

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50312996 ((R,S)-3-(5-chloro-1-methyl-1H-indol-3-yl)-4-(3-(2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313013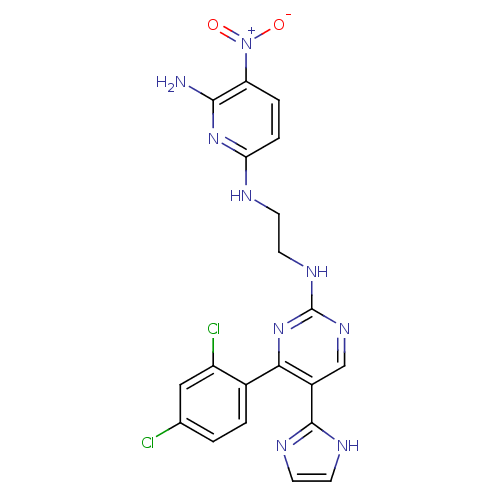 (CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50312997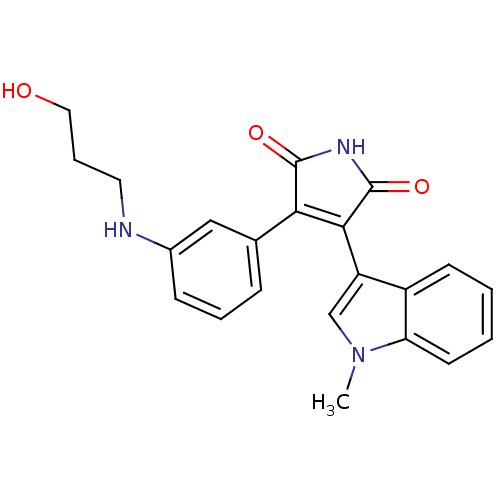 (3-(3-(3-hydroxypropylamino)phenyl)-4-(1-methyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50312998 ((R,S)-3-(3-(2,3-dihydroxypropyl)phenyl)-4-(5-fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM2647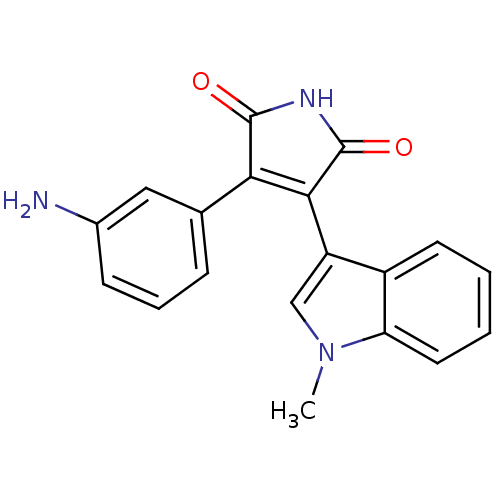 ((Phenylindolyl)maleimide deriv. 69 | 3-(3-aminophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313010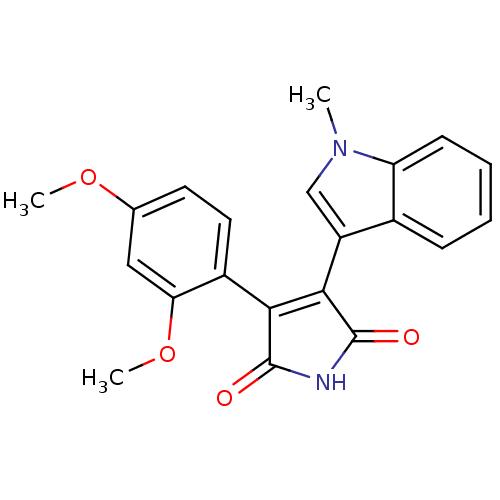 (3-(2,4-dimethoxyphenyl)-4-(1-methyl-1H-indol-3-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27614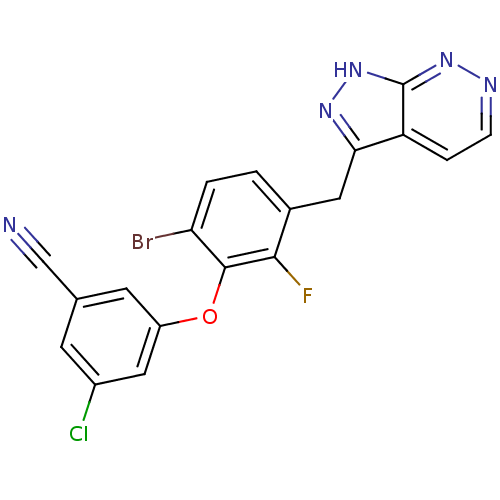 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | n/a | n/a | 3 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27614 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | 4 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27615 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyrazin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | >25 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313000 ((R)-3-(3-(2,3-dihydroxypropyl)phenyl)-4-(1-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM2651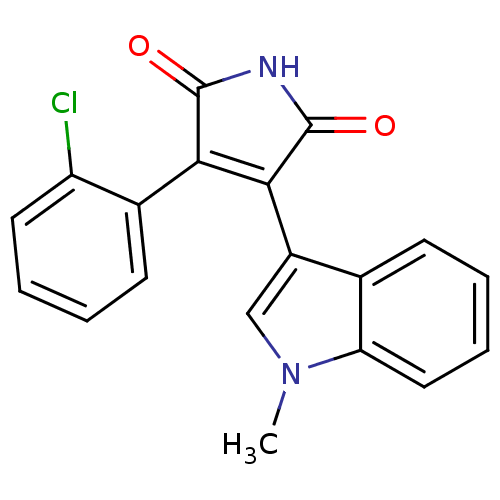 ((Phenylindolyl)maleimide deriv. 