

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50370582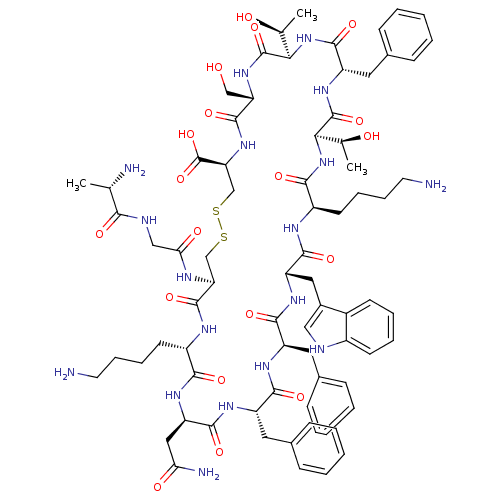 (CHEMBL1791306) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 2 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085050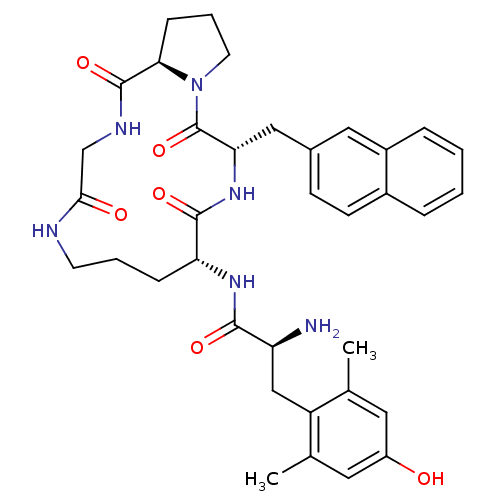 (CHEMBL152690 | H-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085050 (CHEMBL152690 | H-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DAMGO at Opioid receptor mu 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50370578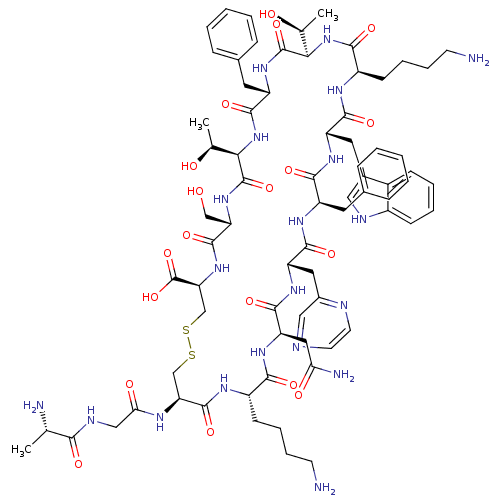 (CHEMBL1791304) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 2 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50370577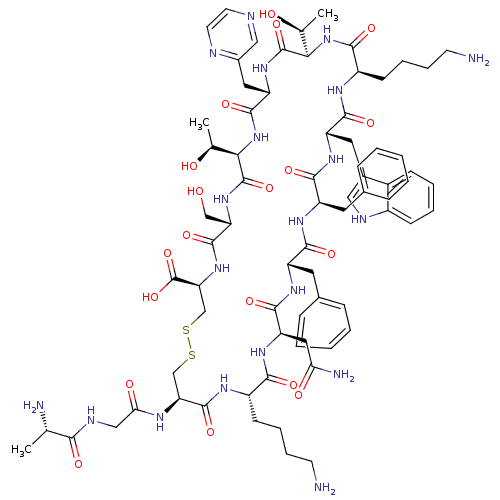 (CHEMBL1791312) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 2 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50370582 (CHEMBL1791306) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 4 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404821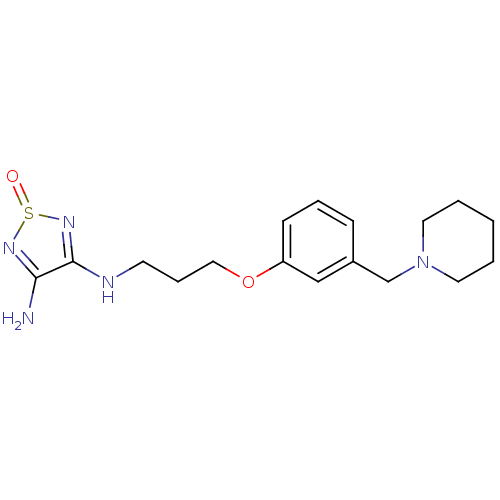 (CHEMBL306465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404822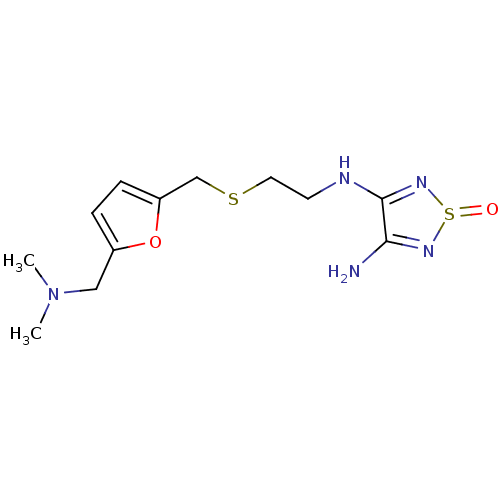 (CHEMBL8982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404823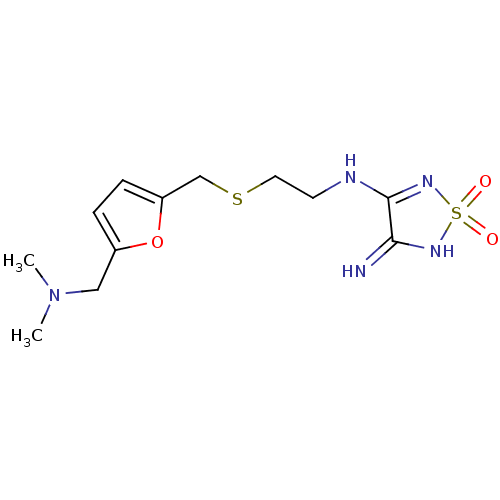 (CHEMBL63299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50370580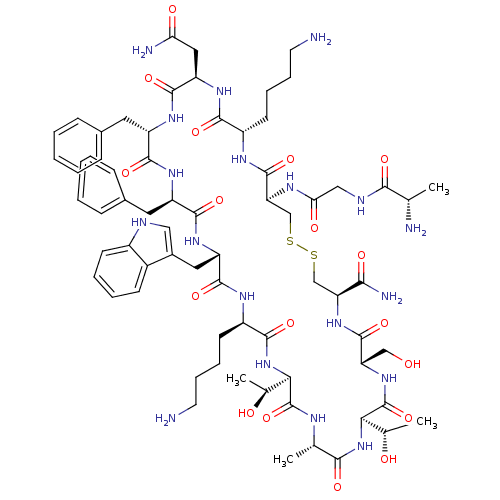 (CHEMBL1791307) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 2 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50370578 (CHEMBL1791304) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 2 (n=6) | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50370583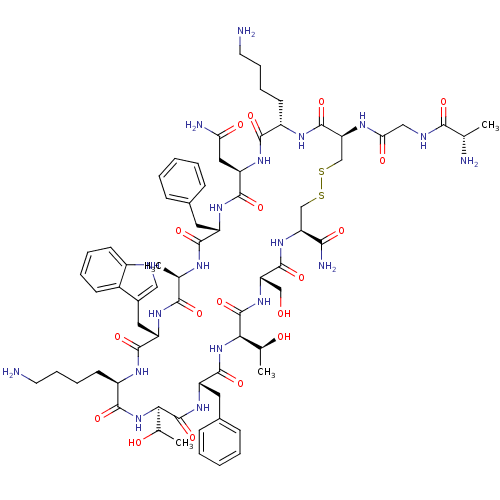 (CHEMBL1791311) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 2 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50370581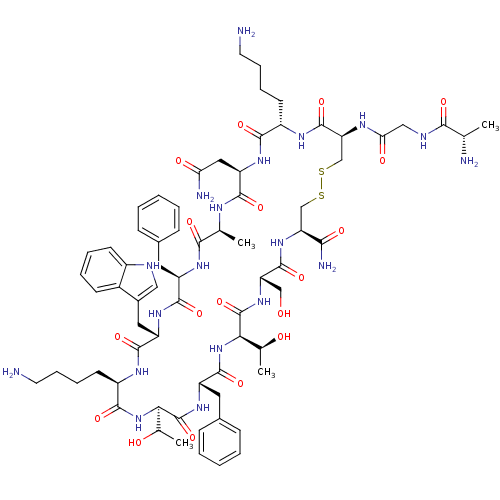 (CHEMBL1791310) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 2 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50064032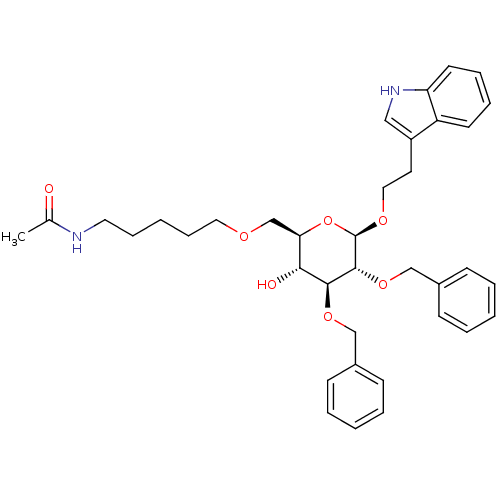 (CHEMBL29161 | N-(5-{(2R,3R,4S,5R,6R)-4,5-Bis-benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity of the compound against Tachykinin receptor 1 | J Med Chem 46: 1858-69 (2003) Article DOI: 10.1021/jm0205088 BindingDB Entry DOI: 10.7270/Q2K936W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085049 (CHEMBL152689 | CHO-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 32.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50127508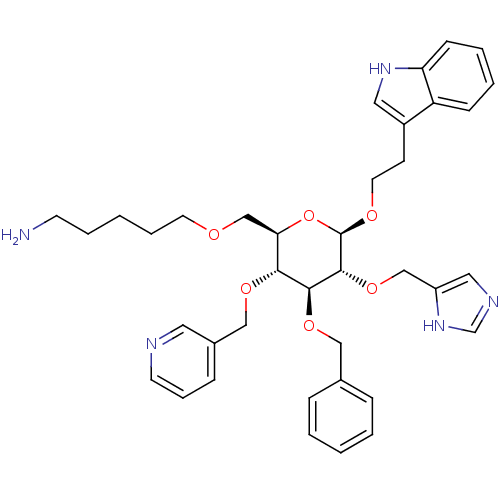 (5-(((2R,3R,4S,5R,6R)-5-((1H-imidazol-5-yl)methoxy)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Tested for binding affinity at human Somatostatin receptor type 4 using ([3-125I-Tyr11)]-SRIF-14 as the radioligand | J Med Chem 46: 1858-69 (2003) Article DOI: 10.1021/jm0205088 BindingDB Entry DOI: 10.7270/Q2K936W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50370578 (CHEMBL1791304) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 4 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50370578 (CHEMBL1791304) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 4 (n=5) | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50370581 (CHEMBL1791310) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 4 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50064027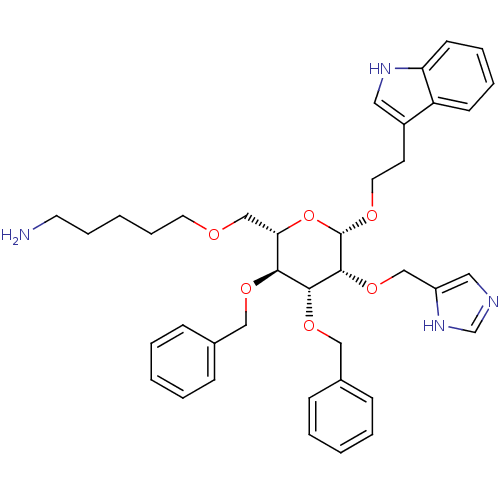 (5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR4. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50127507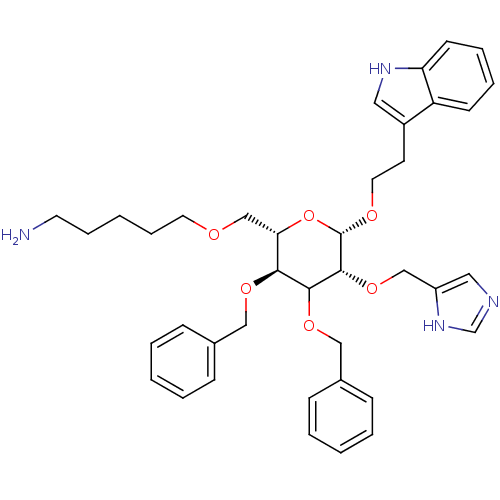 (5-{3,4-Bis-benzyloxy-5-(1H-imidazol-4-ylmethoxy)-6...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Tested for binding affinity at human Somatostatin receptor type 4 using ([3-125I-Tyr11)]-SRIF-14 as the radioligand | J Med Chem 46: 1858-69 (2003) Article DOI: 10.1021/jm0205088 BindingDB Entry DOI: 10.7270/Q2K936W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085055 (CHEMBL151098 | Dhp-c-[-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50370577 (CHEMBL1791312) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 4 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50051567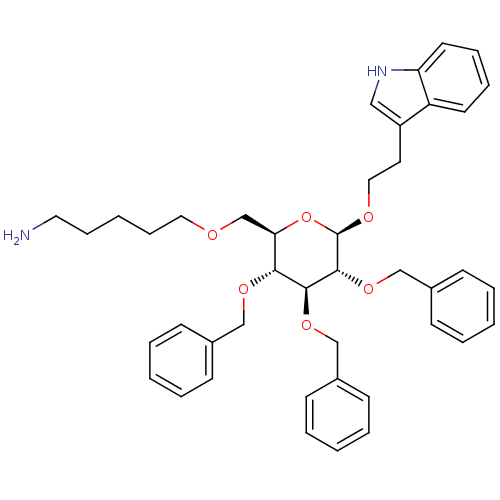 (5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity of the compound against Tachykinin receptor 1 | J Med Chem 46: 1858-69 (2003) Article DOI: 10.1021/jm0205088 BindingDB Entry DOI: 10.7270/Q2K936W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50369778 (CHEMBL1791164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085049 (CHEMBL152689 | CHO-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DAMGO at Opioid receptor mu 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50064027 (5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR1 | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085055 (CHEMBL151098 | Dhp-c-[-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DAMGO at