

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455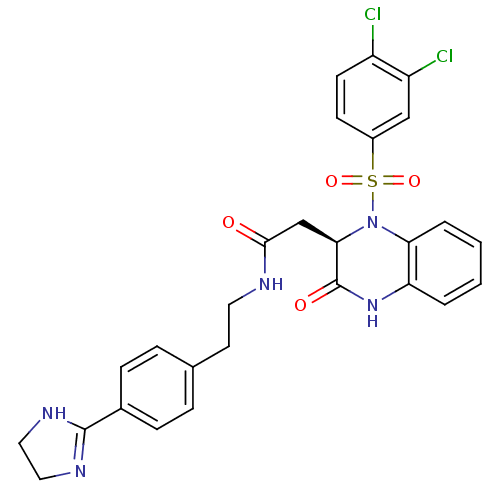 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272453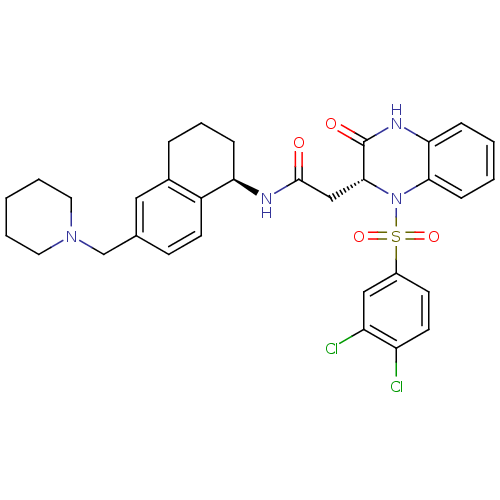 (2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272452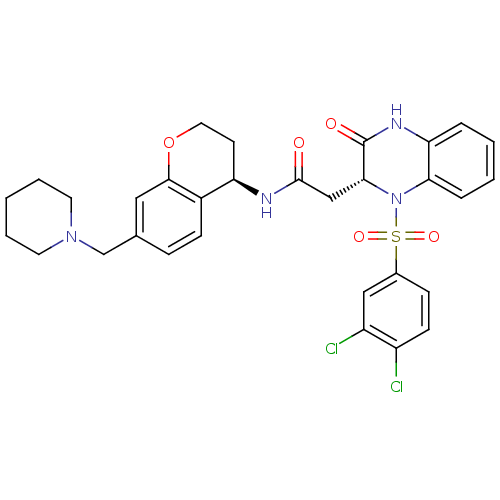 (2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31592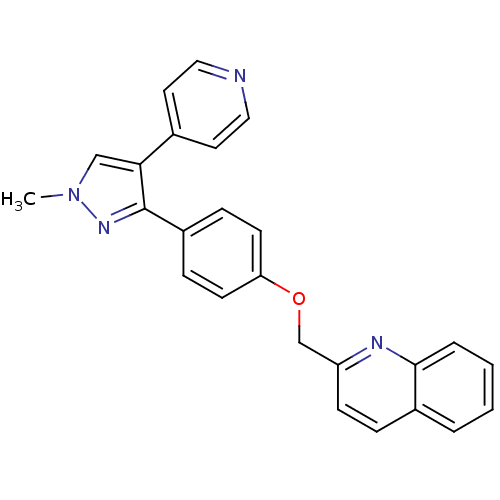 (PF-2545920 | US9138494, MP-10 | substituted pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum | J Med Chem 55: 4776-87 (2012) Article DOI: 10.1021/jm3002372 BindingDB Entry DOI: 10.7270/Q2WM1FFG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272455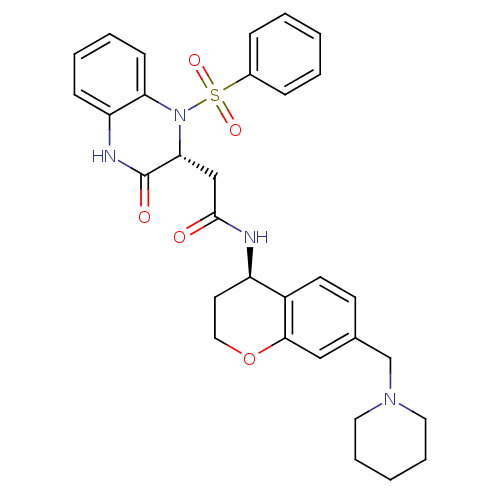 (2-((R)-3-oxo-1-(phenylsulfonyl)-1,2,3,4-tetrahydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272456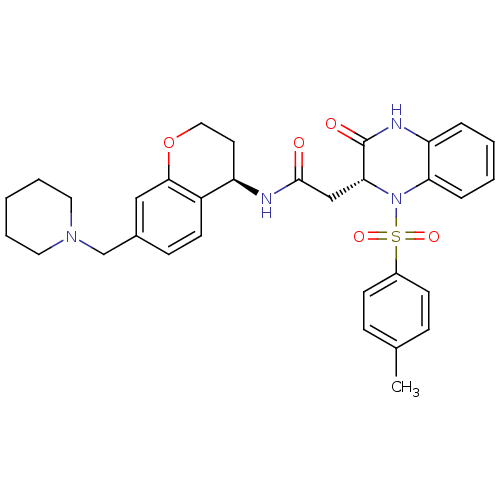 (2-((R)-3-oxo-1-tosyl-1,2,3,4-tetrahydroquinoxalin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 51: 5019-34 (2008) Article DOI: 10.1021/jm800463f BindingDB Entry DOI: 10.7270/Q2W096VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272450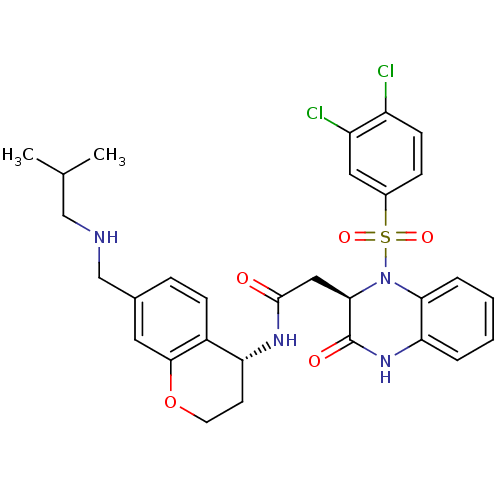 (2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272449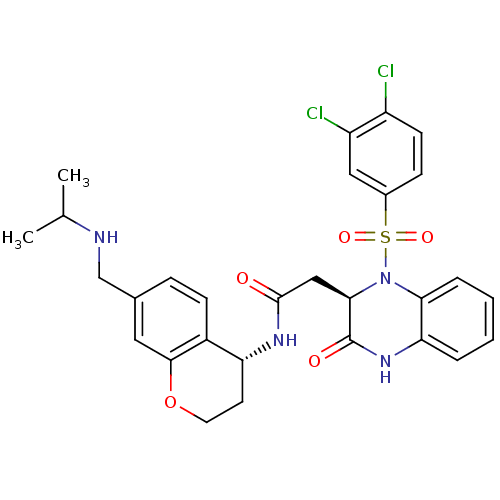 (2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50272598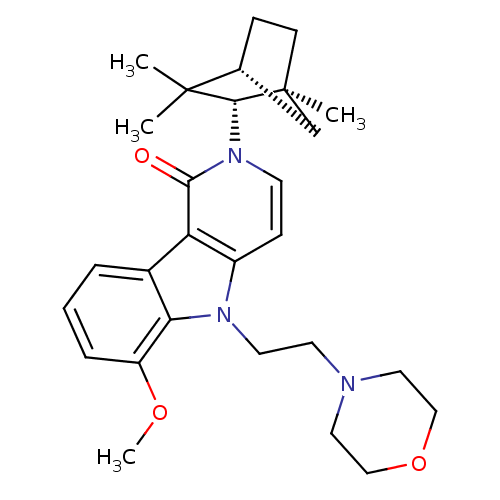 (6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 51: 5019-34 (2008) Article DOI: 10.1021/jm800463f BindingDB Entry DOI: 10.7270/Q2W096VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272451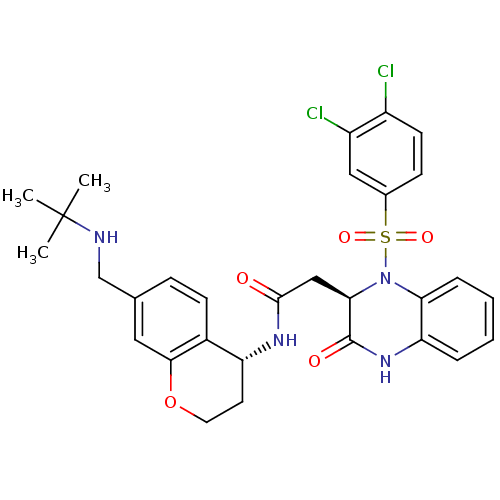 (CHEMBL508043 | N-((R)-7-((tert-butylamino)methyl)c...