73 | 3-(2-chloroph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27613 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | 2 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313003 (3-(5-chloro-1-methyl-1H-indol-3-yl)-4-(3-methoxyph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313009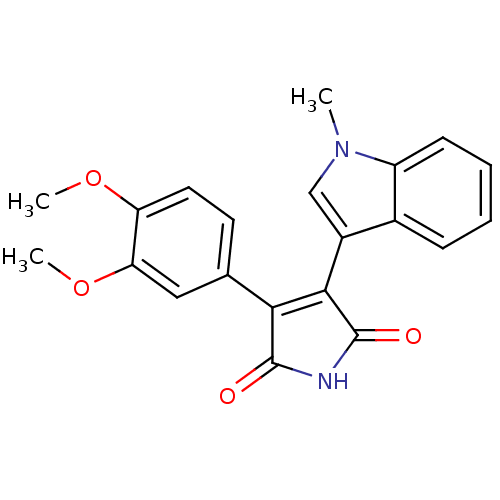 (3-(3,4-dimethoxyphenyl)-4-(1-methyl-1H-indol-3-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313011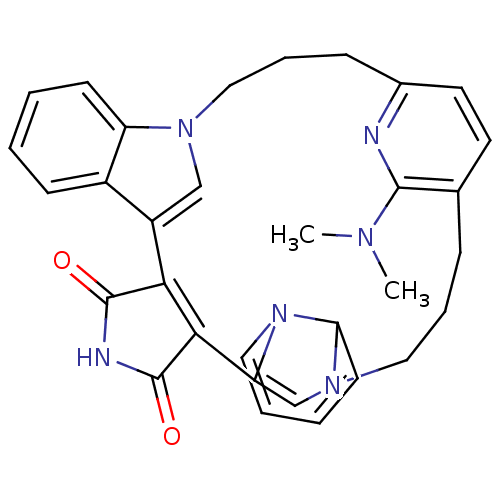 (31-(dimethylamino)-5,11,15,25,30-pentaazaheptacycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27611 (3-[6-bromo-2-fluoro-3-({6-methyl-7-oxo-1H,6H,7H-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | 4 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27612 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50312999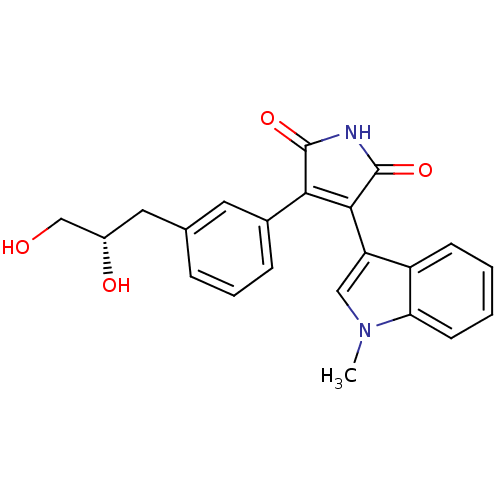 ((S)-3-(3-(2,3-dihydroxypropyl)phenyl)-4-(1-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27613 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | 18 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313012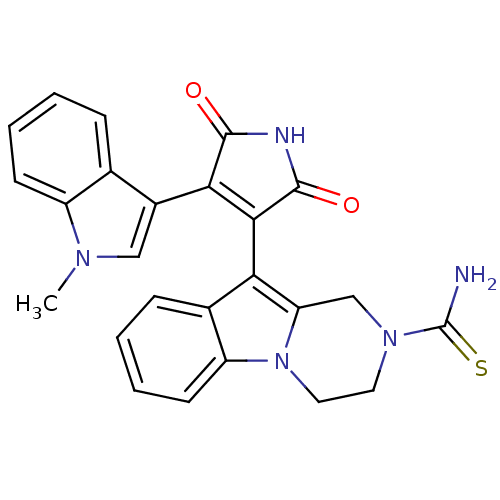 (10-(4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27611 (3-[6-bromo-2-fluoro-3-({6-methyl-7-oxo-1H,6H,7H-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | 97 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27615 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyrazin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | >100 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482300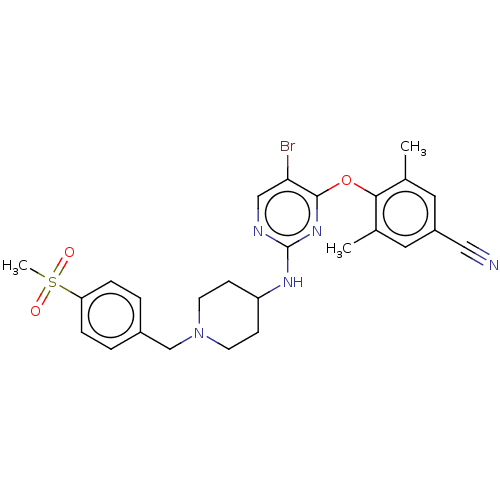 (CHEMBL1170386) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482304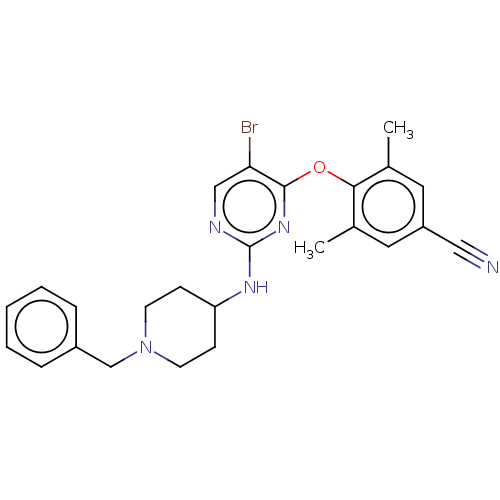 (CHEMBL1170190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27609 (3-{[4-bromo-3-(3-chloro-5-cyanophenoxy)-2-fluoroph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | 53 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482308 (CHEMBL1169643) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482304 (CHEMBL1170190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50282633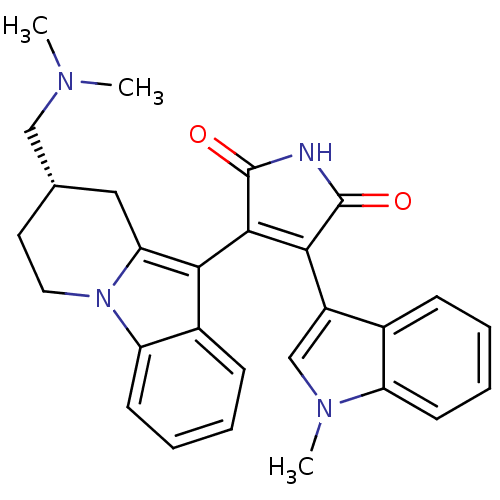 ((S)-3-(8-((dimethylamino)methyl)-6,7,8,9-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482308 (CHEMBL1169643) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482298 (CHEMBL1171403) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482299 (CHEMBL1170387) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27610 (3-{3-[(7-amino-1H-indazol-3-yl)methyl]-6-bromo-2-f...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | 84 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50282633 ((S)-3-(8-((dimethylamino)methyl)-6,7,8,9-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of PKCalpha | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313008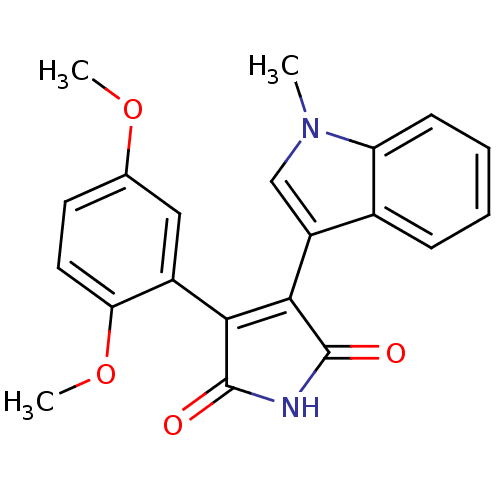 (3-(2,5-dimethoxyphenyl)-4-(1-methyl-1H-indol-3-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM2604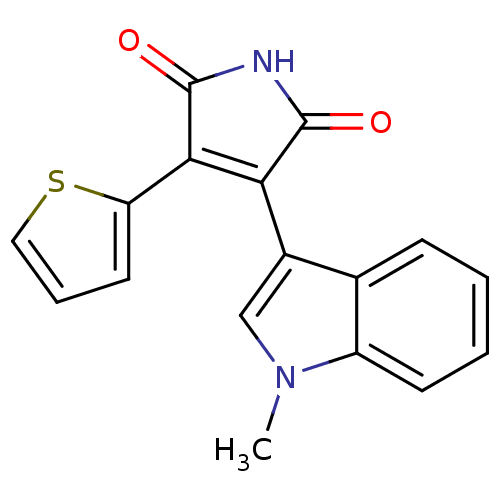 ((Arylindolyl)maleimide deriv. 26 | 3-(1-methyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482301 (CHEMBL1170591) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27610 (3-{3-[(7-amino-1H-indazol-3-yl)methyl]-6-bromo-2-f...