Opioid receptor mu 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50369779 (CHEMBL1791163) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 486 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50064025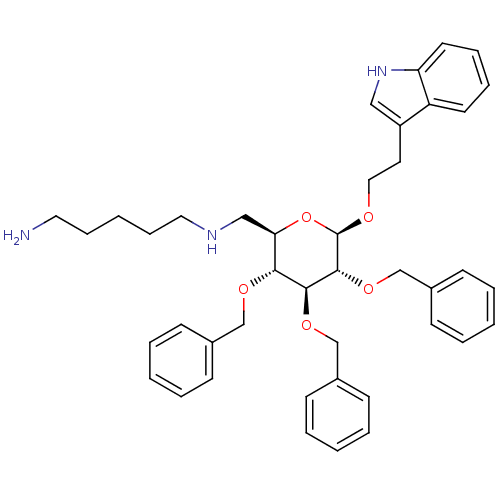 (CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR4. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22893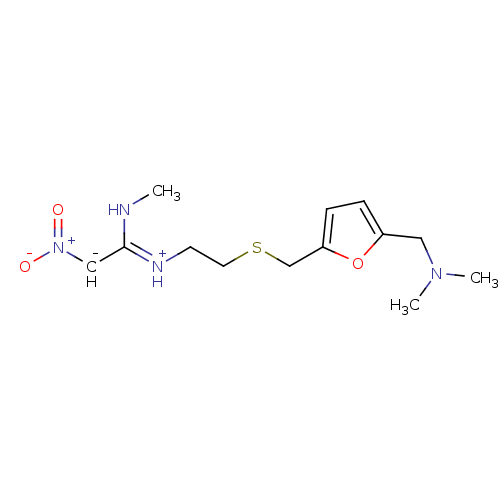 (CHEMBL512 | Ranitidine | ZANTAC | dimethyl[(5-{[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50403559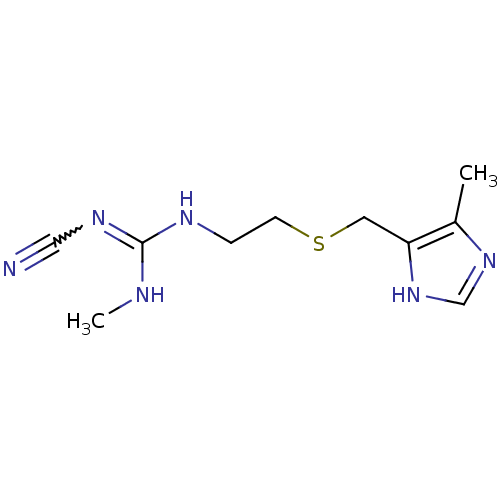 (Brumetadina | CIMETIDINE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50064025 (CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR2 | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50064025 (CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR3. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50370579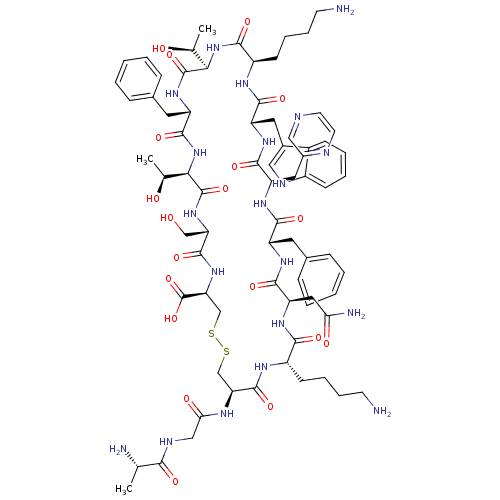 (CHEMBL1791305) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 4 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50369780 (CHEMBL1791161) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50064027 (5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR2 | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50064027 (5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR3. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50370579 (CHEMBL1791305) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human somatostatin receptor type 2 | J Med Chem 48: 4025-30 (2005) Article DOI: 10.1021/jm058184l BindingDB Entry DOI: 10.7270/Q2736RP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50369777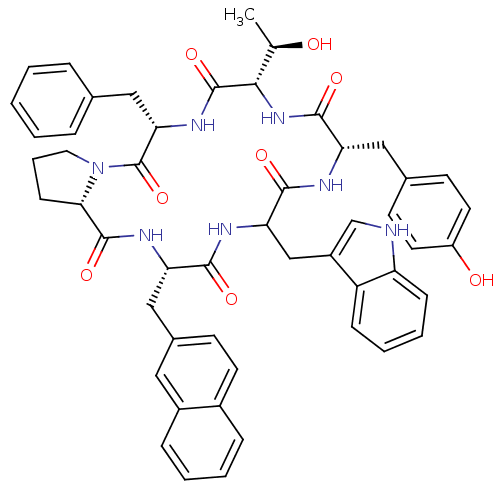 (CHEMBL1791165) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50051567 (5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR4. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50064025 (CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR5. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50051578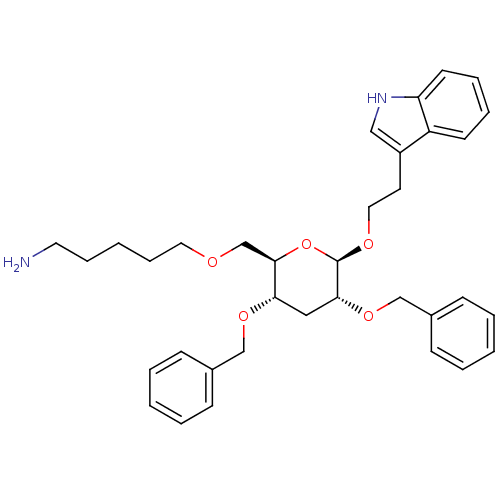 (5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR4. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50051567 (5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR2 | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50064024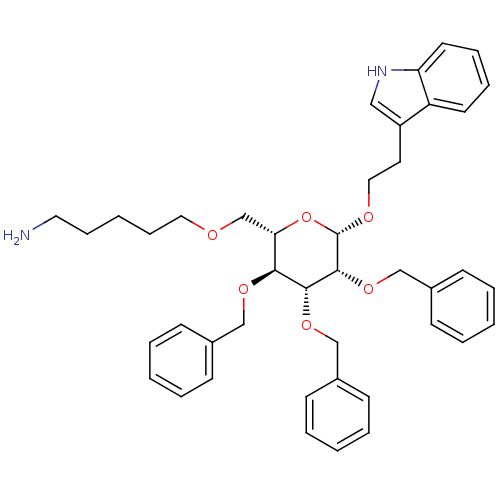 (5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR4. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50064030 (5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR4. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50064026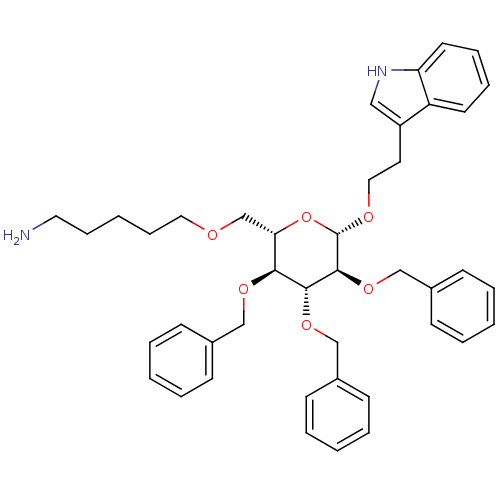 (5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR4. | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50064025 (CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR1 | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM50064030 (5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR1 | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50064024 (5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for human receptor subtype hSSTR2 | J Med Chem 41: 1382-91 (1998) Article DOI: 10.1021/jm9800346 BindingDB Entry DOI: 10.7270/Q28S4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 226 total ) | Next | Last >> |