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM50365964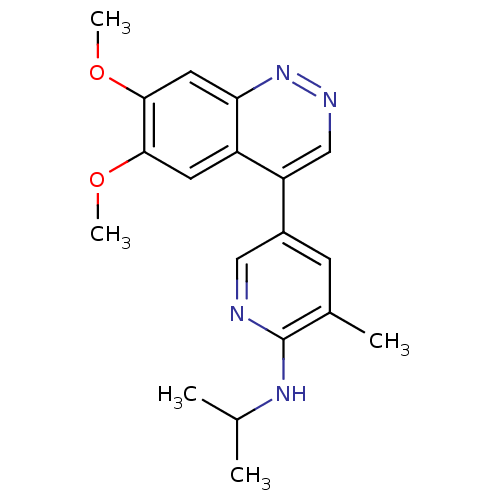 (CHEMBL1956235 | CHEMBL2070530) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum | J Med Chem 55: 4776-87 (2012) Article DOI: 10.1021/jm3002372 BindingDB Entry DOI: 10.7270/Q2WM1FFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272447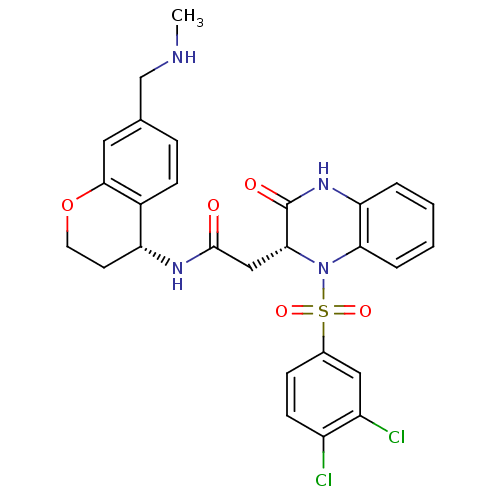 (2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272448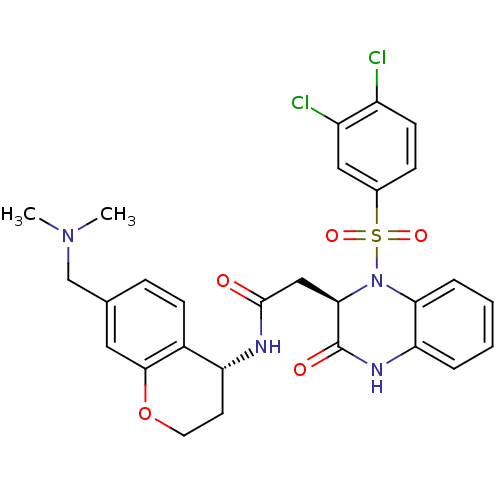 (2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272454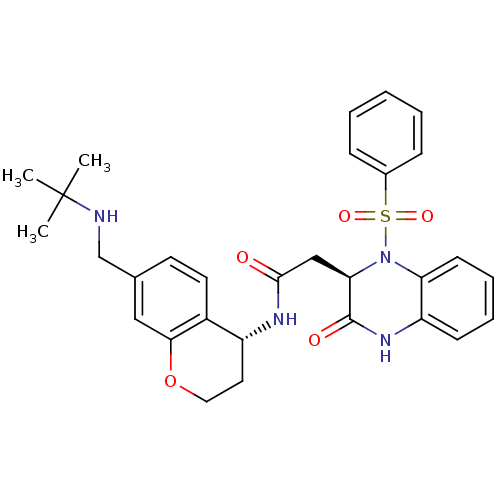 (CHEMBL525092 | N-((R)-7-((tert-butylamino)methyl)c...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | J Med Chem 51: 5019-34 (2008) Article DOI: 10.1021/jm800463f BindingDB Entry DOI: 10.7270/Q2W096VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272416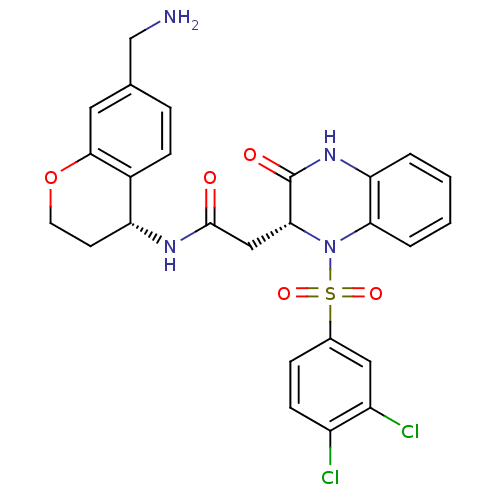 (CHEMBL496459 | N-((R)-7-(aminomethyl)chroman-4-yl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 6 (Homo sapiens (Human)) | BDBM412953 (US10406157, Compound Formula 1) | UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The inhibition constant (Ki) from a GPR6 competition binding assay and the IC50 values from a hERG functional assay for the compound of Formula 1 (Ex... | US Patent US10406157 (2019) BindingDB Entry DOI: 