

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM161396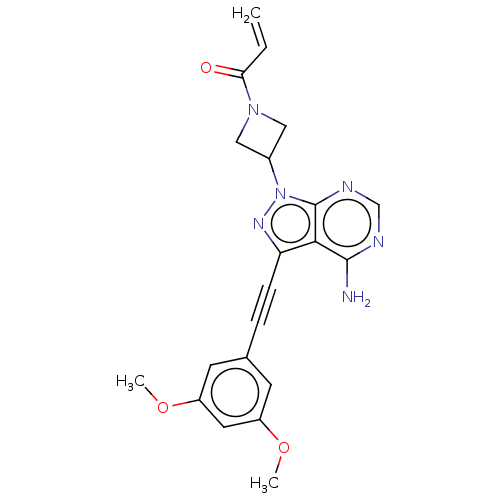 (US10124003, Ex. Compound 9 | US10835536, Ex. Comp ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory effect of the compounds on FGFR3 kinase activity was measured according to the method of Test Example 3. Purified recombinant human FG... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM161392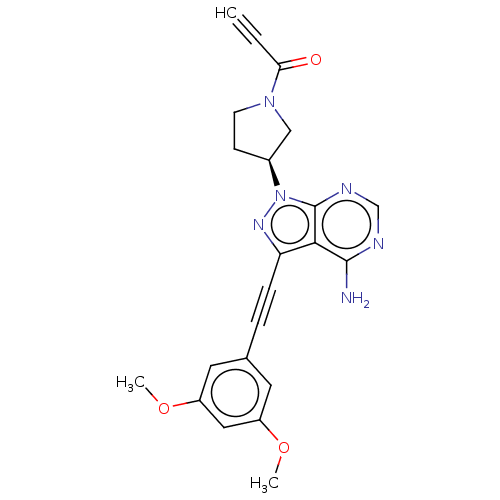 (US10124003, Ex. Compound 5 | US10835536, Ex. Comp ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory effect of the compounds on FGFR3 kinase activity was measured according to the method of Test Example 3. Purified recombinant human FG... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM161396 (US10124003, Ex. Compound 9 | US10835536, Ex. Comp ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory effect of the compounds on FGFR3 kinase activity was measured according to the method of Test Example 3. Purified recombinant human FG... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161396 (US10124003, Ex. Compound 9 | US10835536, Ex. Comp ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM478146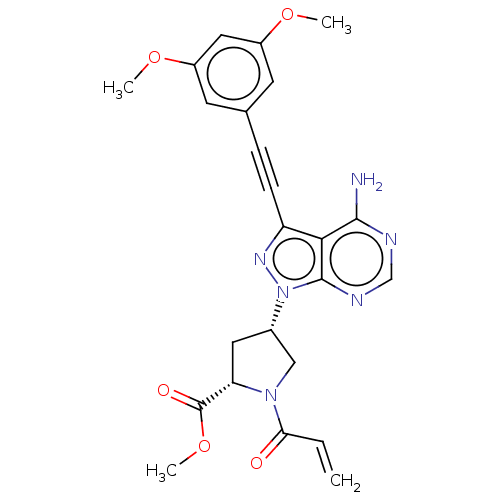 (US10894048, Ex Comp 66) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161419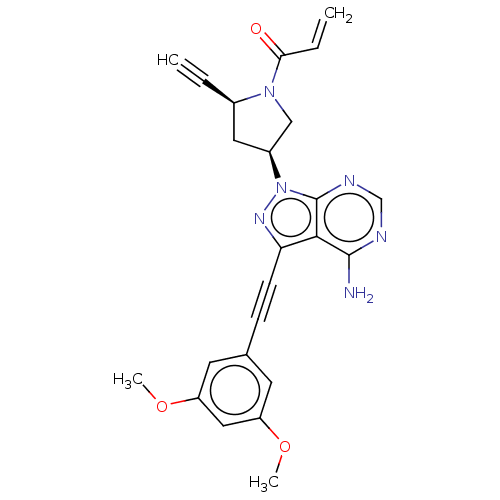 (US10124003, Ex. Compound 32 | US10835536, Ex. Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155568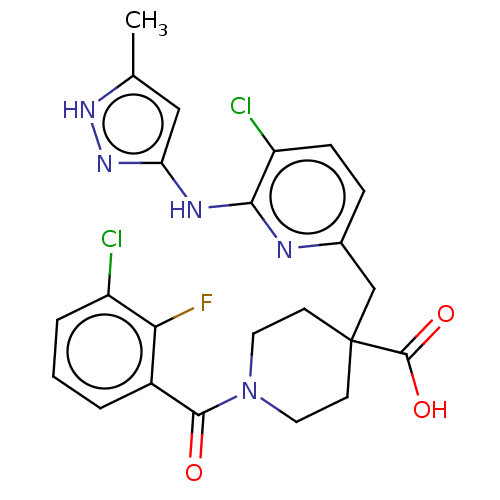 (US10092556, Example 12 | US9012475, 12 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161426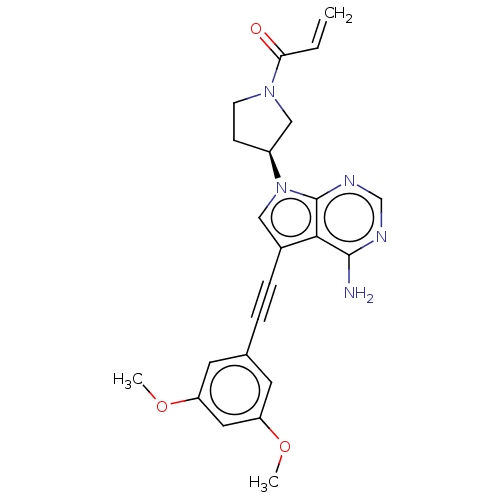 (US10124003, Ex. Compound 39 | US10894048, Ex Comp ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155565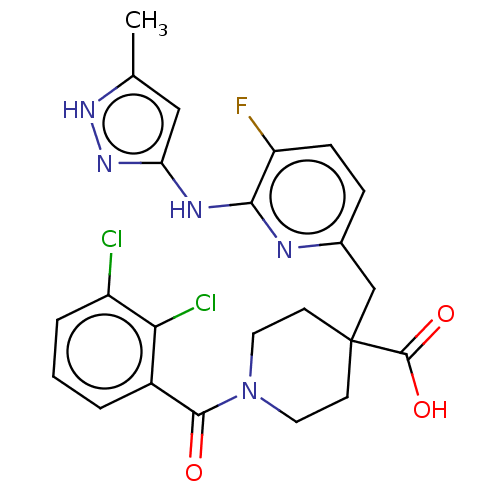 (US10092556, Example 1 | US9012475, 1 | US9346787, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | CHEMBL5291403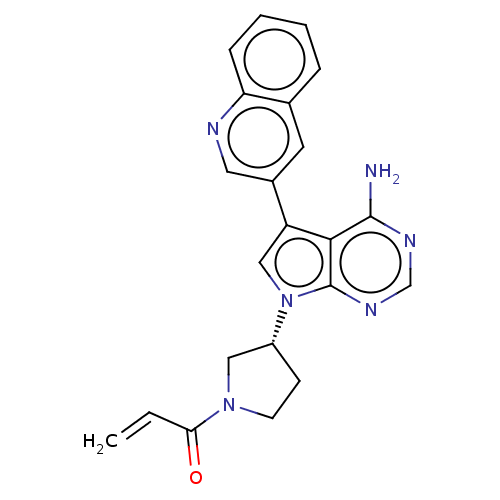 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155569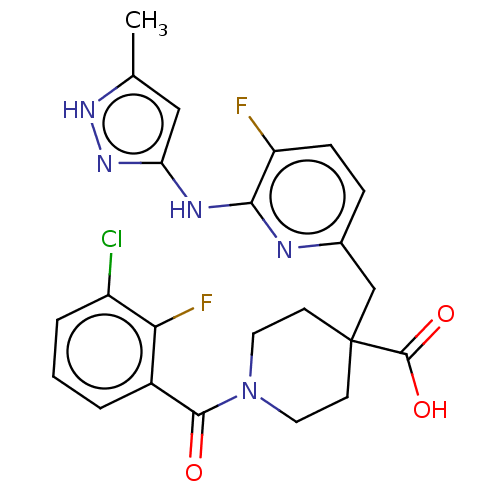 (US10092556, Example 13 | US9012475, 13 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155574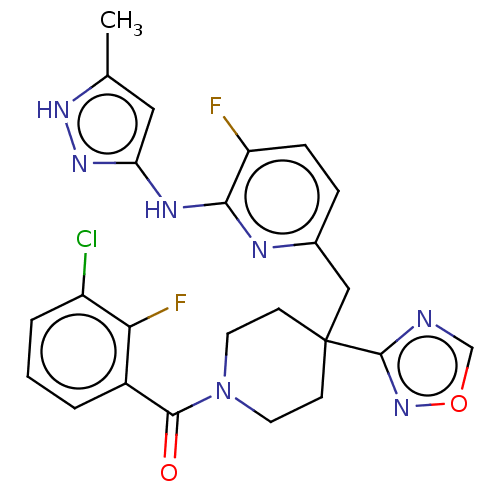 (US10092556, Example 24 | US9012475, 24 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155566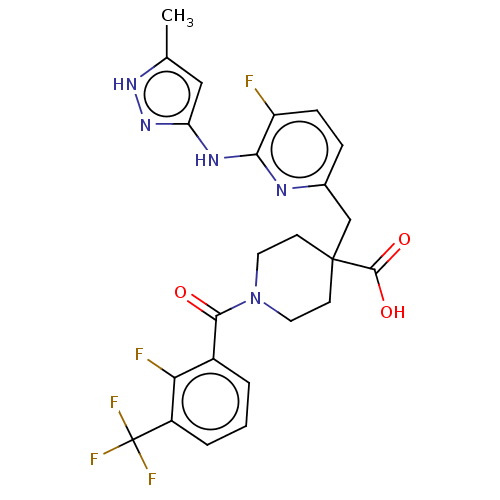 (US10092556, Example 2 | US9012475, 2 | US9346787, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM161389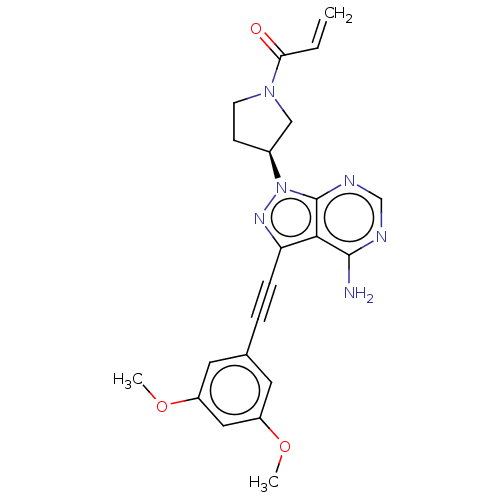 (US10124003, Ref. Ex. Compound 3 | US10835536, Ex. ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory effect of the compounds on FGFR3 kinase activity was measured according to the method of Test Example 3. Purified recombinant human FG... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM161392 (US10124003, Ex. Compound 5 | US10835536, Ex. Comp ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory effect of the compounds on FGFR4 kinase activity was measured according to the method of Test Example 3. Purified recombinant human FG... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM161434 (US10894048, Ex Comp 47 | US9108973, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory effect of the compounds on FGFR3 kinase activity was measured according to the method of Test Example 3. Purified recombinant human FG... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM478136 (US10894048, Ex Comp 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM47238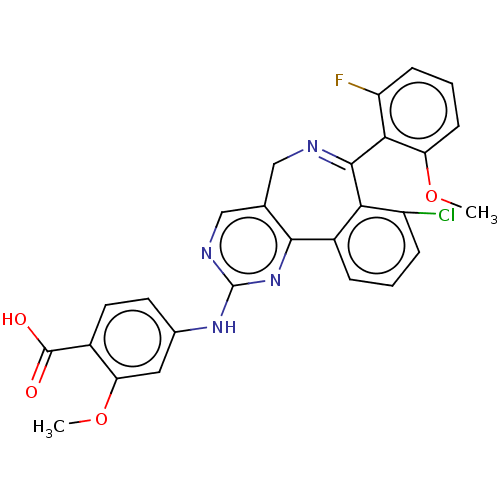 (US9012475, MLN8237) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The method for measuring the in-vitro inhibitory activity of a test compound against Aurora B kinase activity was substantially the same as in the ca... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155567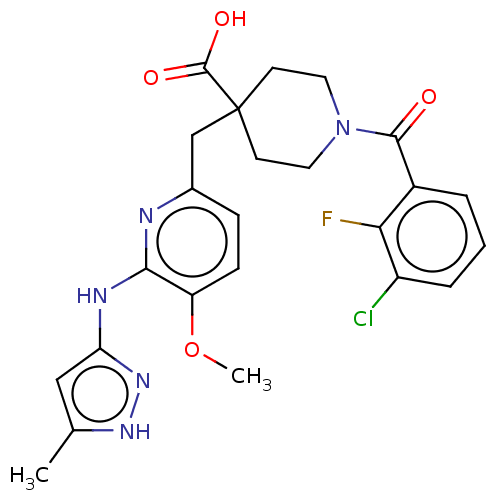 (US10092556, Example 11 | US9012475, 11 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155575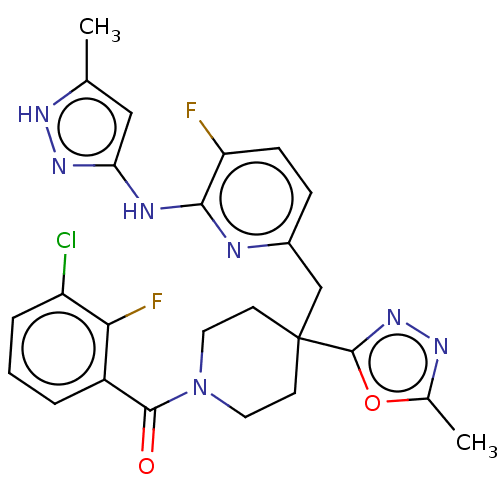 (US10092556, Example 28 | US9012475, 28 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM161450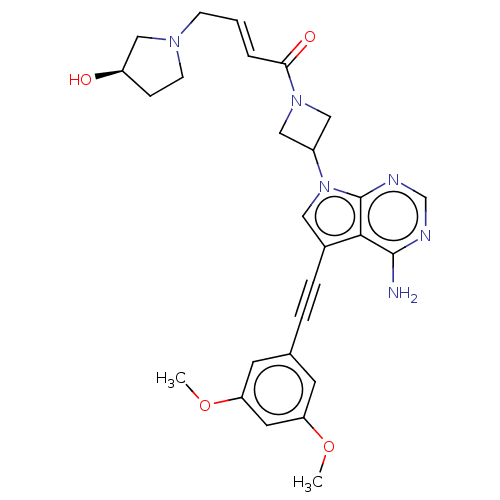 (US10124003, Ex. Compound 63 | US10835536, Ex. Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory effect of the compounds on FGFR3 kinase activity was measured according to the method of Test Example 3. Purified recombinant human FG... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155573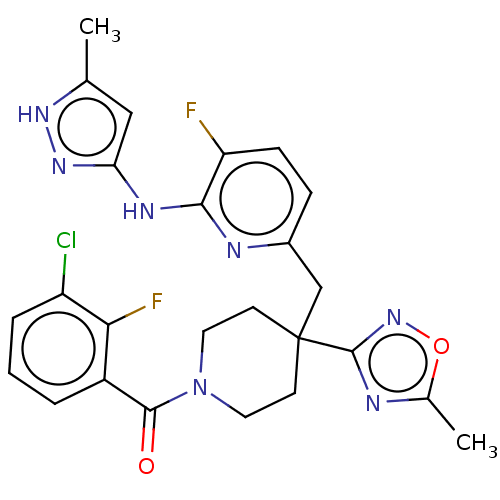 (US10092556, Example 22 | US9012475, 22 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM161439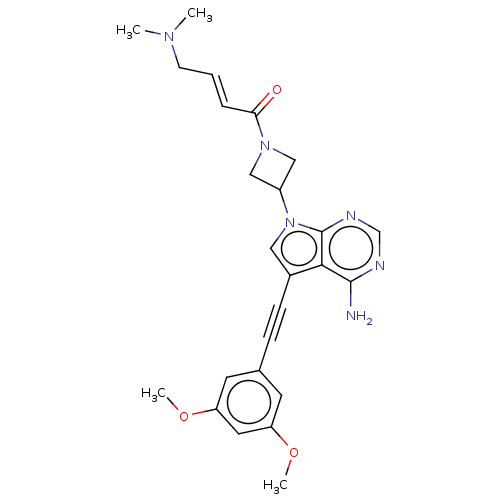 (US10124003, Ex. Compound 52 | US10894048, Ex Comp ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory effect of the compounds on FGFR3 kinase activity was measured according to the method of Test Example 3. Purified recombinant human FG... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155576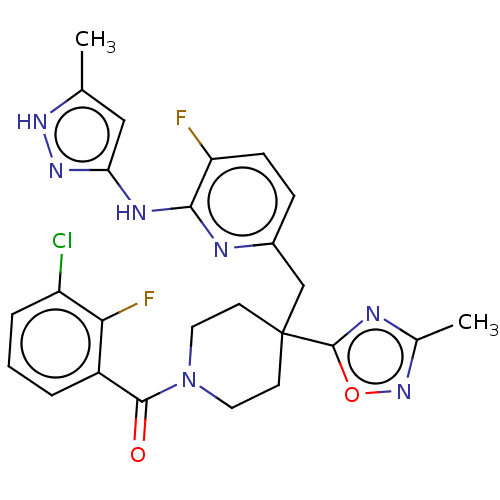 (US10092556, Example 29 | US9012475, 29 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155571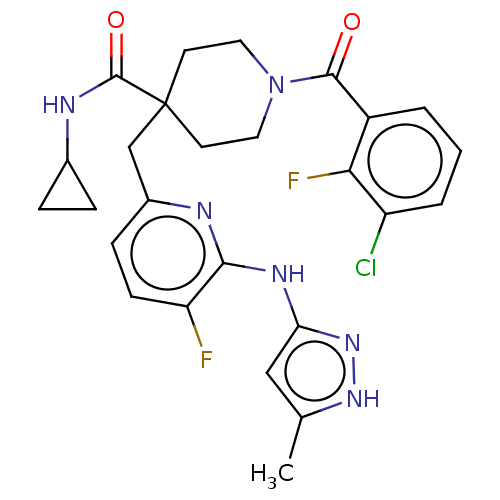 (US10092556, Example 17 | US9012475, 17 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155570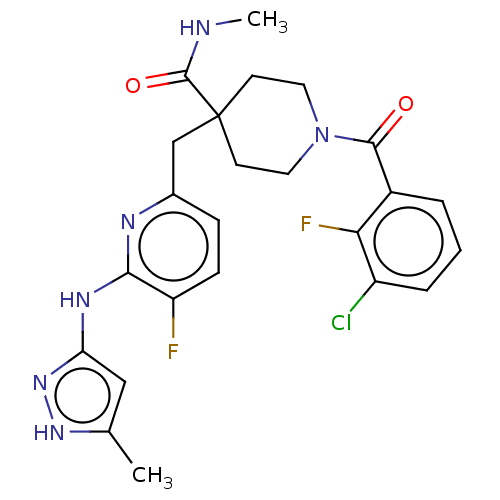 (US10092556, Example 14 | US9012475, 14 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50192071 ((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CDK1 | Bioorg Med Chem Lett 16: 5122-6 (2006) Article DOI: 10.1016/j.bmcl.2006.07.026 BindingDB Entry DOI: 10.7270/Q2K35VF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM155572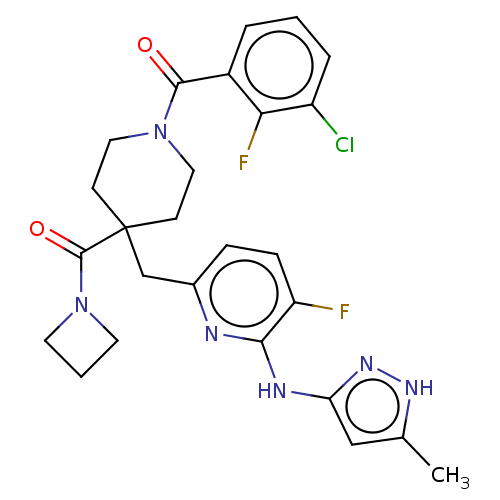 (US10092556, Example 19 | US9012475, 19 | US9346787...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description An inhibitory activity of the above compound in-vitro against Aurora A kinase activity was measured with reference to a method described in JP-A-2008... | US Patent US9012475 (2015) BindingDB Entry DOI: 10.7270/Q2MW2FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161439 (US10124003, Ex. Compound 52 | US10894048, Ex Comp ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161389 (US10124003, Ref. Ex. Compound 3 | US10835536, Ex. ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161450 (US10124003, Ex. Compound 63 | US10835536, Ex. Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161392 (US10124003, Ex. Compound 5 | US10835536, Ex. Comp ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161389 (US10124003, Ref. Ex. Compound 3 | US10835536, Ex. ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161396 (US10124003, Ex. Compound 9 | US10835536, Ex. Comp ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM161396 (US10124003, Ex. Compound 9 | US10835536, Ex. Comp ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR1 kinase activity, a biotinylated peptide (biotin-EEPLYW... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161397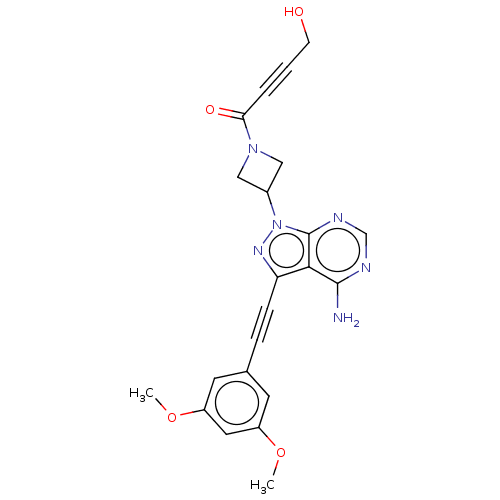 (US10124003, Ex. Compound 10 | US10835536, Ex. Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | CHEMBL5265881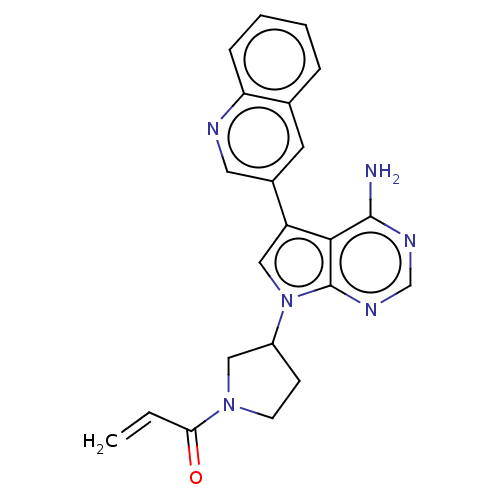 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161446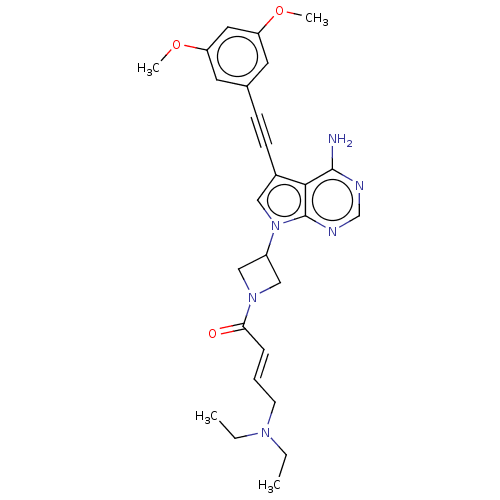 (US10124003, Ex. Compound 59 | US10835536, Ex. Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM478147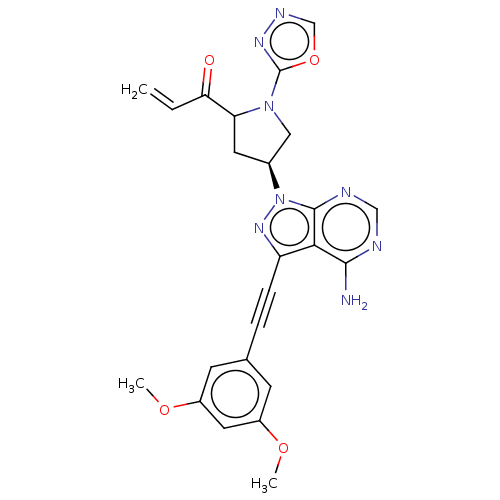 (US10894048, Ex Comp 68) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM161392 (US10124003, Ex. Compound 5 | US10835536, Ex. Comp ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR1 kinase activity, a biotinylated peptide (biotin-EEPLYW... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161447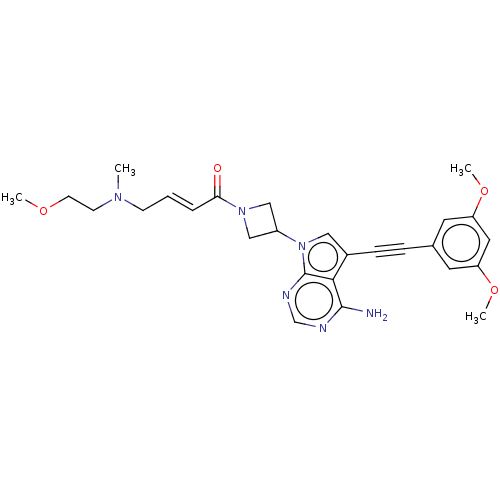 (US10124003, Ex. Compound 60 | US10835536, Ex. Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161444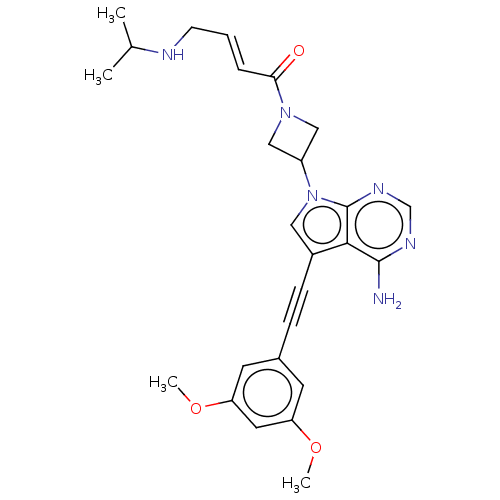 (US10124003, Ex. Compound 57 | US10835536, Ex. Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161439 (US10124003, Ex. Compound 52 | US10894048, Ex Comp ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161396 (US10124003, Ex. Compound 9 | US10835536, Ex. Comp ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM161389 (US10124003, Ref. Ex. Compound 3 | US10835536, Ex. ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM161396 (US10124003, Ex. Compound 9 | US10835536, Ex. Comp ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory effect of the compounds on FGFR4 kinase activity was measured according to the method of Test Example 3. Purified recombinant human FG... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM161389 (US10124003, Ref. Ex. Compound 3 | US10835536, Ex. ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161389 (US10124003, Ref. Ex. Compound 3 | US10835536, Ex. ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM161434 (US10894048, Ex Comp 47 | US9108973, 47) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR2 kinase activity, FL-Peptide 22 (Caliper Life Sciences,... | US Patent US10894048 (2021) BindingDB Entry DOI: 10.7270/Q2319ZZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50096797 (CHEMBL2370665 | macrocyclic lipopeptidolactone der...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans | Bioorg Med Chem Lett 11: 395-8 (2001) BindingDB Entry DOI: 10.7270/Q28051W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 303 total ) | Next | Last >> |