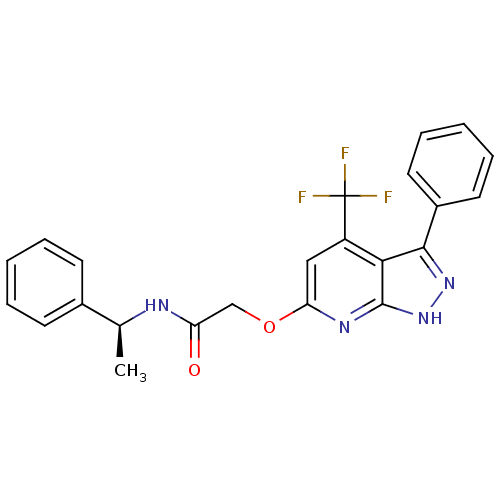 (US8742106, 1.2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123358 (US8742106, 4.8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Mus musculus) | BDBM50441375 (CHEMBL2435402) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse OX2 receptor expressed in HEK cells assessed as inhibition of orexin A-induced Ca2+ accumulation after 1 hr by Fluo-4-AM... | J Med Chem 56: 7590-607 (2013) Article DOI: 10.1021/jm4007627 BindingDB Entry DOI: 10.7270/Q2M90B3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 496 total ) | Next | Last >> |