10.7270/Q2J105HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50272567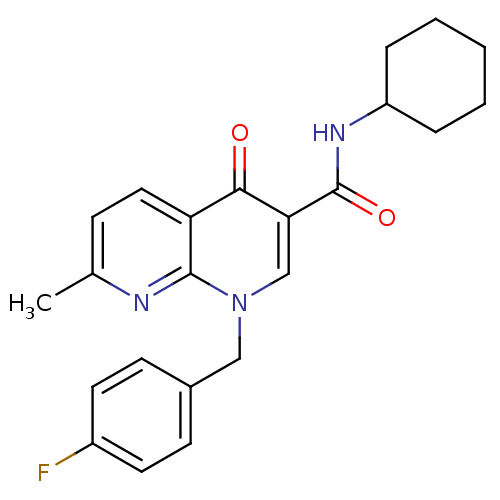 (1-(4-fluorobenzyl)-N-cyclohexyl-7-methyl-4-oxo-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | J Med Chem 51: 5019-34 (2008) Article DOI: 10.1021/jm800463f BindingDB Entry DOI: 10.7270/Q2W096VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 6 (Homo sapiens (Human)) | BDBM191037 (US10077266, Example 149 | US10406157, Compound A |...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The inhibition constant (Ki) from a GPR6 competition binding assay and the IC50 values from a hERG functional assay for the compound of Formula 1 (Ex... | US Patent US10406157 (2019) BindingDB Entry DOI: 10.7270/Q2J105HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 6 (Homo sapiens (Human)) | BDBM50582430 (CHEMBL4598390) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 3H-RL338 from human GPR6 expressed in T-REx-CHO-GPR6 cells assessed as inhibition constant by competition binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02081 BindingDB Entry DOI: 10.7270/Q2JW8JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 6 (Homo sapiens (Human)) | BDBM50582450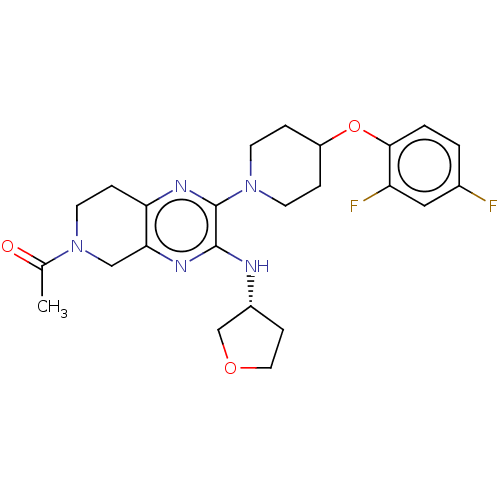 (CHEMBL4778540) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 3H-RL338 from human GPR6 expressed in T-REx-CHO-GPR6 cells assessed as inhibition constant by competition binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02081 BindingDB Entry DOI: 10.7270/Q2JW8JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263478 ((s)-2-(6,8-dimethyl-4-oxobenzo[d][1,2,3]triazin-3(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263478 ((s)-2-(6,8-dimethyl-4-oxobenzo[d][1,2,3]triazin-3(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263499 ((s)?n-(1-(2-fluoro-4-(trifluoromethyl)phenyl)ethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263499 ((s)?n-(1-(2-fluoro-4-(trifluoromethyl)phenyl)ethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50272567 (1-(4-fluorobenzyl)-N-cyclohexyl-7-methyl-4-oxo-1,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | J Med Chem 51: 5019-34 (2008) Article DOI: 10.1021/jm800463f BindingDB Entry DOI: 10.7270/Q2W096VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 6 (Homo sapiens (Human)) | BDBM50582449 (CHEMBL5093828) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 3H-RL338 from human GPR6 expressed in T-REx-CHO-GPR6 cells assessed as inhibition constant by competition binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02081 BindingDB Entry DOI: 10.7270/Q2JW8JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50272598 (6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | J Med Chem 51: 5019-34 (2008) Article DOI: 10.1021/jm800463f BindingDB Entry DOI: 10.7270/Q2W096VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50182221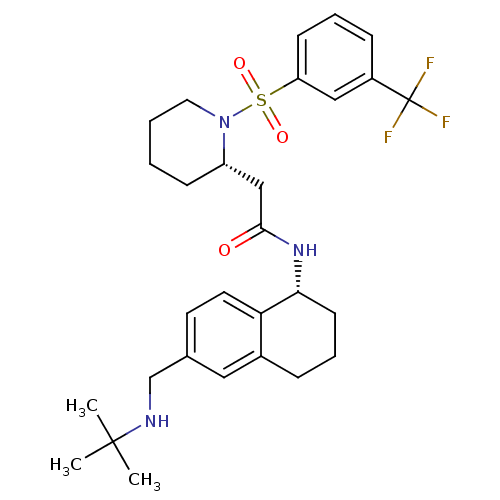 (CHEMBL381366 | N-((R)-6-((tert-butylamino)methyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263493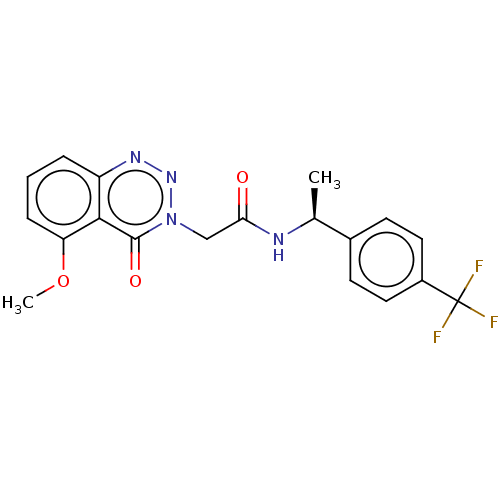 ((s)-2-(5-methoxy-4-oxobenzo[d][1,2,3]triazin-3 (4h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26 | -40.