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | 8 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27609 (3-{[4-bromo-3-(3-chloro-5-cyanophenoxy)-2-fluoroph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | 5 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482310 (CHEMBL1170006) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482301 (CHEMBL1170591) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482300 (CHEMBL1170386) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482300 (CHEMBL1170386) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y188L mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482313 (CHEMBL1170005) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482306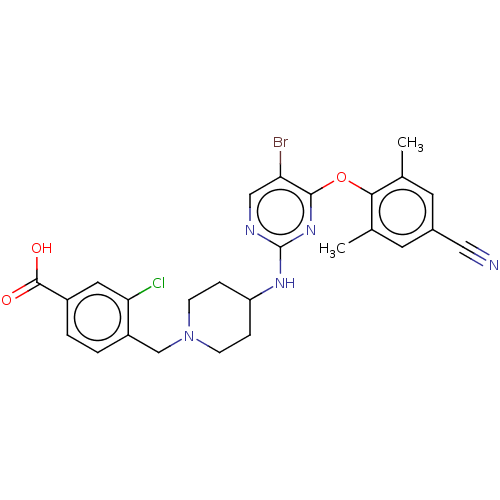 (CHEMBL1172334) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482302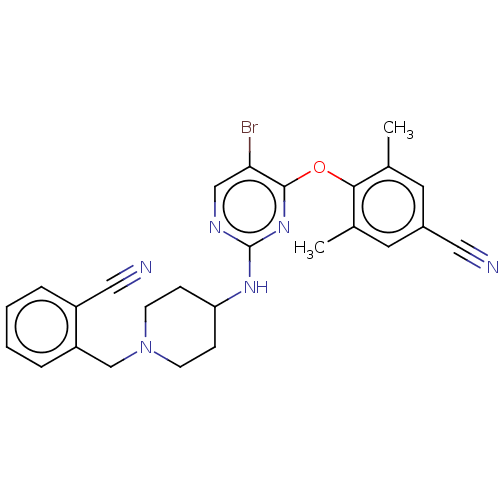 (CHEMBL1170378) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482299 (CHEMBL1170387) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313004 (3-(6-fluoro-1-methyl-1H-indol-3-yl)-4-(3-methoxyph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482306 (CHEMBL1172334) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482307 (CHEMBL1169811) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50313002 (3-(1,6-dimethyl-1H-indol-3-yl)-4-(3-methoxyphenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta assessed as [gamma33]ATP transfer to biotinylated CREB-peptide substrate after 1 hr by scintillation counti... | Bioorg Med Chem Lett 20: 1693-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.038 BindingDB Entry DOI: 10.7270/Q2ZK5HNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 192 total ) | Next | Last >> |