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263461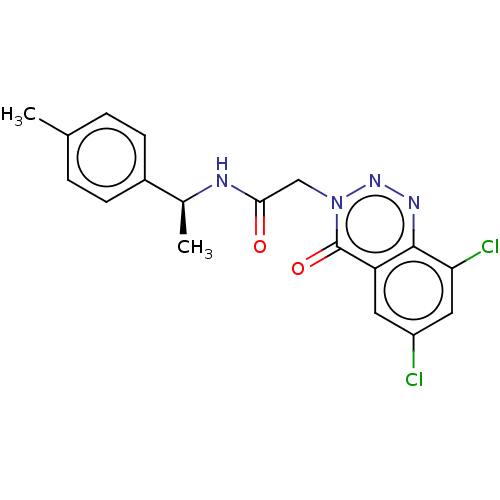 ((s)-2-(6,8-dichloro-4-oxobenzo[d][1,2,3]triazin-3(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26 | -40.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263461 ((s)-2-(6,8-dichloro-4-oxobenzo[d][1,2,3]triazin-3(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263493 ((s)-2-(5-methoxy-4-oxobenzo[d][1,2,3]triazin-3 (4h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263504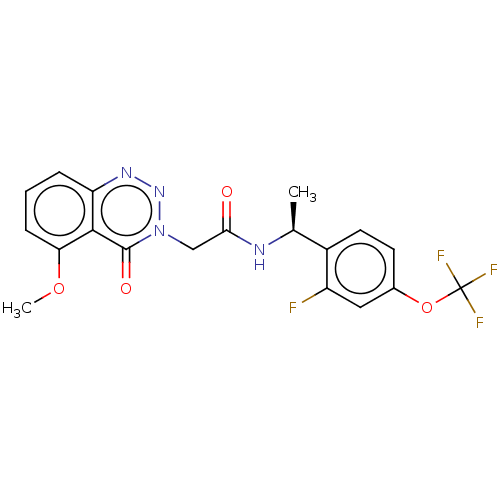 ((s)?n-(1-(2-fluoro-4-(trifluoromethoxy)phenyl)ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263504 ((s)?n-(1-(2-fluoro-4-(trifluoromethoxy)phenyl)ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of (S)-N-(1-(2-[3H]-4-methoxyphenyl)propan-2-yl)-2-(2,3-dimethyl-7-oxothieno[2,3-d]pyridazin-6(7H)-yl)acetamide from human GPR139 expres... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00820 BindingDB Entry DOI: 10.7270/Q25B068P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263504 ((s)?n-(1-(2-fluoro-4-(trifluoromethoxy)phenyl)ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 33 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263490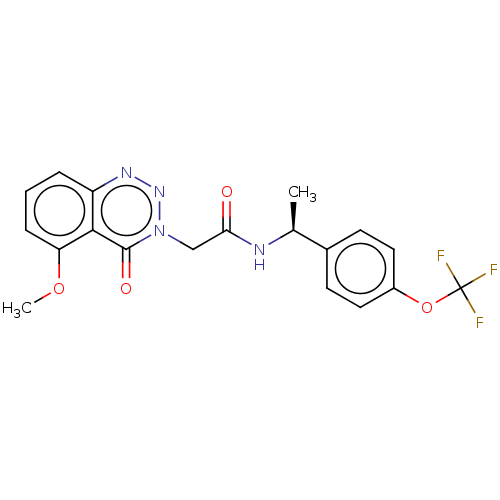 ((s)-2-(5-methoxy-4-oxobenzo[d][1,2,3]triazin-3(4h)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 33 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263490 ((s)-2-(5-methoxy-4-oxobenzo[d][1,2,3]triazin-3(4h)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263484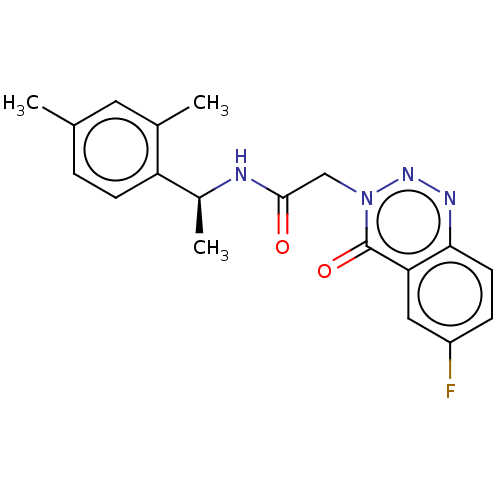 ((s)?n-(1-(2,4-dimethylphenyl)ethyl)-2-(6-fluoro-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 39 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263484 ((s)?n-(1-(2,4-dimethylphenyl)ethyl)-2-(6-fluoro-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263487 ((s)-2-(6-fluoro-4-oxobenzo[d][1,2,3]triazin-3(4h)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263487 ((s)-2-(6-fluoro-4-oxobenzo[d][1,2,3]triazin-3(4h)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 42 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263369 ((s)-2-(5-methoxy-4-oxobenzo[d][1,2,3]triazin-3(4h)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of (S)-N-(1-(2-[3H]-4-methoxyphenyl)propan-2-yl)-2-(2,3-dimethyl-7-oxothieno[2,3-d]pyridazin-6(7H)-yl)acetamide from human GPR139 expres... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00820 BindingDB Entry DOI: 10.7270/Q25B068P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263369 ((s)-2-(5-methoxy-4-oxobenzo[d][1,2,3]triazin-3(4h)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 48 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263369 ((s)-2-(5-methoxy-4-oxobenzo[d][1,2,3]triazin-3(4h)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272478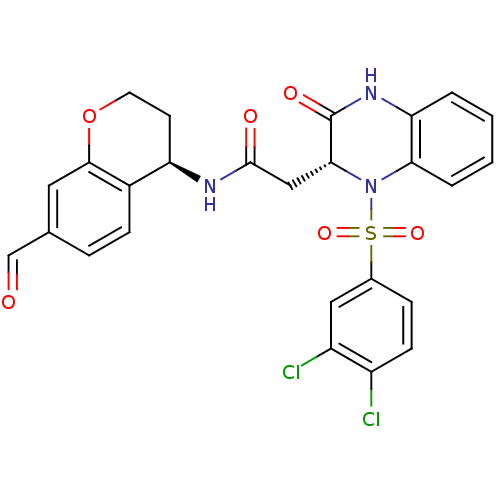 (2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique | Bioorg Med Chem Lett 18: 4477-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.055 BindingDB Entry DOI: 10.7270/Q2F76CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263453 ((s)-2-(6-methoxy-4-oxobenzo[d][1,2,3]triazin-3(4h)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 58 | -38.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description This membrane based assay measures the ability of compounds to competitively bind GPR139 in stably transfected CHO-TRex membranes. CHO-TRex (Life Tec... | US Patent US9556130 (2017) BindingDB Entry DOI: 10.7270/Q29025SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263453 ((s)-2-(6-methoxy-4-oxobenzo[d][1,2,3]triazin-3(4h)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Membranes were removed from −80° C., thawed and diluted in cold radioligand assay buffer (20 mM HEPES pH 7.4/5 mM MgCl2/1 mM CaCl2/Roche protea... | Citation and Details BindingDB Entry DOI: 10.7270/Q28D00DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable G-protein coupled receptor 139 (Homo sapiens (Human)) | BDBM263453 ((s)-2-(6-methoxy-4-oxobenzo[d][1,2,3]triazin-3(4h)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of (S)-N-(1-(2-[3H]-4-methoxyphenyl)propan-2-yl)-2-(2,3-dimethyl-7-oxothieno[2,3-d]pyridazin-6(7H)-yl)acetamide from human GPR139 expres... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00820 BindingDB Entry DOI: 10.7270/Q25B068P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2218 total ) | Next | Last >> |